Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States
Abstract
1. Introduction
2. Materials and Methods
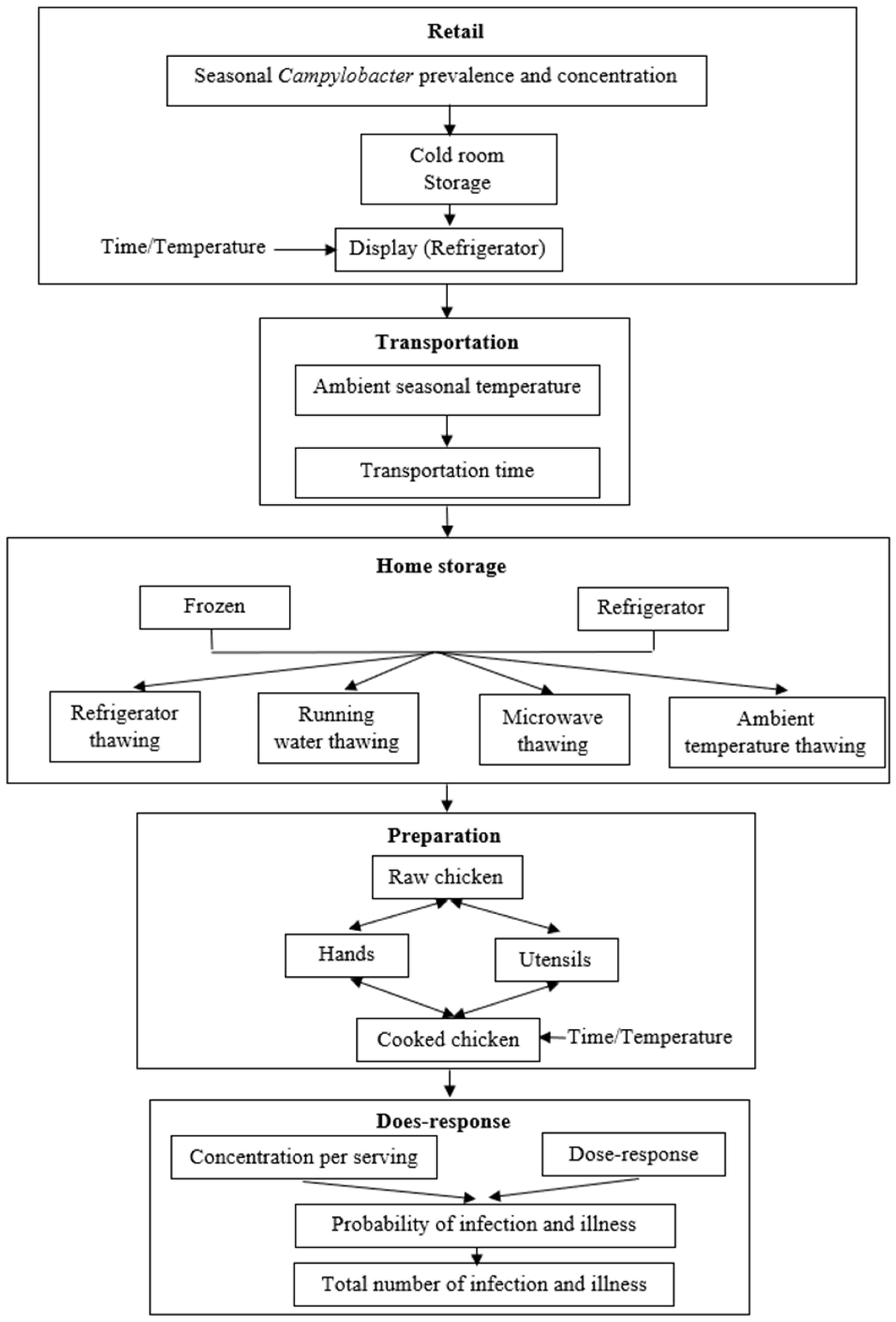
2.1. QMRA Overview
2.2. Campylobacter Growth Kinetics
2.3. Product Temperature Change
2.4. Retail Prevalence
2.5. Retail Storage
2.6. Transportation and Home Storage
2.7. Cross-Contamination during Preparation
2.8. Cooking
2.9. Dose–Response Modeling and Risk Characterization
2.10. “What-If” Scenarios
2.11. Risk Modeling
3. Results and Discussion
3.1. Seasonal Effect on Presence of Campylobacter in Chicken
3.2. Growth Rates
3.3. Effects of Ambient Temperature
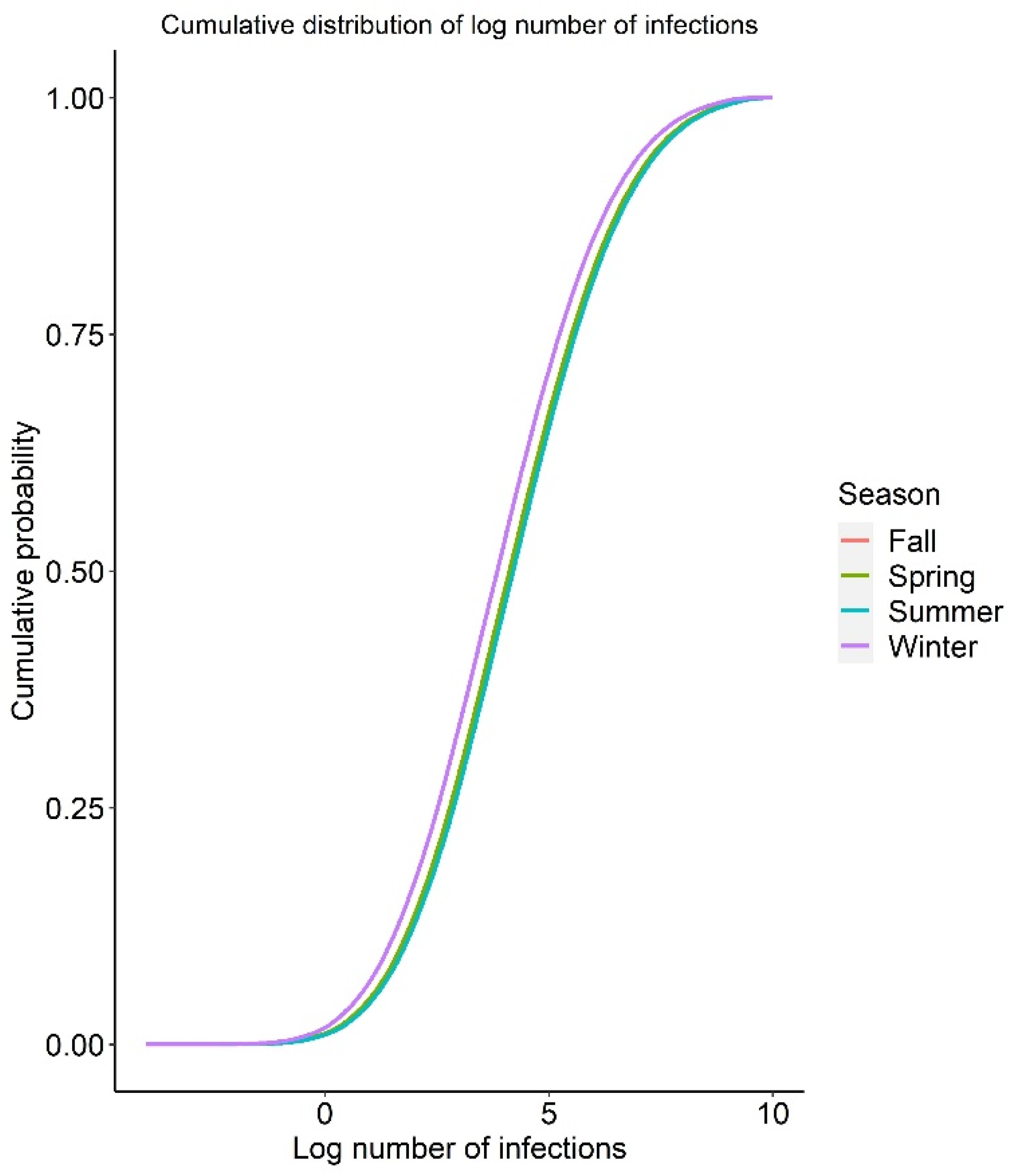
