Nutritional Composition of Hass Avocado Pulp
Abstract
1. Introduction
2. Unique Nutritional Physiology and Ripening of Hass Avocados
3. Nutritional Composition of Ripe Hass Avocado Pulp
3.1. Energy and Water
3.2. Lipids
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| g/100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Total Fat | 15.4 | 8.4, 23.2 | 31 | 17.77 | 12.9, 26.7 | 28 | [16,17,18,19,20,21,23,24] |
| Saturated fatty acids | 2.13 | NA | 1 | 3.18 | 0.85, 6.3 | 18 | [16,17,18,19,20,21,24] |
| 16:0 Palmitic acid | 2.08 | 1.73, 2.54 | 8 | 1.27 | 0.54, 4.32 | 121 | [17,18,24,33,34] |
| 18:0 Stearic acid | 0.05 | 0.007, 0.082 | 8 | 0.03 | 0, 1.98 | 202 | [17,18,24,34] |
| Monounsaturated fatty acids | 9.8 | NA | 1 | 12.37 | 8.48, 19.51 | 18 | [16,17,18,19,20,21,24] |
| 16:1n-7 Palmitoleic acid | 0.698 | 0.5, 0.881 | 8 | 0.53 | 0.11, 1.98 | 121 | [17,18,24,33,34] |
| 17:1 | 0.01 | 0, 0.016 | 8 | 0 | NA | 2 | [18] |
| 18:1n-9 Oleic acid | 9.07 | 7.44, 10.9 | 8 | 4.07 | 1.5, 19.4 | 121 | [17,18,24,33,34] |
| 18:1n-7 Cis-Vaccenic acid | NA | 0.627 | 0.35, 0.84 | 109 | [34] | ||
| 20:1n-9 Gondoic acid | 0.025 | 0.02, 0.033 | 8 | 0.02 | 0.02, 0.02 | 2 | [18] |
| Polyunsaturated fatty acids | 1.82 | NA | 1 | 2.46 | 0.46, 4.55 | 18 | [16,17,18,19,20,21,24] |
| 18:2n-6 Linoleic acid | 1.67 | 1.44, 1.97 | 8 | 0.93 | 0.29, 2.68 | 121 | [17,18,24,33,34] |
| 18:2n-6 Linolelaidic acid | NA | 1.60 | 1.54, 1.66 | 6 | [24] | ||
| 18:3n-3 α-Linolenic acid | 0.11 | 0.096, 0.128 | 4 | 0.135 | 0, 0.33 | 13 | [17,18,24,33] |
| 18:3n-6 γ-Linolenic acid | 0.015 | 0.015, 0.015 | 4 | 0.068 | 0, 0.1 | 110 | [18,34] |
| 20:3n-6 | 0.016 | 0, 0.04 | 8 | NA | |||
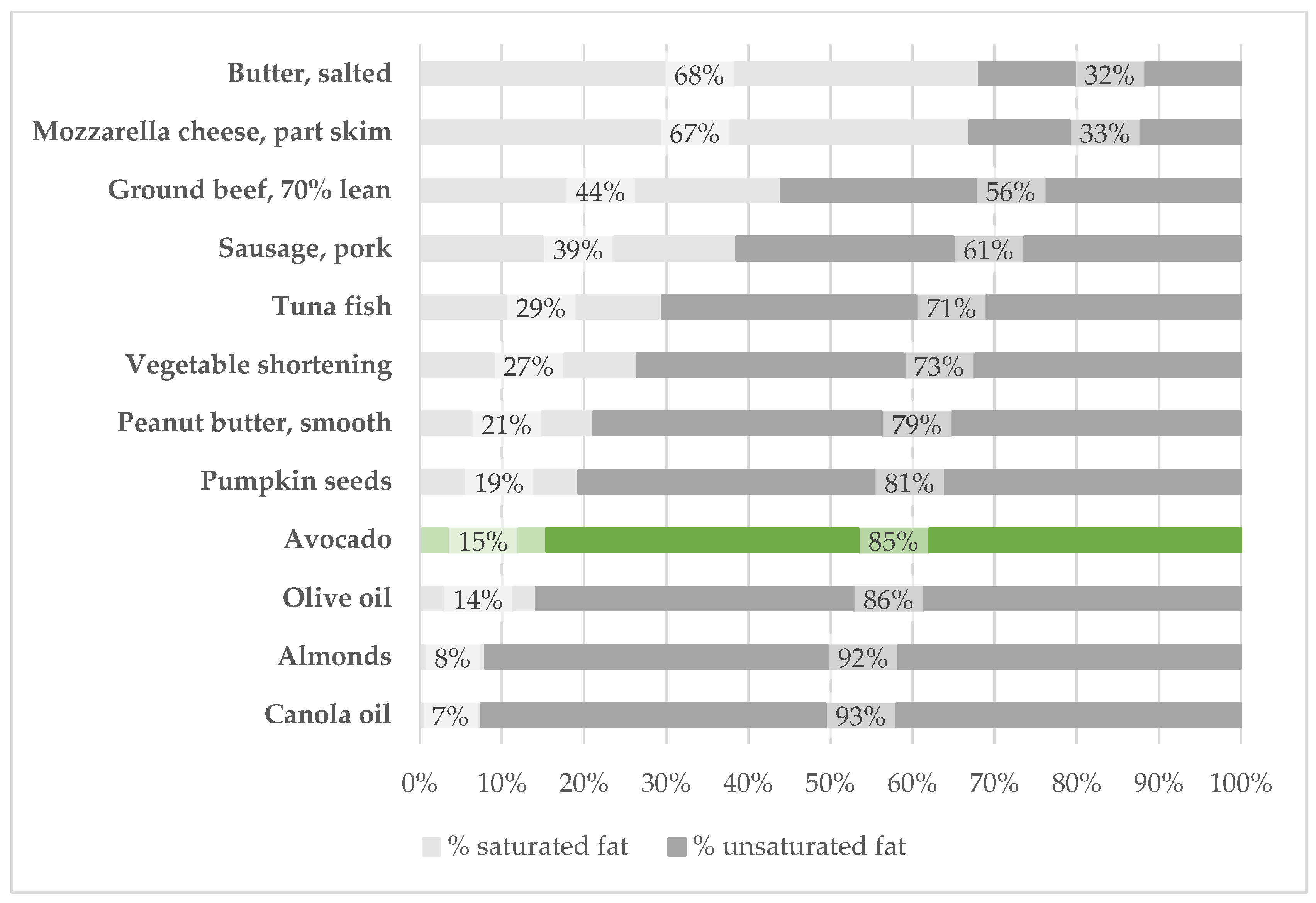
3.3. Carbohydrates
