Growth Kinetics of Listeria monocytogenes and Salmonella enterica on Dehydrated Vegetables during Rehydration and Subsequent Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Preparation and Dehydration
2.2. Strains, Culture Conditions, and Inoculum Preparation
2.3. Inoculation of Dehydrated Vegetables
2.4. Rehydration of Dehydrated Vegetables
2.5. Storage of Rehydrated Vegetables
2.6. Measurement of pH, Water Activity (aw), and Moisture Content
2.7. Enumeration of L. monocytogenes and S. enterica
2.8. Modeling of Growth Kinetics and Statistical Analysis
3. Results
3.1. Characteristics of the Fresh, Dehydrated, and Rehydrated Vegetables
3.2. Survival of L. monocytogenes and S. enterica on Dehydrated Vegetables during Rehydration
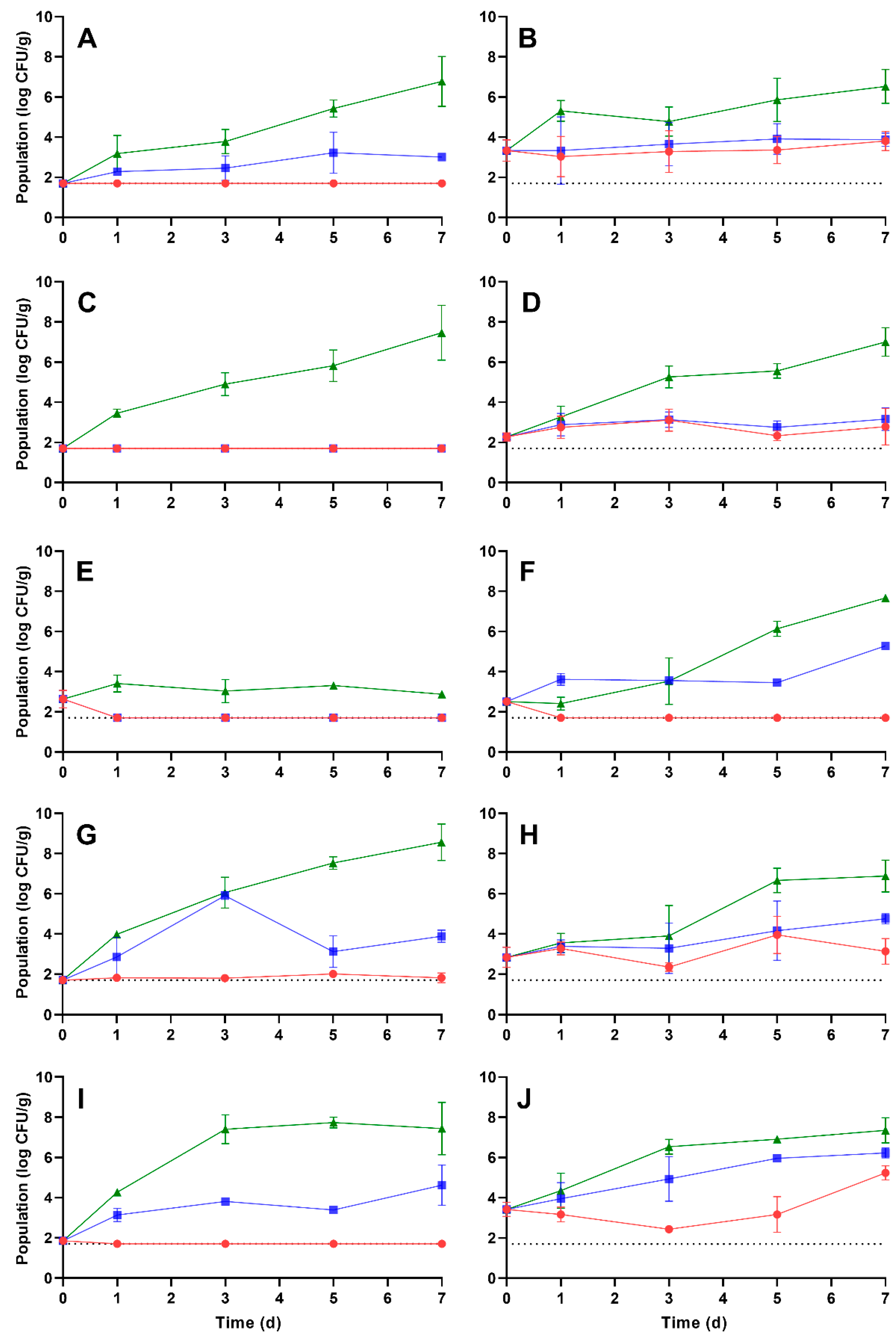
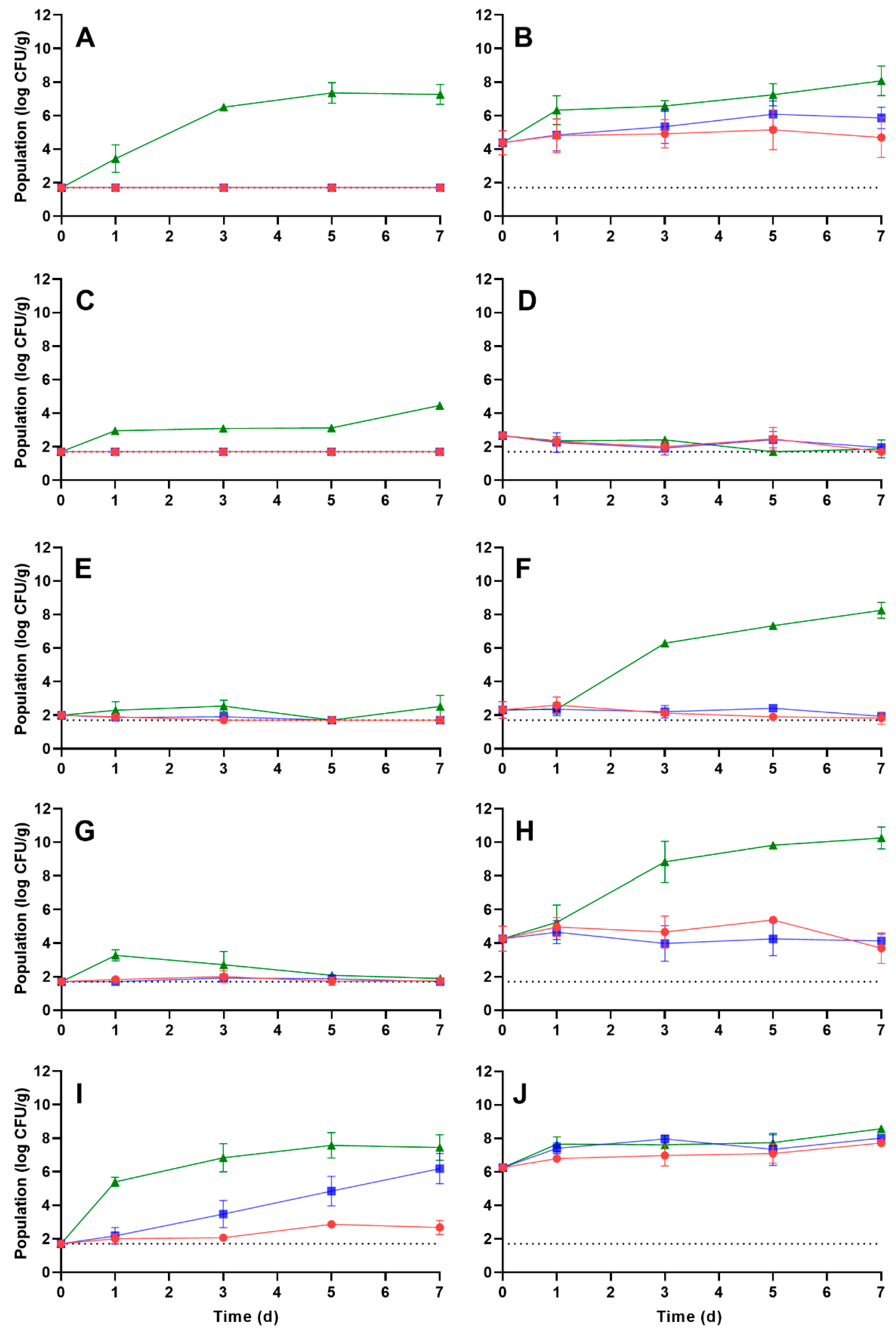
3.3. Growth Kinetics of L. monocytogenes and S. enterica on Rehydrated Vegetables during Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of Drying Technologies to Ensure Microbial Safety of Dried Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Dehydrated foods: Are they microbiologically safe? Crit. Rev. Food Sci. Nutr. 2019, 59, 2734–2745. [Google Scholar] [CrossRef]
- Blessington, T.; Mitcham, E.J.; Harris, L.J. Survival of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes on inoculated walnut kernels during storage. J. Food Prot. 2012, 75, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.K.; Natarajan, V.; Stewart, D.; Suehr, Q.; Mhetras, T.; Gonsalves, L.J.; Tortorello, M.L. Survival kinetics of Listeria monocytogenes on chickpeas, sesame seeds, pine nuts, and black pepper as affected by relative humidity storage conditions. PLoS ONE 2019, 14, e0226362. [Google Scholar] [CrossRef] [PubMed]