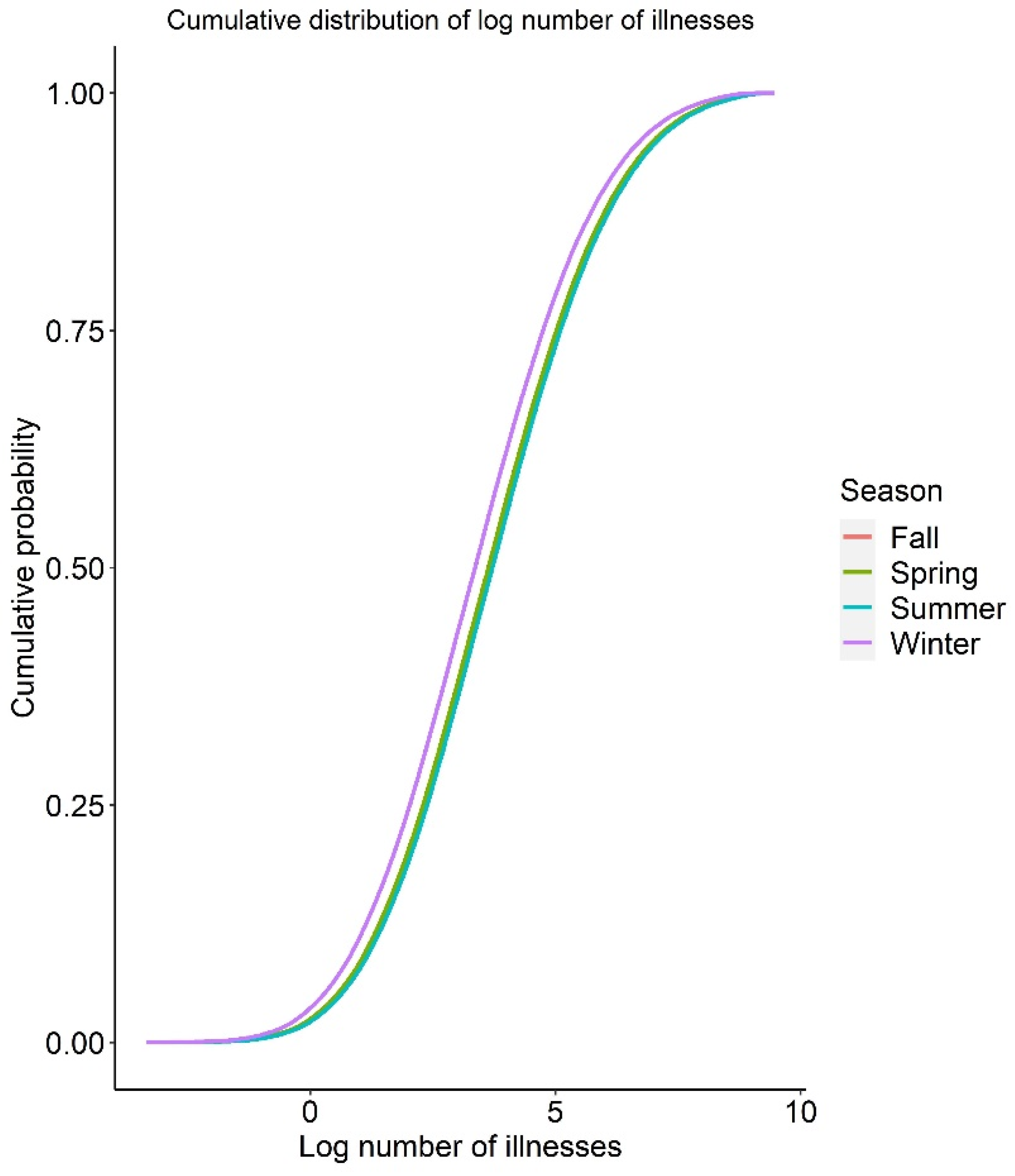
3.4. Baseline QMRA Model
3.5. Uncertainty Analysis
3.6. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Doorduyn, Y.; Van Den Brandhof, W.E.; Van Duynhoven, Y.T.; Breukink, B.J.; Wagenaar, J.A.; Van Pelt, W. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in The Netherlands: A case-control study. Epidemiol. Infect. 2010, 138, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- CDC. Centers for Disease Control and Prevention—National Outbreak Reporting System (NORS), Outbreaks Per State, Campylobacter, Chicken. 2020. Available online: https://wwwn.cdc.gov/norsdashboard/ (accessed on 19 September 2022).