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| g/100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Total carbohydrates | 8.64 | NA | 1 | 5.82 | 3, 12.2 | 16 | [17,18,19,20,21,24] |
| Dietary fiber | 6.8 | 3.2, 12.7 | 21 | 3.87 | 2.2, 7.5 | 17 | [17,18,19,20,21,24] |
| Insoluble fiber | NA | 2.63 | 2.56, 2.7 | 2 | [16] | ||
| Soluble fiber | NA | 2.05 | 1.99, 2.11 | 2 | [16] | ||
| Total sugars | 0.3 | 0, 0.55 | 11 | 0.1 | 0, 0.8 | 8 | [18,19,20,21] |
| Sucrose | 0.06 | 0, 0.15 | 9 | 0.11 | 0.002, 0.43 | 25 | [33,40,41] |
| Glucose | 0.08 | 0.06, 0.24 | 9 | 0.03 | 0.002, 0.1 | 22 | [40,41] |
| Fructose | 0.08 | 0.07, 0.15 | 9 | 0.04 | 0.01, 0.1 | 22 | [40,41] |
| Galactose | 0.08 | 0, 0.3 | 8 | NA | |||
| Starch | 0.11 | 0.05, 0.17 | 4 | NA | |||
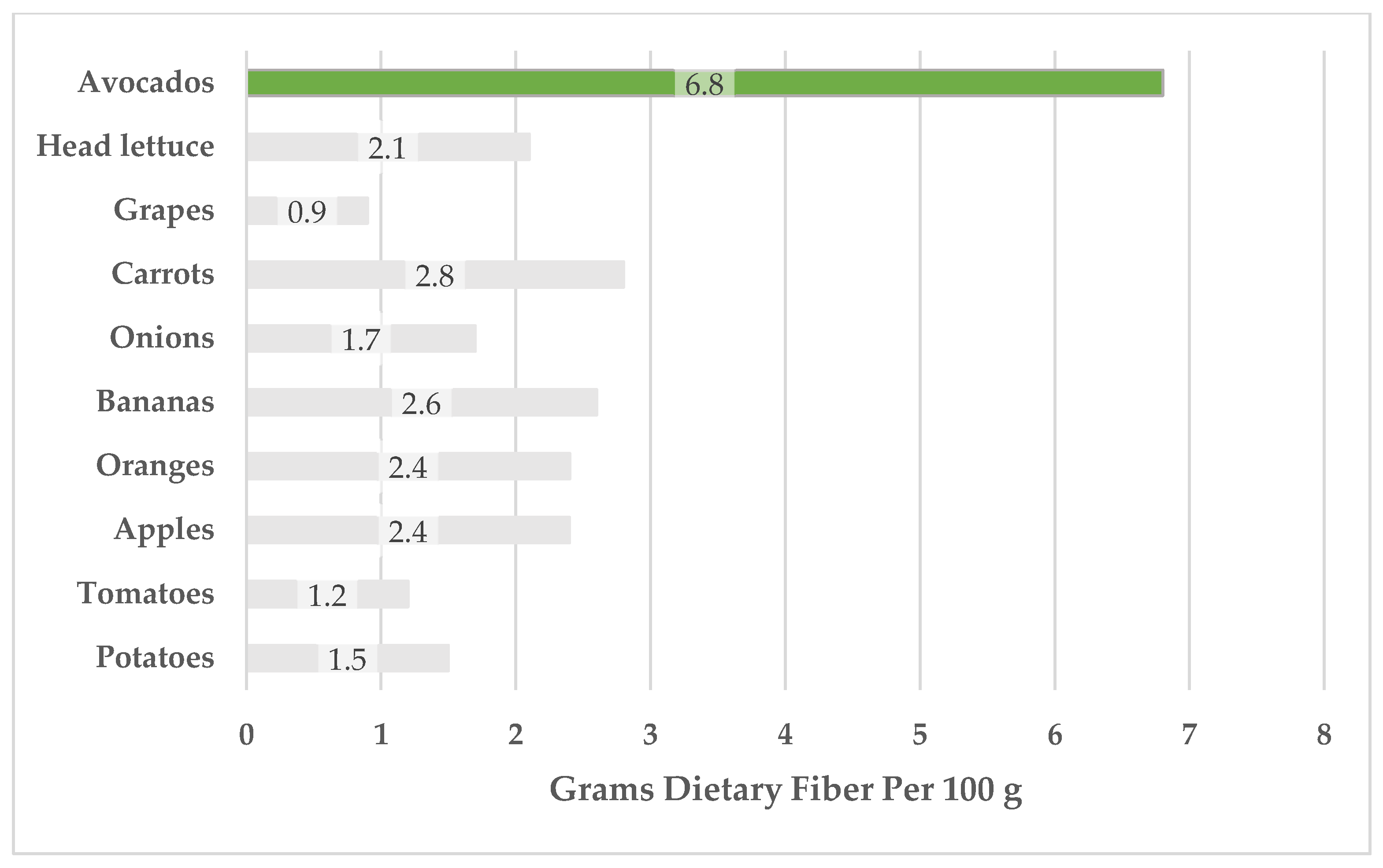
3.4. Protein and Amino Acids
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| g/100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Total protein | 1.96 | 1.53, 3 | 30 | 1.59 | 1.05, 2.4 | 45 | [17,18,19,20,21,23,24,40] |
| Amino acids | |||||||
| Taurine | NA | 0.02 | NA | 1 | |||
| Hydroxyproline | NA | 0.04 | NA | 1 | |||
| Aspartic acid | 0.232 | 1 | 0.14 | 0.12, 0.15 | 2 | [18] | |
| Threonine * | 0.072 | 1 | 0.07 | 0.06, 0.08 | 2 | [18] | |
| Serine | 0.112 | 1 | 0.08 | 0.08, 0.08 | 2 | [18] | |
| Glutamic acid | 0.28 | 1 | 0.15 | 0.14, 0.16 | 2 | [18] | |
| Proline | 0.096 | 1 | 0.07 | 0.06, 0.08 | 2 | [18] | |
| Lanthionine | NA | 0.04 | 1 | ||||
| Glycine | 0.102 | 1 | 0.08 | 0.07,0.09 | 2 | [18] | |
| Alanine | 0.11 | 1 | 0.09 | 0.07, 0.1 | 2 | [18] | |
| Cysteine | NA | 0.03 | 0.03, 0.04 | 2 | [43] | ||
| Cystine | 0.027 | 1 | ND | ||||
| Valine * | 0.11 | 1 | 0.09 | 0.08, 0.1 | 2 | [18] | |
| Methionine * | 0.04 | 1 | 0.03 | 0.02, 0.04 | 3 | [18,43] | |
| Isoleucine * | 0.08 | 1 | 0.07 | 0.06, 0.08 | 2 | [18] | |