- Brar, P.K.; Proano, L.G.; Friedrich, L.M.; Harris, L.J.; Danyluk, M.D. Survival of Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes on raw peanut and pecan kernels stored at -24, 4, and 22 degrees C. J. Food Prot. 2015, 78, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Farakos, S.M.; Frank, J.F.; Schaffner, D.W. Modeling the influence of temperature, water activity and water mobility on the persistence of Salmonella in low-moisture foods. Int. J. Food Microbiol. 2013, 166, 280–293. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Carminati, J.A.; Morishita, K.N.; Amorim Neto, D.P.; Pinheiro, H.P.; Maia, R.P. Long-term kinetics of Salmonella Typhimurium ATCC 14028 survival on peanuts and peanut confectionery products. PLoS ONE 2018, 13, e0192457. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.E.; VanDoren, J.M.; Grasso, E.M.; Halik, L.A. Growth and survival of Salmonella in ground black pepper (Piper nigrum). Food Microbiol. 2013, 34, 182–188. [Google Scholar] [CrossRef]
- Bowman, L.S.; Waterman, K.M.; Williams, R.C.; Ponder, M.A. Inoculation Preparation Affects Survival of Salmonella enterica on Whole Black Peppercorns and Cumin Seeds Stored at Low Water Activity. J. Food Prot. 2015, 78, 1259–1265. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Mann, D.A. Survival of Salmonella on dried fruits and in aqueous dried fruit homogenates as affected by temperature. J. Food Prot. 2014, 77, 1102–1109. [Google Scholar] [CrossRef]
- Canakapalli, S.S.; Sheng, L.N.; Wang, L.X. Survival of common foodborne pathogens on dates, sundried tomatoes, and dried pluots at refrigerated and ambient temperatures. LWT—Food Sci. Technol. 2022, 154, 112632. [Google Scholar] [CrossRef]