- Hermans, D.; Pasmans, F.; Messens, W.; Martel, A.; Van Immerseel, F.; Rasschaert, G.; Heyndrickx, M.; Van Deun, K.; Haesebrouck, F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 2012, 12, 89–98. [Google Scholar] [CrossRef]
- Awada, R.; Ghssein, G.; Roz, A.E.; Farhat, M.; Nehme, N.; Hassan, H.F. Prevalence of Campylobacter spp. in broilers in North Lebanon. Vet. World 2023, 16, 322–328. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Mohnl, M.; Schatzmayr, G.; Böhm, J. Control strategies for Campylobacter infection in poultry production. World’s Poult. Sci. J. 2019, 69, 57–76. [Google Scholar] [CrossRef]
- Keener, K.M.; Bashor, M.P.; Curtis, P.A.; Sheldon, B.W.; Kathariou, S. Comprehensive Review of Campylobacter and Poultry Processing. Compr. Rev. Food Sci. Food Saf. 2004, 3, 105–116. [Google Scholar] [CrossRef]
- Stern, N.J. Influence of season and refrigerated storage on Campylobacter spp. contamination of broiler carcasses. J. Appl. Poult. Res. 1995, 4, 235–238. [Google Scholar] [CrossRef]
- Willis, W.L.; Murray, C. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poult. Sci. 1997, 76, 314–317. [Google Scholar] [CrossRef]
- Allain, V.; Chemaly, M.; Laisney, M.J.; Rouxel, S.; Quesne, S.; Le Bouquin, S. Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. Br. Poult. Sci. 2014, 55, 452–459. [Google Scholar] [CrossRef]
- Jorgensen, F.; Ellis-Iversen, J.; Rushton, S.; Bull, S.A.; Harris, S.A.; Bryan, S.J.; Gonzalez, A.; Humphrey, T.J. Influence of season and geography on Campylobacter jejuni and C. coli subtypes in housed broiler flocks reared in Great Britain. Appl. Environ. Microbiol. 2011, 77, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Nylen, G.; Dunstan, F.; Palmer, S.R.; Andersson, Y.; Bager, F.; Cowden, J.; Feierl, G.; Galloway, Y.; Kapperud, G.; Megraud, F.; et al. The seasonal distribution of campylobacter infection in nine European countries and New Zealand. Epidemiol. Infect. 2002, 128, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.N.; Eghnatios, E.; El Roz, A.; Fardoun, T.; Ghssein, G. Prevalence, antimicrobial resistance and risk factors for campylobacteriosis in Lebanon. J. Infect. Dev. Ctries. 2019, 13, 11–20. [Google Scholar] [CrossRef]
- Louis, V.R.; Gillespie, I.A.; O’Brien, S.J.; Russek-Cohen, E.; Pearson, A.D.; Colwell, R.R. Temperature-driven Campylobacter seasonality in England and Wales. Appl. Environ. Microbiol. 2005, 71, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Dunn, G.M.; Smith-Palmer, A.; Ogden, I.D.; Strachan, N.J. Human campylobacteriosis in Scotland: Seasonality, regional trends and bursts of infection. Epidemiol. Infect. 2004, 132, 585–593. [Google Scholar] [CrossRef]
- Dogan, O.B.; Clarke, J.; Mattos, F.; Wang, B. A quantitative microbial risk assessment model of Campylobacter in broiler chickens: Evaluating processing interventions. Food Control 2019, 100, 97–110. [Google Scholar] [CrossRef]
- Membre, J.M.; Boue, G. Quantitative microbiological risk assessment in food industry: Theory and practical application. Food Res. Int. 2018, 106, 1132–1139. [Google Scholar] [CrossRef]
- Haas, C.N.; Joan, B.R.; Charles, P.G. Quantitative Microbial Risk Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lindqvist, R.; Lindblad, M. Quantitative risk assessment of thermophilic Campylobacter spp. and cross-contamination during handling of raw broiler chickens evaluating strategies at the producer level to reduce human campylobacteriosis in Sweden. Int. J. Food Microbiol. 2008, 121, 41–52. [Google Scholar] [CrossRef]
- Hartnett, E.; Kelly, L.; Newell, D.; Wooldridge, M.; Gettinby, G. A quantitative risk assessment for the occurrence of Campylobacter in chickens at the point of slaughter. Epidemiol. Infect. 2001, 127, 195–206. [Google Scholar] [CrossRef]
- Blankenship, L.C.; Craven, S.E. Campylobacter jejuni survival in chicken meat as a function of temperature. Appl. Environ. Microbiol. 1982, 44, 88–92. [Google Scholar] [CrossRef]
- Nicorescu, I.; Crivineanu, M. The influence of temperature and gas mixtures on growth and survival of Campylobacter jejuni in chicken meat. Sci. Works-Univ. Agron. Sci. Vet. Med. Buchar. Ser. C Vet. Med. 2009, 55, 77–82. [Google Scholar]
- Solow, B.T.; Cloak, O.M.; Fratamico, P.M. Effect of temperature on viability of Campylobacter jejuni and Campylobacter coli on raw chicken or pork skin. J. Food Prot. 2003, 66, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Hazeleger, W.C.; Wouters, J.A.; Rombouts, F.M.; Abee, T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 1998, 64, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Park, S.F. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 2002, 74, 177–188. [Google Scholar] [CrossRef]
- Golden, C.E.; Mishra, A. Assessing the risk of salmonellosis from consumption of conventionally and alternatively produced broiler meat prepared in-Home in the United States. Microb. Risk Anal. 2021, 18, 100160. [Google Scholar] [CrossRef]
- Hinton, J.A.; Cason, J.A.; Hume, M.E.; Ingram, K.D. Spread of Campylobacter spp. during poultry processing in different seasons. Int. J. Poult. Sci. 2004, 3, 432–437. [Google Scholar]
- USDA. United States Department of Agriculture. The Nationwide Microbiological Baseline Data Collection Program: Raw Chicken Parts Survey. 2012. Available online: https://www.fsis.usda.gov/shared/PDF/Baseline_Data_Raw_Chicken_Parts.pdf (accessed on 2 October 2022).
- Derens, E.; Palagos, B.; Guilpart, J. The cold chain of chilled products under supervision in France. In Proceedings of the 13th World Congress of Food Science & Technology, Nantes, France, 17–21 September 2006. [Google Scholar]
- CR. Current Results—Average Temperatures for Large US Cities. 2022. Available online: https://www.currentresults.com/Weather/US/average-city-temperatures-in-january.php (accessed on 10 October 2022).
- Ecosure. 2007 U.S. Cold Temperature Evaluation Design and Summary Pages. 2008. Available online: http://old.foodrisk.org/default/assets/File/EcoSure%202007%20Cold%20Temperature%20Report.pdf (accessed on 16 September 2022).
- Mazengia, E.; Fisk, C.; Liao, G.; Huang, H.; Meschke, J. Direct observational study of the risk of cross-contamination during raw poultry: Handling practices in private homes. Food Prot. Trends 2015, 35, 8–23. [Google Scholar]
- Booten, C.; Robertson, J.; Chritensen, D.; Heaney, M.; Brown, D.; Norton, P.; Smith, C. Residential Indoor Temperature Study. 2017. Available online: https://www.nrel.gov/docs/fy17osti/68019.pdf (accessed on 15 September 2022).