| Leucine * | 0.14 | 1 | 0.11 | 0.1, 0.1 | 2 | [18] | |
| Tyrosine | 0.05 | 1 | 0.1 | 0.04, 0.15 | 2 | [18] | |
| Phenylalanine * | 0.1 | 1 | 0.07 | 0.06, 0.07 | 2 | [18] | |
| Hydroxylysine | NA | 0.03 | 1 | ||||
| Lysine * | 0.13 | 1 | 0.09 | 0.08, 0.1 | 2 | [18] | |
| Histidine * | 0.05 | 1 | 0.03 | 0.03, 0.04 | 2 | [18] | |
| Arginine | 0.09 | 1 | 0.08 | 0.07, 0.09 | 2 | [18] | |
| Tryptophan * | 0.03 | 1 | 0.02 | 0.02, 0.02 | 2 | [18] | |
3.5. Vitamins
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| Per 100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Vitamin C (mg) | 8.8 | 6.3, 13.9 | 16 | 6.19 | 1.9, 13 | 15 | [16,17,18,19,20,21,41] |
| Thiamin (mg) | 0.075 | 0.052, 0.1 | 12 | 0.069 | 0.03, 0.119 | 10 | [17,18,19,20,21] |
| Riboflavin (mg) | 0.143 | 0.119, 0.18 | 12 | 0.139 | 0.12, 0.183 | 10 | [17,18,19,20,21] |
| Niacin (mg) | 1.91 | 1.46, 2.51 | 12 | 2.07 | 1.59, 2.6 | 10 | [17,18,19,20,21] |
| Pantothenic acid (mg) | 1.46 | 0.93, 2.71 | 12 | 0.89 | 0.65, 1.2 | 6 | [17,18,19,20,21] |
| Pyroxidine (mg) | 0.287 | 0.196, 0.452 | 11 | 0.28 | 0.1, 0.69 | 10 | [17,18,19,20,21] |
| Folate (µg) | 89 | 71, 155 | 20 | 90 | 61,120 | 10 | [17,18,19,20,21] |
| Biotin (µg) | NA | 2.73 | 0, 5.6 | 6 | [17,18,19,20,21] | ||
| Vitamin A * (µg) | 7 | NA | NA | 10.5 | 6, 16 | 4 | [18,19,20,21] |
| α-tocopherol (mg) | 1.97 | 0.66, 3.28 | 22 | 2.13 | 0.94, 3.28 | 20 | [17,18,41,47] |
| β-tocopherol (mg) | 0.04 | 0.02, 0.06 | 9 | 0.01 | 0, 0.05 | 5 | [18] |
| γ-tocopherol (mg) | 0.32 | 0.09, 0.75 | 18 | 0.25 | 0, 0.75 | 14 | [18,47] |
| δ-tocohpherol (mg) | 0.02 | 0.01, 0.03 | 9 | 0.03 | 0,0.13 | 9 | [18,41] |
| Vitamin K (µg) | 21 | 15.7, 27 | 8 | 16.55 | 5, 25 | 6 | [19,20,21] |
| Other | |||||||