- Lian, F.; Zhao, W.; Yang, R.J.; Tang, Y.; Katiyo, W. Survival of Salmonella enterica in skim milk powder with different water activity and water mobility. J. Food Cont. 2015, 47, 1–6. [Google Scholar] [CrossRef]
- Koseki, S.; Nakamura, N.; Shiina, T. Comparison of desiccation tolerance among Listeria monocytogenes, Escherichia coli O157:H7, Salmonella enterica, and Cronobacter sakazakii in powdered infant formula. J. Food Prot. 2015, 78, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; den Bakker, M.; Liao, J.Y.; Payton, A.S.; Futral, A.N.; Diez-Gonzalez, F. Salmonella and Enterohemorrhagic Escherichia coli Serogroups O45, O121, O145 in Wheat Flour: Effects of Long-Term Storage and Thermal Treatments. Front. Microbiol. 2019, 10, 323. [Google Scholar] [CrossRef]
- Taylor, M.H.; Tsai, H.C.; Rasco, B.; Tang, J.; Zhu, M.J. Stability of Listeria monocytogenes in wheat flour during extended storage and isothermal treatment. Food Control. 2018, 91, 434–439. [Google Scholar] [CrossRef]
- Komitopoulou, E.; Penaloza, W. Fate of Salmonella in dry confectionery raw materials. J. Appl. Microbiol. 2009, 106, 1892–1900. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Mann, D.A. Survival of Salmonella in Cookie and Cracker Sandwiches Containing Inoculated, Low-Water Activity Fillings. J. Food Prot. 2015, 78, 1828–1834. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevention. Multistate Outbreak of Salmonella Typhimurium Infections Linked to Dried Coconut (Final Update). 2018. Available online: https://www.cdc.gov/salmonella/typhimurium-03-18/index.html (accessed on 27 March 2023).