- Bruhn, C.M. Chicken preparation in the home: An observational study. Food Prot. Trends 2014, 34, 318–330. [Google Scholar]
- Byrd-Bredbenner, C.; Maurer, J.; Wheatley, V.; Cottone, E.; Clancy, M. Food safety hazards lurk in the kitchens of young adults. J. Food Prot. 2007, 70, 991–996. [Google Scholar] [CrossRef]
- Evison, L.; Sunna, N. Microbial regrowth in household water storage tanks. J.-Am. Water Work. Assoc. 2001, 93, 85–94. [Google Scholar] [CrossRef]
- Taher, B.J.; Farid, M.M. Cyclic microwave thawing of frozen meat: Experimental and theoretical investigation. Chem. Eng. Process. Process Intensif. 2001, 40, 379–389. [Google Scholar] [CrossRef]
- Chen, Y.; Jackson, K.M.; Chea, F.P.; Schaffner, D.W. Quantification and variability analysis of bacterial cross-contamination rates in common food service tasks. J. Food Prot. 2001, 64, 72–80. [Google Scholar] [CrossRef]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of Campylobacter species cross-contamination during handling of contaminated fresh chicken parts in kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff-Bakkenes, L.; Beumer, R.R.; de Jonge, R.; van Leusden, F.M.; de Jong, A.E. Quantification of Campylobacter jejuni cross-contamination via hands, cutlery, and cutting board during preparation of a chicken fruit salad. J. Food Prot. 2008, 71, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. A quantitative risk assessment model for Salmonella and whole chickens. Int. J. Food Microbiol. 2004, 93, 231–247. [Google Scholar] [CrossRef]
- van Asselt, E.D.; Zwietering, M.H. A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int. J. Food Microbiol. 2006, 107, 73–82. [Google Scholar] [CrossRef]
- Kosa, K.M.; Cates, S.; Bradley, S.; Chambers, E.I.; Godwin, S. Consumerreported handling of raw poultry products at home: Results from a national survey. J. Food Prot. 2015, 78, 180–186. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Havelaar, A.H. The Beta Poisson dose-response model is not a single-hit model. Risk Anal. 2000, 20, 513–520. [Google Scholar] [CrossRef]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Hughes, T.P.; Blaser, M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988, 157, 472–479. [Google Scholar] [CrossRef]
- Havelaar, A.H.; de Wit, M.A.; van Koningsveld, R.; van Kempen, E. Health burden in the Netherlands due to infection with thermophilic Campylobacter spp. Epidemiol. Infect. 2000, 125, 505–522. [Google Scholar] [CrossRef]
- Nauta, M.J.; Jacobs-Reitsma, W.F.; Havelaar, A.H. A risk assessment model for Campylobacter in broiler meat. Risk Anal. 2007, 27, 845–861. [Google Scholar] [CrossRef]
- USDA/ERS. United States Department of Agriculture/Economic Research Service. Food Availability (Per Capita) Data System. 2019. Available online: https://www.ers.usda.gov/data-products/foodavailability-per-capita-data-system/ (accessed on 16 September 2022).
- NCC. Nationtal Chicken Council. US Chicken Consumption. 2015. Available online: https://www.nationalchickencouncil.org/wp-content/uploads/2015/07/2015-US-chicken-consumption-NCC.pdf (accessed on 5 October 2022).
- NCC. National Chicken Council. Chicken Usage Summary. 2014. Available online: https://www.nationalchickencouncil.org/wp-content/uploads/2014/07/Consumer-Research-2014-Presentation-Final-0717141-1.pdf (accessed on 16 September 2022).
- Buchanan, R.L.; Whiting, R.C.; Damert, W.C. When is simple good enough: A comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997, 14, 313–326. [Google Scholar] [CrossRef]
- Huang, L. IPMP 2013—A comprehensive data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2014, 171, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Guo, M.; Buchanan, R.L.; Schaffner, D.W.; Pradhan, A.K. Prediction of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes growth in leafy greens without temperature control. J. Food Prot. 2016, 80, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E. What are the seasons? Bull. Am. Meteorol. Soc. 1983, 64, 1276–1282. [Google Scholar] [CrossRef]
- Ritz, M.; Nauta, M.J.; Teunis, P.F.; van Leusden, F.; Federighi, M.; Havelaar, A.H. Modelling of Campylobacter survival in frozen chicken meat. J. Appl. Microbiol. 2007, 103, 594–600. [Google Scholar] [CrossRef]
- USDA. United States Department of Agriculture. The Big Thaw—Safe Defrosting Methods for Consumers. 2013. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/safe-food-handling/the-big-thaw-safe-defrosting-methods-for-consumers (accessed on 10 October 2022).
- CLS. Cornell Law School—9 CFR § 381.412—Reference Amounts Customarily Consumed Per Eating Occasion. 2020. Available online: https://www.law.cornell.edu/cfr/text/9/381.412 (accessed on 11 October 2022).
- Medema, G.J.; Teunis, P.F.; Havelaar, A.H.; Haas, C.N. Assessment of the dose-response relationship of Campylobacter jejuni. Int. J. Food Microbiol. 1996, 30, 101–111. [Google Scholar] [CrossRef]
- Pang, H.; Lambertini, E.; Buchanan, R.L.; Schaffner, D.W.; Pradhan, A.K. Quantitative microbial risk assessment for Escherichia coli O157:H7 in fresh-cut lettuce. J. Food Prot. 2017, 80, 302–311. [Google Scholar] [CrossRef]
- Nannapaneni, R.; Hanning, I.; Wiggins, K.C.; Story, R.P.; Ricke, S.C.; Johnson, M.G. Ciprofloxacin-resistant Campylobacter persists in raw retail chicken after the fluoroquinolone ban. Food Addit. Contam. 2009, 26, 1348–1353. [Google Scholar] [CrossRef]
- Berrang, M.E.; Meinersmann, R.J.; Ladely, S.R.; Cox, N.A. Campylobacter detection in broiler ceca at processing: A three-year, 211-flock survey. J. Appl. Poult. Res. 2017, 26, 154–158. [Google Scholar] [CrossRef]
- Williams, A.; Oyarzabal, O.A. Prevalence of Campylobacter spp. in skinless, boneless retail broiler meat from 2005 through 2011 in Alabama. BMC Microbiol. 2012, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Boysen, L.; Vigre, H.; Rosenquist, H. Seasonal influence on the prevalence of thermotolerant Campylobacter in retail broiler meat in Denmark. Food Microbiol. 2011, 28, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Franklin-Hayes, P.; Koolman, L.; Egan, J.; Gutierrez, M.; Byrne, W.; Golden, O.; Bolton, D.; Reid, P.; Coffey, A.; et al. Prevalence and levels of Campylobacter in broiler chicken batches and carcasses in Ireland in 2017–2018. Int. J. Food Microbiol. 2022, 372, 109693. [Google Scholar] [CrossRef]
- Garcia-Sanchez, L.; Melero, B.; Diez, A.M.; Jaime, I.; Canepa, A.; Rovira, J. Genotyping, virulence genes and antimicrobial resistance of Campylobacter spp.isolated during two seasonal periods in Spanish poultry farms. Prev. Vet. Med. 2020, 176, 104935. [Google Scholar] [CrossRef]
- Mercier, S.; Villeneuve, S.; Mondor, M.; Uysal, I. Time-Temperature Management Along the Food Cold Chain: A Review of Recent Developments. Compr. Rev. Food Sci. Food Saf. 2017, 16, 647–667. [Google Scholar] [CrossRef]
- Kim, S.A.; Yun, S.J.; Lee, S.H.; Hwang, I.G.; Rhee, M.S. Temperature increase of foods in car trunk and the potential hazard for microbial growth. Food Control 2013, 29, 66–70. [Google Scholar] [CrossRef]
- USDA/FSIS. Kitchen Companion: Your Safe Food Handbook. 2008. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-12/Kitchen-Companion.pdf (accessed on 12 October 2022).