| Choline (mg) | 14.2 | NA | NA | 19.5 | 19.3, 19.6 | 2 | [17] |
3.6. Minerals
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| Per 100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Calcium (mg) | 13 | 8, 19 | 24 | 11.7 | 8, 15 | 43 | [17,18,19,20,21,24,53] |
| Iron (mg) | 0.61 | 0.29, 1.06 | 34 | 0.65 | 0.4, 2.3 | 43 | [17,18,19,20,21,24,53] |
| Magnesium (mg) | 29 | 19, 34 | 12 | 30.64 | 19, 64 | 43 | [17,18,19,20,21,24,53] |
| Phosphorus (mg) | 54 | 41, 70 | 12 | 44.0 | 26.3, 55 | 43 | [17,18,19,20,21,24,53] |
| Potassium (mg) | 507 | 356, 691 | 24 | 478.0 | 408, 1010 | 44 | [16,17,18,19,20,21,24,53] |
| Sodium (mg) | 8 | 2, 17 | 18 | 3.57 | 1.5, 18 | 43 | [17,18,19,20,21,24,53] |
| Zinc (mg) | 0.68 | 0.49, 0.83 | 12 | 0.52 | 0.35, 1.1 | 41 | [18,19,20,21,24,53] |
| Copper (mg) | 0.17 | 0.09, 0.38 | 12 | 0.25 | 0.15, 0.34 | 43 | [17,18,19,20,21,24,53] |
| Manganese (mg) | 0.149 | 0.106, 0.19 | 12 | 0.17 | 0.08, 0.4 | 43 | [17,18,19,20,21,24,53] |
| Selenium (µg) | 0.4 | 0.2, 0.6 | 5 | 0.1 | 0, 0.9 | 31 | [18,19,20,21,53] |
| Fluoride (µg) | NA | 230 | 1 | [18] | |||
| Iodine (µg) | NA | 0.08 | 0, 1.5 | 31 | [18,19,20,21,53] | ||
| Nickle (mg) | NA | 0.03 | 0, 0.21 | 30 | [17,18,53] | ||
| Chloride (mg) | NA | 30 | 1 | [18] | |||
| Chromium (mg) | NA | 0.001 | 0, 0.018 | 30 | [17,18,53] | ||
| Molybdenum (µg) | NA | 0.0003 | 0.0002, 0.0003 | 28 | [18,53] | ||
| Silicon (mg) | NA | 31 | 10, 51 | 2 | [17] | ||
| Boron (mg) | NA | 3.7 | 2.6, 4.8 | 2 | [17] | ||
| Strontium (mg) | NA | 0.15 | 0.11,0.97 | 29 | [17,53] | ||
4. Bioactive Compounds in Hass Avocado Pulp
4.1. Fatty Alcohols
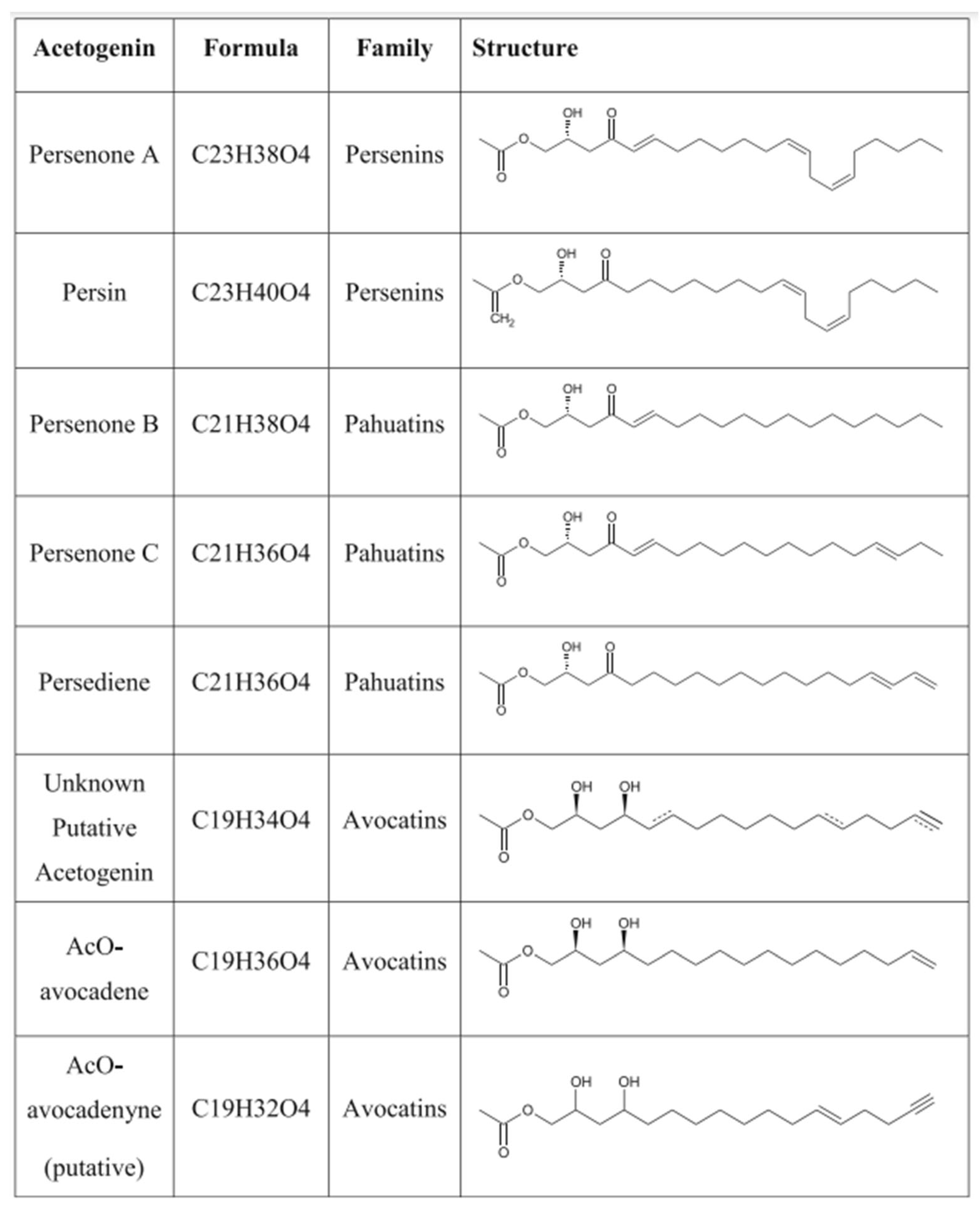
| mg/100 g | Pooled Mean | Min, Max | n | Refs. |
|---|---|---|---|---|
| Avocadyne | 4.99 * | NA | 3 | [63] |
| Avocadene | 6.09 * | NA | 3 | [63] |