- U.S. Centers for Disease Control and Prevention. Outbreak of Salmonella Stanley Infections Linked to Wood Ear Mushrooms. 2020. Available online: https://www.cdc.gov/salmonella/stanley-09-20/index.html (accessed on 4 September 2022).
- U.S. Food and Drug Administration. Outbreak Investigation of Salmonella Stanley: Wood Ear Mushrooms—Dried Fungus (September 2020). 2020. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-stanley-wood-ear-mushrooms-dried-fungus-september-2020 (accessed on 4 September 2022).
- Jernberg, C.; Hjertqvist, M.; Sundborger, C.; Castro, E.; Lofdahl, M.; Paajarvi, A.; Sundqvist, L.; Lof, E. Outbreak of Salmonella Enteritidis phage type 13a infection in Sweden linked to imported dried-vegetable spice mixes, December 2014 to July 2015. Eurosurveillance 2015, 20, 21194. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention. Multistate Outbreak of Human Salmonella Montevideo Infections (Final Update). 2010. Available online: https://www.cdc.gov/salmonella/2010/montevideo-5-4-2010.html (accessed on 3 February 2019).
- Van Doren, J.M.; Neil, K.P.; Parish, M.; Gieraltowski, L.; Gould, L.H.; Gombas, K.L. Foodborne illness outbreaks from microbial contaminants in spices, 1973–2010. Food Microbiol. 2013, 36, 456–464. [Google Scholar] [CrossRef]
- Vij, V.; Ailes, E.; Wolyniak, C.; Angulo, F.J.; Klontz, K.C. Recalls of spices due to bacterial contamination monitored by the US Food and Drug Administration: The predominance of Salmonellae. J. Food Prot. 2006, 69, 233–237. [Google Scholar] [CrossRef] [PubMed]
- FSN. Organic Muesli, Granola Recalled for Listeria Risk in Ingredient. 2017. Available online: https://www.foodsafetynews.com/2017/06/organic-muesli-granola-recalled-for-listeria-risk-in-ingredient/#:~:text=New%20England%20Natural%20Bakers%20is,possible%20contamination%20with%20Listeria%20monocytogenes (accessed on 4 March 2023).
- Hughlett, M. Elgin Nut Company Sanfilippo Issues Recall. 2009. Available online: https://www.chicagotribune.com/news/ct-xpm-2009-10-15-0910140663-story.html (accessed on 4 March 2023).
- FSN. Publix Recalls Fruit Mix because of Listeria Risk in Apricots. 2017. Available online: https://www.foodsafetynews.com/2017/06/publix-recalls-fruit-mix-because-of-listeria-risk-in-apricots/ (accessed on 4 March 2023).
- Krokida, M.K.; Marinos-Louris, D. Rehydration kinetics of dehydrated products. J. Food Eng. 2003, 57, 1–7. [Google Scholar] [CrossRef]
- Maldonado, S.; Arnau, E.; Bertuzzi, M.A. Effect of temperature and pretreatment on water diffusion during rehydration of dehydrated mangoes. J. Food Eng. 2010, 96, 333–341. [Google Scholar] [CrossRef]
- Cunningham, S.E.; Mcminn, W.A.M.; Magee, T.R.A.; Richardson, P.S. Experimental study of rehydration kinetics of potato cylinders. Food Bioprod. Process. 2008, 86, 15–24. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Food Code. 2022. Available online: https://www.fda.gov/food/retail-food-protection/fda-food-code (accessed on 14 April 2023).
- Sagar, V.R.; Kumar, P.S. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Tech. Mys 2010, 47, 15–26. [Google Scholar] [CrossRef]
- USDA. Food Data Central. 2020. Available online: https://fdc.nal.usda.gov/index.html (accessed on 8 April 2023).