- IBIS. Public Health Indicator Based Information System. Complete Health Indicator Report of Foodborne Illness—Campylobacter Infections. 2021. Available online: https://ibis.health.utah.gov/ibisph-view/indicator/complete_profile/FooPoiCampy.html (accessed on 13 October 2022).
- Wagenaar, J.A.; French, N.P.; Havelaar, A.H. Preventing Campylobacter at the source: Why is it so difficult? Clin. Infect. Dis. 2013, 57, 1600–1606. [Google Scholar] [CrossRef]
- Hall, A.J.; Wikswo, M.E.; Manikonda, K.; Roberts, V.A.; Yoder, J.S.; Gould, L.H. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013, 19, 1305–1309. [Google Scholar] [CrossRef]
- Batz, M.B.; Hoffmann, S.; Morris, J.G., Jr. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef]
- Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K.; Tauxe, R.V. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. sites, 1996–2012. Morb. Mortal. Wkly. Rep. 2013, 62, 283. [Google Scholar]
- Engberg, J. Contributions to the Epidemiology of Campylobacter infections, A review of clinical and microbiological studies. Dan. Med. Bull. 2006, 53, 361–389. Available online: https://www.ncbi.nlm.nih.gov/pubmed/17150145 (accessed on 13 October 2022). [PubMed]
- Hall, G.V.; Kirk, M.D.; Ashbolt, R.; Stafford, R.; Lalor, K. Frequency of infectious gastrointestinal illness in Australia, 2002: Regional, seasonal and demographic variation. Epidemiol. Infect. 2006, 134, 111–118. [Google Scholar] [CrossRef]
- Bull, S.A.; Allen, V.M.; Domingue, G.; Jorgensen, F.; Frost, J.A.; Ure, R.; Whyte, R.; Tinker, D.; Corry, J.E.; Gillard-King, J.; et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 2006, 72, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mkhungo, M.C.; Oyedeji, A.B.; Ijabadeniyi, O.A. Food safety knowledge and microbiological hygiene of households in selected areas of Kwa-Zulu Natal, South Africa. Ital. J. Food Saf. 2018, 7, 6887. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Pouillot, R.; Garin, B.; Ravaonindrina, N.; Diop, K.; Ratsitorahina, M.; Ramanantsoa, D.; Rocourt, J. A risk assessment of campylobacteriosis and salmonellosis linked to chicken meals prepared in households in Dakar, Senegal. Risk Anal. 2012, 32, 1798–1819. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; van Asselt, E.D.; Beumer, R.R.; Zwietering, M.H. A quantitative analysis of cross-contamination of Salmonella and Campylobacter spp. via domestic kitchen surfaces. J. Food Prot. 2004, 67, 1892–1903. [Google Scholar] [CrossRef]
- Lopez, G.U.; Kitajima, M.; Sherchan, S.P.; Sexton, J.D.; Sifuentes, L.Y.; Gerba, C.P.; Reynolds, K.A. Impact of disinfectant wipes on the risk of Campylobacter jejuni infection during raw chicken preparation in domestic kitchens. J. Appl. Microbiol. 2015, 119, 245–252. [Google Scholar] [CrossRef]
- Yu, B.J.; Kim, J.A.; Pan, J.G. Signature gene expression profile of triclosan-resistant Escherichia coli. J. Antimicrob. Chemother. 2010, 65, 1171–1177. [Google Scholar] [CrossRef]
- de Jong, A.E.; Verhoeff-Bakkenes, L.; Nauta, M.J.; de Jonge, R. Cross-contamination in the kitchen: Effect of hygiene measures. J. Appl. Microbiol. 2008, 105, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Montville, R.; Chen, Y.; Schaffner, D.W. Glove barriers to bacterial cross-contamination between hands to food. J. Food Prot. 2001, 64, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Maughan, C.; Chambers, E.I.; Godwin, S.; Chambers, D.; Cates, S.; Koppel, K. Food Handling Behaviors Observed in Consumers When Cooking Poultry and Eggs. J. Food Prot. 2016, 79, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Signorini, M.L.; Zbrun, M.V.; Romero-Scharpen, A.; Olivero, C.; Bongiovanni, F.; Soto, L.P.; Frizzo, L.S.; Rosmini, M.R. Quantitative risk assessment of human campylobacteriosis by consumption of salad cross-contaminated with thermophilic Campylobacter spp. from broiler meat in Argentina. Prev. Vet. Med. 2013, 109, 37–46. [Google Scholar] [CrossRef] [PubMed]

| Variable | Cell | Distribution, Value, or Formula | Unit | Source |
|---|---|---|---|---|
| Growth Parameter | ||||
| Growth model, b | B4 | =0.04673 | No unit | [22,23,24] |
| Growth model, Tmin | B5 | =31.96 | °C | [22,23,24] |
| Observed Tmin | B6 | =31 | °C | [25,26] |
| Newton heating constant, B | B7 | =2.026 | h−1 | [27] |
| Retail | ||||
| Retail Campylobacter prevalence, Spring | B9 | =RiskPert(0.41877,0.48933,0.48933) | Proportion | [10,28] |
| Retail Campylobacter prevalence, Summer | B10 | =RiskTriang(0.546,0.546,0.608227) | Proportion | [10,28] |
| Retail Campylobacter prevalence, Fall | B11 | =RiskUniform(0.507695,0.546205) | Proportion | [10,28] |
| Retail Campylobacter prevalence, Winter | B12 | =RiskUniform(0.24443,0.268024) | Proportion | [10,28] |
| Campylobacter concentration, if positive at purchase | B13 | =RiskWeibull(2.5448,1.9265,RiskShift(−1.4281)) | log CFU/g | [29] |
| Retail cold room storage time | B14 | =RiskExpon(0.58736,RiskShift(0.00027443)) 4 | h | [30] |
| Retail cold room storage temperature | B15 | =RiskNormal(3.3188,1.7533) | °C | [30] |
| Retail display storage time | B16 | =RiskExpon(0.22461,RiskShift(−0.0000889766)) 24 | h | [30] |
| Retail display storage temperature | B17 | =RiskNormal(3.2321,1.3117) | °C | [30] |
| Growth rate during retail storage | B18 | =IF(B15 < B6,0,(B4 (B15 − B5))2) + IF(B17 < B6,0,(B4 (B17 − B5))2) | log CFU/h | Calculated |
| Change during retail storage | B19 | =B18 × (B14 + B16) | log CFU/g | Calculated |
| Concentration at point of purchase | B20 | =IF((B19 + B13) > 5,5,B19 + B13) | log CFU/g | Calculated |
| Transportation | ||||