| Personone A | 172.5 | 163, 182 | 6 | [13,64] |
| Persenone B | 56.5 | 34, 79 | 6 | [13,64] |
| Persenone C | 31 | 26, 36 | 6 | [13,64] |
| Persediene | 4 | NA | 3 | [13] |
| Acetylated-avocadene | 1 | NA | 3 | [13] |
| Acetylated-avocadyne | 40.5 | 30, 51 | 6 | [13,64] |
| Avoenin | 0.98 * | NA | 1 | [65] |
4.2. Seven-Carbon Carbohydrates
4.3. Phenolics and Organic Acids
| mg/100 g | Pooled Mean | Min, Max | n | Refs. |
|---|---|---|---|---|
| Total Phenolic Content (GAE) | 20 § | 13.3, 26 | 15 | [23,41,73] |
| Total Phenolic Content | 6.1 * | 1.5, 10.7 | 2 | [74,75] |
| Epicatechin | 0.48 | 0.08, 1.11 | 12 | [74,76,77] |
| Epigallocatechin | 1.03 | 0.96, 1.1 | 2 | [76] |
| Cyanadin | 0.5 | 0.42, 0.58 | 4 | [76] |
| Nargenin | 0.007 | NA | 1 | [77] |
| Quercetin | 0.557 | NA | 1 | [77] |
| Rutin | 0.006 | NA | 1 | [74] |
| Taxifolin | 0.005 | NA | 1 | [74] |
| Vanillin | 0.002 | NA | 1 | [74] |
| 4-hydroxybenzoic acid | 0.02 | 0.005, 0.03 | 2 | [74,77] |
| Caffeic acid | 0.02 | NA | 1 | [74] |
| Caffeic acid glucoside | 0.27 *,§ | NA | 1 | [75] |
| Chlorogenic acid | 0.015 | 0.006, 0.023 | 2 | [74,77] |
| Ferulic acid | 0.19 | 0.15, 0.23 | 2 | [74,77] |
| Ferulic acid glucoside isomers | 0.75 *,§ | NA | 1 | [75] |
| 3-feruloylquinic acid | 0.21 § | NA | 1 | [75] |
| 5-feruloylquinic acid | 2.11 § | NA | 1 | [75] |
| 4-feruloylquinic acid | 0.22 § | NA | 1 | [75] |
| Gentisic acid | 0.02 | NA | 1 | [74] |
| Isoramnetin | 0.003 | NA | 1 | [74] |
| Coumaric acid | 0.64 * | 0.47, 0.82 | 2 | [75,77] |
| p-coumaric acid | 0.58 | 0.36, 0.79 | 2 | [74,77] |
| m-coumaric acid | 0.032 | NA | 1 | [77] |
| p-coumaric acid glucoside isomers | 2.62 *,§ | NA | 1 | [75] |
| p-coumaric acid pentoside | 0.29 *,§ | NA | 1 | [75] |
| p-coumaric acid rutinoside | 0.45 *,§ | NA | 1 | [75] |