- de Niederhäusern, S.; Bondi, M.; Camellini, S.; Sabia, C.; Messi, P.; Iseppi, R. Plant Extracts for the Control of Listeria monocytogenes in Meat Products. Appl. Sci. 2021, 11, 10820. [Google Scholar] [CrossRef]
- Pinton, S.C.; Bardsley, C.A.; Marik, C.M.; Boyer, R.R.; Strawn, L.K. Fate of Listeria monocytogenes on Broccoli and Cauliflower at Different Storage Temperatures. J. Food Prot. 2020, 83, 858–864. [Google Scholar] [CrossRef]
- Salazar, J.K.; Sahu, S.N.; Hildebrandt, I.M.; Zhang, L.; Qi, Y.; Liggans, G.; Datta, A.R.; Tortorello, M.L. Growth Kinetics of Listeria monocytogenes in Cut Produce. J. Food Prot. 2017, 80, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Sapers, G.M. Influence of soft rot bacteria on growth of Listeria monocytogenes on potato tuber slices. J. Food Prot. 1999, 62, 343–348. [Google Scholar] [CrossRef]
- Bottichio, L.; Medus, C.; Sorenson, A.; Donovan, D.; Sharma, R.; Dowell, N.; Williams, I.; Wellman, A.; Jackson, A.; Tolar, B.; et al. Outbreak of Salmonella Oslo Infections Linked to Persian Cucumbers—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1430–1433. [Google Scholar] [CrossRef]
- De Corcuera, J.I.R.; Cavalieri, R.P.; Powers, J.R. Blanching of Foods. In Encyclopedia of Agri, Food and Biological Engineering; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Koutsoumanis, K.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The public health risk posed by Listeria monocytogenes in frozen fruit and vegetables including herbs, blanched during processing. EFSA J. 2020, 18, e06092. [Google Scholar] [CrossRef]
| Vegetable | Fresh 1 | Dehydrated 2 | ||
|---|---|---|---|---|
| pH | aw | pH | aw | |
| Carrot | 7.00 ± 0.10 aA | 0.956 ± 0.002 aA | 6.50 ± 0.22 aB | 0.250 ± 0.070 aB |
| Corn | 6.90 ± 0.14 aA | 0.951 ± 0.015 aA | 6.77 ± 0.02 bB | 0.261 ± 0.008 aB |
| Onion | 6.79 ± 0.57 aA | 0.952 ± 0.014 aA | 5.73 ± 0.07 cB | 0.244 ± 0.011 aB |
| Pepper | 6.15 ± 0.06 bA | 0.956 ± 0.010 aA | 6.01 ± 0.04 dB | 0.262 ± 0.050 aB |
| Potato | 6.77 ± 0.09 aA | 0.960 ± 0.008 aA | 6.42 ± 0.04 aB | 0.221 ± 0.025 aB |
| Vegetable | Moisture (%) | |||
|---|---|---|---|---|
| Fresh 1 | Dehydrated 2 | Rehydrated 3 | ||
| 5 °C | 25 °C | |||
| Carrot | 88.61 ± 1.00 aA | 8.20 ± 1.13 aB | 88.52 ± 1.59 aA | 88.16 ± 1.46 aA |
| Corn | 82.25 ± 0.22 bA | 7.27 ± 0.84 aB | 79.39 ± 0.92 bC | 83.36 ± 1.13 bD |
| Onion | 91.90 ± 1.45 cA | 15.58 ± 0.94 bB | 92.94 ± 1.40 cAC | 94.19 ± 0.91 cC |
| Pepper | 93.69 ± 1.28 dA | 11.40 ± 1.83 cB | 90.38 ± 0.26 acC | 92.08 ± 1.41 cA |
| Potato | 78.00 ± 0.15 eA | 4.50 ± 0.68 dB | 68.00 ± 4.39 dC | 68.42 ± 4.27 dC |
| Vegetable | Initial Population 1 (log CFU/g ± SD 2) | Population after Rehydration 3 (log CFU/g ± SD) | |