| Ambient temperature during transportation, Spring | B22 | =RiskPert(1.7239,21.039,35.837) | °C | [31] |
| Ambient temperature during transportation, Summer | B23 | =RiskPert(17.826,30.476,42.062) | °C | [31] |
| Ambient temperature during transportation, Fall | B24 | =RiskPert(3.9191,21.889,39.226) | °C | [31] |
| Ambient temperature during transportation, Winter | B25 | =RiskPert(−6.2993,10.263,29.747) | °C | [31] |
| Transportation time | B26 | =RiskLoglogistic(0.0063772,1.0915,4.6212,RiskTruncate(0.3,18.45)) | h | [32] |
| Transportation growth rate, Spring | B27 | =IF(B22 < B6,0,(B4 ((B22 − (EXP(−B7 B26) (B22 − B17))) − B5))2) | log CFU/h | Calculated |
| Transportation growth rate, Summer | B28 | =IF(B23 < B6,0,(B4 ((B23 − (EXP(−B7 B26) (B23 − B17))) − B5))2) | log CFU/h | Calculated |
| Transportation growth rate, Fall | B29 | =IF(B24 < B6,0,(B4 × ((B24 − (EXP(−B7 B26) (B24 − B17))) − B5))2) | log CFU/h | Calculated |
| Transportation growth rate, Winter | B30 | =IF(B25 < B6,0,(B4 ((B25 − (EXP(−B7 B26) (B25 − B17))) − B5))2) | log CFU/h | Calculated |
| Change during transportation, Spring | B31 | =B27 B26 | log CFU/g | Calculated |
| Change during transportation, Summer | B32 | =B28 B26 | log CFU/g | Calculated |
| Change during transportation, Fall | B33 | =B29 B26 | log CFU/g | Calculated |
| Change during transportation, Winter | B34 | =B30 B26 | log CFU/g | Calculated |
| Concentration after transportation, Spring | B35 | =B31 + B20 | log CFU/g | Calculated |
| Concentration after transportation, Summer | B36 | =B32 + B20 | log CFU/g | Calculated |
| Concentration after transportation, Fall | B37 | =B33 + B20 | log CFU/g | Calculated |
| Concentration after transportation, Winter | B38 | =B34 + B20 | log CFU/g | Calculated |
| Home storage | ||||
| Does chicken get frozen? | B40 | =RiskBernoulli(0.4) | No unit | [33] |
| If frozen: | ||||
| Time until frozen | B42 | =RiskBetaGeneral(0.0067951,0.59992,0,2) | h | [33] |
| Ambient room temperature | B43 | =RiskNormal(22.3107,5.8722,RiskTruncate(15,30)) | °C | [34] |
| Growth rate before products were put in freezer | B44 | =IF(B43 < B6,0,(B4 (B43 − B5))2) | log CFU/h | Calculated |
| Change before frozen | B45 | =B44 B43 | log CFU/g | Calculated |
| Concentration before frozen, Spring | B46 | =B45 + B35 | log CFU/g | Calculated |
| Concentration before frozen, Summer | B47 | =B45 + B36 | log CFU/g | Calculated |
| Concentration before frozen, Fall | B48 | =B45 + B37 | log CFU/g | Calculated |
| Concentration before frozen, Winter | B49 | =B45 + B38 | log CFU/g | Calculated |
| Home refrigerator temperature | B50 | =RiskLaplace(4.4444,2.5231) | °C | [35] |
| Home freezer temperature | B51 | =RiskNormal(−9.275,5.2857,RiskTruncate(−25,0)) | °C | [36] |
| Thawing method | B52 | =RiskDiscrete({1,2,3,4},{0.48,0.14,0.24,0.14}) | No unit | [33] |
| If thaw method =1: | ||||
| Thaw time | B54 | =RiskTriang(2,24,72) | h | |
| Growth rate during refrigerated thawing | B55 | =IF(B50 < B6,0,(B4 ((B50 − (EXP(−B7 B54) (B50 − B51))) − B5))2) | log CFU/h | Calculated |
| Change during refrigerated thawing | B56 | =IF(B52 = 1,B54 B55,0) | log CFU/g | Calculated |
| If thaw method = 2: | ||||
| Running water temperature | B58 | =RiskPert(14,22.9,30) | °C | [37] |
| Thaw time | B59 | =RiskTriang(0.25,1,2) | ||
| Growth rate during running water thawing | B60 | =(B4 ((B58 − (EXP(−B7 B59) (B58 − B51))) − B5))2 | log CFU/h | Calculated |
| Change during running water thawing | B61 | =IF(B52 = 2,B59 B60,0) | log CFU/g | Calculated |
| If thaw method = 3: | ||||
| Temperature of meat during microwave thawing | B63 | =RiskPert(−8,−4,8) | °C | [38] |
| Thaw time | B64 | =RiskUniform(8,20)/60 | h | |
| Growth rate during microwave thawing | B65 | =IF(B63 < B6,0,(B4 ((B63 − (EXP(−B7 B64) (B63 − B51))) − B5))2) | log CFU/h | Calculated |
| Change during microwave thawing | B66 | =IF(B52 = 3,B64 B65,0) | log CFU/g | Calculated |
| If thaw method = 4: | ||||
| Ambient room temperature | B68 | =RiskNormal(22.3107,5.8722,RiskTruncate(15,30)) | °C | [34] |
| Thaw time | B69 | =RiskUniform(1,10) | h | |
| Growth during room temperature thawing | B70 | =(B4 ((B68 − (EXP(−B7 B69) (B68 − B51))) − B5))2 | log CFU/h | Calculated |
| Change during room temperature thawing | B71 | =IF(B52 = 4,B69 × B70,0) | log CFU/g | Calculated |
| Concentration after thawing, Spring | B72 | =IF(B40 = 1,B46 + B56 + B61 + B66 + B71,0) | log CFU/g | Calculated |
| Concentration after thawing, Summer | B73 | =IF(B40 = 1,B47 + B56 + B61 + B66 + B71,0) | log CFU/g | Calculated |
| Concentration after thawing, Fall | B74 | =IF(B40 = 1,B48 + B56 + B61 + B66 + B71,0) | log CFU/g | Calculated |
| Concentration after thawing, Winter | B75 | =IF(B40 = 1,B49 + B56 + B61 + B66 + B71,0) | log CFU/g | Calculated |
| If not frozen: | ||||
| Refrigerator storage time | B78 | =RiskPareto(3.4887,2,RiskTruncate(0,5)) 24 | h | [33] |
| Growth rate during refrigerated storage | B79 | =IF(B50 < B6,0,(B4 (B50 − B5))2) | log CFU/h | Calculated |
| Change during storage | B80 | =B77 B78 | log CFU/g | Calculated |
| Concentration after storage, Spring | B81 | =IF(B40 = 1,0,B35 + B79) | log CFU/g | Calculated |
| Concentration after storage, Summer | B82 | =IF(B40 = 1,0,B36 + B79) | log CFU/g | Calculated |
| Concentration after storage, Fall | B83 | =IF(B40 = 1,0,B37 + B79) | log CFU/g | Calculated |
| Concentration after storage, Winter | B84 | =IF(B40 = 1,0,B38 + B79) | log CFU/g | Calculated |
| Concentration before preparation, Spring | B85 | =B72 + B80 | log CFU/g | Calculated |
| Concentration before preparation, Summer | B86 | =B73 + B81 | log CFU/g | Calculated |