| Sinapic acid-C-hexoside | 0.21 *,§ | NA | 1 | [75] |
| Sinapic acid | 0.03 | NA | 1 | [77] |
| Tyrosol-hexoside-pentoside | 0.63 *,§ | NA | 1 | [75] |
| Octyl gallate | 0.26 *,§ | NA | 1 | [75] |
| Trans-cinnamic acid | 0.052 | 0.005, 0.98 | 2 | [74,77] |
| Sinapinic acid | 0.04 | NA | 1 | [73,74] |
| mg/100 g | Pooled Mean | Min, Max | n | Refs. |
|---|---|---|---|---|
| Succinic acid | 1 *,§ | 0.3, 1.2 | 5 | [41,75] |
| Fumaric acid | 26.9 | NA | 4 | [41] |
| Quinic acid | 20.6 *,§ | 0.03, 30.9 | 6 | [41,75,77] |
| Malic acid | 119.2 | NA | 4 | [41] |
| Citric acid | 216 * | NA | 5 | [41,75] |
| Oxalic acid | ND | 4 | [41] | |
| Benzoic acid | 0.11 | 0.1, 0.13 | 2 | [74,77] |
| Abscisic acid | 0.267 | NA | 1 | [77] |
| Homovanillic acid | 0.002 | NA | 1 | [77] |
4.4. Carotenoids and Other Pigments
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| µg/100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| Lutein and Zeaxanthin | 271 | 170, 379 | 16 | 541 | 223, 874 | 209 | [41,47,83] |
| Lutein | NA | 514 | 140, 842 | 224 | [41,47,82,83] | ||
| Zeaxanthin | NA | 8 | 1, 100 | 209 | [41,47,83] | ||
| β-cryptoxanthin | 27 | 0, 120 | 25 | 23 | 17, 64 | 206 | [18,47,83] |
| Neoxanthin | NA | 448 | 46, 1190 | 192 | [83] | ||
| Lutein-5,6-epoxide | NA | 402 | 2, 899 | 196 | [41,83] | ||
| 9′-cis-neoxanthin | NA | 102 | 6, 216 | 196 | [41,83] | ||
| cis-violaxanthin | NA | 202 | 44, 475 | 192 | [83] | ||
| Neochrome | NA | 96 | 37, 161 | 192 | [83] | ||
| Chrysanthemaxanthin | NA | 159 | 31, 272 | 192 | [83] | ||
| 15-cis-zeaxanthin | NA | 13 | NA | 4 | [41] | ||
| 13-cis-lutein | NA | 6 | NA | 4 | [41] | ||
| 15-cis-lutein | NA | 36 | NA | 4 | [41] | ||
| Alpha-carotene | 24 | 0, 100 | 27 | 40 | 3, 89 | 206 | [18,47,83] |