|---|---|---|---|
| 5 °C | 25 °C | ||
| Carrot | 2.70 ± 0.20 aA | <1.70 | 3.34 ± 0.54 aB |
| Corn | 1.99 ± 0.18 bA | <1.70 | 2.28 ± 0.20 bB |
| Onion | 2.50 ± 0.90 abA | <1.70 | 2.51 ± 0.11 bcA |
| Pepper | 2.80 ± 0.07 aA | <1.70 | 2.84 ± 0.50 acA |
| Potato | 2.62 ± 0.19 aA | 1.85 ± 0.16 B | 3.42 ± 0.35 aC |
| Vegetable | Initial Population 1 (log CFU/g ± SD 2) | Population after Rehydration 3 (log CFU/g ± SD) | |
|---|---|---|---|
| 5 °C | 25 °C | ||
| Carrot | 1.82 ± 0.51 aA | <1.70 | 4.38 ± 0.73 aB |
| Corn | 2.54 ± 0.23 bA | <1.70 | 2.66 ± 0.13 bA |
| Onion | 2.46 ± 0.40 bA | 1.99 ± 0.15 B | 2.32 ± 0.50 bAB |
| Pepper | 2.82 ± 0.08 bA | <1.70 | 4.27 ± 0.75 aB |
| Potato | 2.83 ± 0.09 bA | <1.70 | 6.25 ± 0.10 cB |
| Vegetable | Rehydration Temperature (°C) 1 | Storage Temperature (°C) | Growth Rate (log CFU/g per d ± SE 2) | Time (d) to a 1 log CFU/g Increase 4 | Population after 7 d Storage (log CFU/g ± SD 3) |
|---|---|---|---|---|---|
| Carrot | 5 | 5 | ND 5 | NA 6 | <1.70 |
| 10 | ND | NA | 3.01 ± 0.18 a | ||
| 25 | 0.63 ± 0.07 a | 1.59 | 6.78 ± 1.24 b | ||
| 25 | 5 | 0.07 ± 0.05 b | 14.29 | 3.81 ± 0.48 c | |
| 10 | 0.10 ± 0.07 b | 10.00 | 3.88 ± 0.33 c | ||
| 25 | 0.39 ± 0.06 c | 2.56 | 6.53 ± 0.84 b | ||
| Corn | 5 | 5 | ND | NA | <1.70 |
| 10 | ND | NA | <1.70 | ||
| 25 | 0.67 ± 0.06 a | 1.49 | 7.46 ± 1.37 a | ||
| 25 | 5 | 0.03 ± 0.04 b | 33.33 | 2.79 ± 0.91 b | |
| 10 | 0.51 ± 0.22 a | 1.96 | 3.16 ± 0.56 b | ||
| 25 | 0.63 ± 0.04 a | 1.59 | 7.00 ± 0.71 a | ||
| Onion | 5 | 5 | ND | NA | <1.70 |
| 10 | ND | NA | <1.70 | ||
| 25 | 0.04 ± 0.05 a | 25 | 2.88 ± 0.08 a | ||
| 25 | 5 | ND | NA | <1.70 | |
| 10 | 0.32 ± 0.05 b | 3.13 | 5.29 ± 0.06 b | ||
| 25 | 1.05 ± 0.15 c | 2.80 7 | 7.67 ± 0.07 c | ||
| Pepper | 5 | 5 | 0.02 ± 0.02 a | 50.00 | 1.82 ± 0.24 a |
| 10 | 1.20 ± 1.18 b | 0.83 | 3.89 ± 0.30 bc | ||
| 25 | 1.02 ± 0.11 b | 0.98 | 8.56 ± 0.91 d | ||
| 25 | 5 | 0.05 ± 0.04 a | 20.00 | 3.14 ± 0.64 b | |
| 10 | 0.24 ± 0.06 b | 4.17 | 4.75 ± 0.25 c | ||
| 25 | 1.66 ± 0.60 b | 3.35 8 | 6.88 ± 0.79 d | ||
| Potato | 5 | 5 | ND | NA | <1.70 |
| 10 | 1.35 ± 0.79 a | 1.74 | 4.62 ± 1.00 a | ||
| 25 | 2.37 ± 0.61 a | 0.42 | 7.44 ± 1.30 b | ||
| 25 | 5 | 0.08 ± 0.08 b | 12.50 | 5.24 ± 0.35 a | |
| 10 | 0.43 ± 0.04 c | 2.33 | 6.23 ± 0.24 b | ||
| 25 | 1.07 ± 0.11 a | 0.93 | 7.35 ± 0.63 b |
| Vegetable | Rehydration Temperature (°C) 1 | Storage Temperature (°C) | Growth Rate (log CFU/g per d ± SE 2) | Time (d) to a 1 log CFU/g Increase 4 | Population after 7 d Storage (log CFU/g ± SD 3) |
|---|---|---|---|---|---|
| Carrot | 5 | 5 | 0.01 ± 0.03 a | 100 | <1.70 |