| Concentration before preparation, Fall | B87 | =B74 + B82 | log CFU/g | Calculated |
| Concentration before preparation, Winter | B88 | =B75 + B83 | log CFU/g | Calculated |
| Preparation | ||||
| Raw chicken handling: | ||||
| Transfer rate from raw chicken to hands | B90 | =RiskLognorm(0.15555,1.0547,RiskShift(0.00058696),RiskTruncate(0,1)) | Proportion | [39,40,41] |
| Concentration on hands after handling, Spring | B91 | =LOG10(B90 (10B85)) | log CFU/g | Calculated |
| Concentration on hands after handling, Summer | B92 | =LOG10(B90 (10B86)) | log CFU/g | Calculated |
| Concentration on hands after handling, Fall | B93 | =LOG10(B90 (10B87)) | log CFU/g | Calculated |
| Concentration on hands after handling, Winter | B94 | =LOG10(B90 (10B88)) | log CFU/g | Calculated |
| Concentration left on chicken, Spring | B95 | =IF(10B85–10B91 = 0,0,LOG10(10B85–10B91)) | log CFU/g | Calculated |
| Concentration left on chicken, Summer | B96 | =IF(10B86–10B92 = 0,0,LOG10(10B86–10B92)) | log CFU/g | Calculated |
| Concentration left on chicken, Fall | B97 | =IF(10B87–10B93 = 0,0,LOG10(10B87–10B93)) | log CFU/g | Calculated |
| Concentration left on chicken, Winter | B98 | =IF(10B88–10B94 = 0,0,LOG10(10B88–10B94)) | log CFU/g | Calculated |
| Transfer rate from raw chicken to utensils | B99 | =RiskLognorm(0.0064271,0.28575,RiskShift(0.00000124688),RiskTruncate(0,1)) | Proportion | [39,40,41] |
| Concentration on utensils after handling, Spring | B100 | =LOG10((10B95) B99) | log CFU/g | Calculated |
| Concentration on utensils after handling, Summer | B101 | =LOG10((10B96) B99) | log CFU/g | Calculated |
| Concentration on utensils after handling, Fall | B102 | =LOG10((10B97) B99) | log CFU/g | Calculated |
| Concentration on utensils after handling, Winter | B103 | =LOG10((10B98) B99) | log CFU/g | Calculated |
| Concentration on chicken, Spring | B104 | =LOG10(10B95–10B100) | log CFU/g | Calculated |
| Concentration on chicken, Summer | B105 | =LOG10(10B96–10B101) | log CFU/g | Calculated |
| Concentration on chicken, Fall | B106 | =LOG10(10B97–10B102) | log CFU/g | Calculated |
| Concentration on chicken, Winter | B107 | =LOG10(10B98–10B103) | log CFU/g | Calculated |
| Cooking: | ||||
| Is chicken undercooked? | B109 | =RiskBernoulli(0.399) | No unit | [32] |
| Cooking time | B110 | =RiskPert(15,30,45,RiskCorrmat(NewMatrix1,1)) | Min | [42] |
| Cooking temperature | B111 | =RiskPert(38.244,82.305,100.48,RiskTruncate(38.244, 73.9),RiskCorrmat(NewMatrix1,2)) | °C | [35] |
| D-value | B112 | =10(−0.96−(B111−70)/12.3) | Min | [43] |
| Change during undercooking | B113 | =B110/B112 | log CFU/g | Calculated |
| Concentration after undercooking, Spring | B114 | =B104 − B113 | log CFU/g | Calculated |
| Concentration after undercooking, Summer | B115 | =B105 − B113 | log CFU/g | Calculated |
| Concentration after undercooking, Fall | B116 | =B106 − B113 | log CFU/g | Calculated |
| Concentration after undercooking, Winter | B117 | =B107 − B113 | log CFU/g | Calculated |
| Cooked product handling: | ||||
| Are hands washed? | B119 | =RiskBernoulli(0.883) | No unit | [44] |
| Hand washing reduction | B120 | =RiskNormal(2.7163,1.2661,RiskTruncate(0.34,5.29)) | log CFU/g | [39] |
| Concentration on hands after washing, Spring | B121 | =B91 − B120 | log CFU/g | Calculated |
| Concentration on hands after washing, Summer | B122 | =B92 − B120 | log CFU/g | Calculated |
| Concentration on hands after washing, Fall | B123 | =B93 − B120 | log CFU/g | Calculated |
| Concentration on hands after washing, Winter | B124 | =B94 − B120 | log CFU/g | Calculated |
| Transfer rate to cooked chicken by hands | B125 | =RiskLevy(−0.0003382,0.0019097,RiskTruncate(0,1)) | Proportion | [39,40] |
| Concentration after handling cooked chicken with hands, Spring | B126 | =LOG10(IF(B119 = 0,(10B91) B125,(10B121) B125) + IF(B109 = 0,0, 10B114)) | log CFU/g | Calculated |
| Concentration after handling cooked chicken with hands, Summer | B127 | =LOG10(IF(B119 = 0,(10B92) B125,(10B122) B125) + IF(B109 = 0,0, 10B115)) | log CFU/g | Calculated |
| Concentration after handling cooked chicken with hands, Fall | B128 | =LOG10(IF(B119 = 0,(10B93) B125,(10B123) B125) + IF(B109 = 0,0, 10B116)) | log CFU/g | Calculated |
| Concentration after handling cooked chicken with hands, Winter | B129 | =LOG10(IF(B119 = 0,(10B94) B125,(10B124) B125) + IF(B109 = 0,0, 10B117)) | log CFU/g | Calculated |
| Are different dishes or utensils used? | B130 | =RiskBernoulli(0.959) | No unit | [44] |
| Transfer rate to cooked chicken by dirty utensils | B131 | =RiskExpon(0.12217,RiskShift(−0.00041787),RiskTruncate(0,1)) | Proportion | [39] |
| Final concentration, Spring | B132 | =LOG10(10B126 + IF(B130 = 0,B131 (10B100),0)) | log CFU/g | Calculated |
| Final concentration, Summer | B133 | =LOG10(10B127 + IF(B130 = 0,B131 (10B101),0)) | log CFU/g | Calculated |
| Final concentration, Fall | B134 | =LOG10(10B128 + IF(B130 = 0,B131 (10B102),0)) | log CFU/g | Calculated |
| Final concentration, Winter | B135 | =LOG10(10^B129 + IF(B130 = 0,B131 (10B103),0)) | log CFU/g | Calculated |
| Dose–response and infection | ||||
| Serving size | B137 | =85 | g | 9 CFR §381.412 |
| Concentration per serving, Spring | B138 | =(10B132) B137 | CFU | Calculated |
| Concentration per serving, Summer | B139 | =(10B133) B137 | CFU | Calculated |
| Concentration per serving, Fall | B140 | =(10B134) B137 | CFU | Calculated |
| Concentration per serving, Winter | B141 | =(10B135) B137 | CFU | Calculated |
| Dose–response infection model parameter alpha | B142 | =0.145 | No unit | [45] |