| Chlorophyll a | NA | 1.84 | NA | 1 | [41] | ||
| Chlorophyll b | NA | 1.16 | NA | 1 | [41] | ||
| Pheophorbide a | NA | 0.006 | NA | 1 | [41] | ||
| Pheophytin a | NA | 0.015 | NA | 1 | [41] | ||
4.5. Phytosterols
| USDA Food Data Central | Literature, Other Government Databases and Commercial Analyses | ||||||
|---|---|---|---|---|---|---|---|
| mg/100 g | Mean | Min, Max | n | Pooled Mean | Min, Max | n | Refs. |
| β-sitosterol | 76 | 62, 98 | 6 | 57 | 24, 105 | 85 | [34,41,114] |
| Stigmasterol | 2 | 2, 2 | 6 | 0.94 | 0.14, 10 | 85 | [34,41,114] |
| Campesterol | 5 | 5, 6 | 6 | 6 | 4, 11 | 85 | [34,41,114] |
| Cycloartenol | NA | 17 | NA | 4 | [41] | ||
| Avenasterol | NA | 3.9 | NA | 8 | [114] | ||
| Stanol | NA | 0.5 | NA | 8 | [114] | ||
4.6. Glutathione and Betaine
5. Challenges in Moving toward Precision Nutrition
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FruiTrop. Close-up avocado. In FruiTrop Magazine; Loeillet, D., Imbert, E., Eds.; Cirad: Montpellier, France, 2015; pp. 1–96. [Google Scholar]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Cheng, F.W.; Ford, N.A. A Comprehensive Review of Hass Avocado Clinical Trials, Observational Studies, and Biological Mechanisms. Nutrients 2021, 13, 4376. [Google Scholar] [CrossRef]
- Henning, S.M.; Guzman, J.B.; Thames, G.; Yang, J.; Tseng, C.H.; Heber, D.; Kim, J.; Li, Z. Avocado Consumption Increased Skin Elasticity and Firmness in Women—A Pilot Study. J. Cosmet. Dermatol. 2022, 21, 4028–4034. [Google Scholar] [CrossRef]
- Edwards, C.G.; Walk, A.M.; Thompson, S.V.; Reeser, G.E.; Erdman, J.W., Jr.; Burd, N.A.; Holscher, H.D.; Khan, N.A. Effects of 12-week avocado consumption on cognitive function among adults with overweight and obesity. Int. J. Psychophysiol. 