| 10 | 0.03 ± 0.30 a | 33.33 | <1.70 | ||
| 25 | 1.63 ± 0.18 b | 0.61 | 7.26 ± 0.59 a | ||
| 25 | 5 | 0.05 ± 0.06 a | 20.00 | 4.69 ± 1.19 b | |
| 10 | 0.21 ± 0.06 c | 4.76 | 5.86 ± 0.64 b | ||
| 25 | 0.44 ± 0.05 d | 2.27 | 8.07 ± 0.88 c | ||
| Corn | 5 | 5 | ND 5 | NA 6 | <1.70 |
| 10 | ND | NA | <1.70 | ||
| 25 | 0.31 ± 0.04 a | 3.22 | 4.47 ± 0.09 a | ||
| 25 | 5 | −0.10 ± 0.05 b | NA | <1.70 | |
| 10 | −0.06 ± 0.05 b | NA | 1.95 ± 0.07 b | ||
| 25 | −0.12 ± 0.04 b | NA | 1.88 ± 0.53 b | ||
| Onion | 5 | 5 | −0.05 ± 0.02 a | NA | <1.70 |
| 10 | −0.04 ± 0.02 a | NA | <1.70 | ||
| 25 | 0.04 ± 0.05 b | 25.00 | 2.53 ± 0.66 a | ||
| 25 | 5 | −0.09 ± 0.03 a | NA | 1.82 ± 0.36 a | |
| 10 | −0.04 ± 0.05 a | NA | 1.93 ± 0.04 a | ||
| 25 | 1.36 ± 0.35 c | 0.74 7 | 8.25 ± 0.47 b | ||
| Pepper | 5 | 5 | −0.02 ± 0.03 a | NA | 1.75 ± 0.18 a |
| 10 | 0.01 ± 0.03 a | 100.00 | <1.70 | ||
| 25 | −0.18 ± 0.08 b | NA | 1.89 ± 0.16 a | ||
| 25 | 5 | −0.04 ± 0.09 a | NA | 3.70 ± 0.91 b | |
| 10 | −0.05 ± 0.08 a | NA | 4.13 ± 0.40 b | ||
| 25 | 1.49 ± 0.24 c | 0.67 | 10.25 ± 0.65 c | ||
| Potato | 5 | 5 | 0.14 ± 0.03 a | 7.14 | 2.67 ± 0.42 a |
| 10 | 0.67 ± 0.06 b | 1.49 | 6.18 ± 0.90 b | ||
| 25 | 1.13 ± 0.24 c | 0.89 | 7.44 ± 0.76 bc | ||
| 25 | 5 | 0.16 ± 0.03 a | 6.65 | 7.72 ± 0.13 c | |
| 10 | 0.09 ± 0.05 a | 11.11 | 8.02 ± 0.17 d | ||
| 25 | 0.17 ± 0.04 a | 5.88 | 8.57 ± 0.17 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fay, M.L.; Salazar, J.K.; Ren, Y.; Wu, Z.; Mate, M.; Khouja, B.A.; Lingareddygari, P.; Liggans, G. Growth Kinetics of Listeria monocytogenes and Salmonella enterica on Dehydrated Vegetables during Rehydration and Subsequent Storage. Foods 2023, 12, 2561. https://doi.org/10.3390/foods12132561
Fay ML, Salazar JK, Ren Y, Wu Z, Mate M, Khouja BA, Lingareddygari P, Liggans G. Growth Kinetics of Listeria monocytogenes and Salmonella enterica on Dehydrated Vegetables during Rehydration and Subsequent Storage. Foods. 2023; 12(13):2561. https://doi.org/10.3390/foods12132561
Chicago/Turabian StyleFay, Megan L., Joelle K. Salazar, Yuying Ren, Zihui Wu, Madhuri Mate, Bashayer A. Khouja, Pravalika Lingareddygari, and Girvin Liggans. 2023. "Growth Kinetics of Listeria monocytogenes and Salmonella enterica on Dehydrated Vegetables during Rehydration and Subsequent Storage" Foods 12, no. 13: 2561. https://doi.org/10.3390/foods12132561
APA StyleFay, M. L., Salazar, J. K., Ren, Y., Wu, Z., Mate, M., Khouja, B. A., Lingareddygari, P., & Liggans, G. (2023). Growth Kinetics of Listeria monocytogenes and Salmonella enterica on Dehydrated Vegetables during Rehydration and Subsequent Storage. Foods, 12(13), 2561. https://doi.org/10.3390/foods12132561