| Dose–response infection model parameter, beta | B143 | =7.59 | No unit | [45] |
| Probability of infection, Spring | B144 | =1 − (1 + (B138/B143))−B142 | No unit | Calculated |
| Probability of infection, Summer | B145 | =1 − (1 + (B139/B143))−B142 | No unit | Calculated |
| Probability of infection, Fall | B146 | =1 − (1 + (B140/B143))−B142 | No unit | Calculated |
| Probability of infection, Winter | B147 | =1 − (1 + (B141/B143))−B142 | CFU | Calculated |
| Probability of illness, Spring | B148 | =B144 0.33 | No unit | [46,47,48] |
| Probability of illness, Summer | B149 | =B145 0.33 | No unit | [46,47,48] |
| Probability of illness, Fall | B150 | =B146 0.33 | No unit | [46,47,48] |
| Probability of illness, Winter | B151 | =B147 0.33 | No unit | [46,47,48] |
| Risk of infection per serving, Spring | B152 | =B144 B9 | No unit | Calculated |
| Risk of infection per serving, Summer | B153 | =B145 B10 | No unit | Calculated |
| Risk of infection per serving, Fall | B154 | =B146 B11 | No unit | Calculated |
| Risk of infection per serving, Winter | B155 | =B147 B12 | No unit | Calculated |
| Risk of illness per serving, Spring | B156 | =B148 B9 | No unit | Calculated |
| Risk of illness per serving, Summer | B157 | =B149 B10 | No unit | Calculated |
| Risk of illness per serving, Fall | B158 | =B150 B11 | No unit | Calculated |
| Risk of illness per serving, Winter | B159 | =B151 B12 | No unit | Calculated |
| Total per capita poultry availability per year | B160 | =43,454.15 | g | [49] |
| Total used in raw chicken preparation per year | B161 | =21,727.075 | g | [50] |
| U.S. population | B162 | =325,186,237 | People | [49] |
| Number of consumers who purchased chicken from grocery/supermarket | B163 | =269,904,576.7 | People | [51] |
| Consumed serving per person per season | B164 | =63.90 | Serving | Calculated |
| No. of servings consumed per season in US | B165 | =17,247,755,827 | No unit | Calculated |
| No. of infections per season, Spring | B166 | =B165 B152 | No unit | Calculated |
| No. of infections per season, Summer | B167 | =B165 B153 | No unit | Calculated |
| No. of infections per season, Fall | B168 | =B165 B154 | No unit | Calculated |
| No. of infections per season, Winter | B169 | =B165 B155 | No unit | Calculated |
| No. of illness per season, Spring | B170 | =B165 B156 | No unit | Calculated |
| No. of illness per season, Summer | B171 | =B165 B157 | No unit | Calculated |
| No. of illness per season, Fall | B172 | =B165 B158 | No unit | Calculated |
| No. of illness per season, Winter | B173 | =B165 B159 | No unit | Calculated |
| Total number of infections per year | B174 | =B166 + B167 + B168 + B169 | No unit | Calculated |
| Total number of illnesses per year | B175 | =B170 + B171 + B172 + B173 | No unit | Calculated |
| Spring | Summer | Fall | Winter | |
|---|---|---|---|---|
| Campylobacter prevalence (Average SD) | 0.59 0.32 | 0.56 0.48 | 0.53 0.41 | 0.26 0.32 |
| Campylobacter concentration (log CFU/carcass) (Average SD) | 2.26 0.56 | 1.74 0.89 | 2.35 0.85 | 2.30 1.28 |
| Campylobacter outbreaks * | 17 | 25 | 15 | 10 |
| Seasonal Effect | Risk of Infection per Serving | Risk of Illness per Serving | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | 25% | 75% | Mean | Median | 25% | 75% | |
| Spring | ||||||||
| Summer | ||||||||
| Fall | ||||||||
| Winter | ||||||||
| Seasonal Effect | No. of Infections per Season | No. of Illnesses per Season | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | 25% | 75% | Mean | Median | 25% | 75% | |
| Spring | 22,571,609 | 13,050 | 611 | 318,258 | 7,448,639 | 4306 | 201 | 105,034 |
| Summer | 27,058,680 | 15,895 | 739 | 386,805 | 8,929,364 | 5245 | 244 | 127,646 |
| Fall | 24,941,190 | 14,488 | 676 | 353,010 | 8,230,593 | 4781 | 223 | 116,493 |
| Winter | 12,085,638 | 7008 | 327 | 170,841 | 3,988,261 | 2312 | 108 | 56,378 |
| Scenario | No. of Infections | |||
|---|---|---|---|---|
| Mean | Median | 25% | 75% | |
| Baseline | 86,657,118 | 50,493 | 2355 | 1,228,846 |
| Uncertainty, prevalence: | ||||
| Low | 79,734,367 | 48,471 | 2179 | 1,212,001 |
| Medium | 90,535,132 | 50,282 | 2375 | 1,305,955 |
| High | 92,231,575 | 53,480 | 2525 | 1,349,224 |
| Thawing method: | ||||
| Refrigerator thawing | 53,162,035 | 42,674 | 2014 | 1,010,711 |
| Running water thawing | 68,289,580 | 61,462 | 2887 | 1,487,499 |
| Microwave thawing | 53,007,197 | 42,047 | 2018 | 1,014,576 |
| Ambient room temperature thawing | 286,663,540 | 122,142 | 4714 | 3933 |
| Hand washing: | ||||
| Always wash hands | 41,774,442 | 26,583 | 1569 | 471,588 |
| Never wash hands | 429,585,788 | 11,308,668 | 1,386,150 | 100,598,358 |
| Cleaning: | ||||
| Always use different utensils | 83,552,680 | 42,190 | 2046 | 1,027,979 |
| Never use different utensils | 213,628,883 | 2,694,612 | 312,481 | 25,216,122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Rothrock, M.J., Jr.; Dev Kumar, G.; Mishra, A. Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States. Foods 2023, 12, 2559. https://doi.org/10.3390/foods12132559
Xu X, Rothrock MJ Jr., Dev Kumar G, Mishra A. Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States. Foods. 2023; 12(13):2559. https://doi.org/10.3390/foods12132559
Chicago/Turabian StyleXu, Xinran, Michael J. Rothrock, Jr., Govindaraj Dev Kumar, and Abhinav Mishra. 2023. "Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States" Foods 12, no. 13: 2559. https://doi.org/10.3390/foods12132559
APA StyleXu, X., Rothrock, M. J., Jr., Dev Kumar, G., & Mishra, A. (2023). Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States. Foods, 12(13), 2559. https://doi.org/10.3390/foods12132559