2020, 148, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Bailey, M.A.; Taylor, A.M.; Kaczmarek, J.L.; Mysonhimer, A.R.; Edwards, C.G.; Reeser, G.E.; Burd, N.A.; Khan, N.A.; Holscher, H.D. Avocado Consumption Alters Gastrointestinal Bacteria Abundance and Microbial Metabolite Concentrations among Adults with Overweight or Obesity: A Randomized Controlled Trial. J. Nutr. 2021, 151, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Rasmussen, H.M.; Chen, O.; Johnson, E.J. Avocado Consumption Increases Macular Pigment Density in Older Adults: A Randomized, Controlled Trial. Nutrients 2017, 9, 919. [Google Scholar] [CrossRef]
- Cheng, F.W.; Ford, N.A.; Taylor, M.K. US Older Adults That Consume Avocado or Guacamole Have Better Cognition Than Non-consumers: National Health and Nutrition Examination Survey 2011–2014. Front. Nutr. 2021, 8, 746453. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Forester, S.; Jennings-Dobbs, E.; Heber, D. Perspective: A Comprehensive Evaluation of Data Quality in Nutrient Databases. Adv. Nutr. 2023, 14, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Alvaro, J.E.; Olmedo, P.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Primary Metabolism in Avocado Fruit. Front. Plant. Sci. 2019, 10, 795. [Google Scholar] [CrossRef]
- Ashworth, V.; Rolshausen, P. Avocado Cultivars, Botanical Races and Genetic Footprints. Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=21125 (accessed on 6 March 2023).
- Rodriguez-Lopez, C.E.; Hernandez-Brenes, C.; Trevino, V.; Diaz de la Garza, R.I. Avocado fruit maturation and ripening: Dynamics of aliphatic acetogenins and lipidomic profiles from mesocarp, idioblasts and seed. BMC Plant. Biol. 2017, 17, 159. [Google Scholar] [CrossRef]
- Hernández, I.; Fuentealba, C.; Olaeta, J.A.; Lurie, S.; Defilippi, B.G.; Campos-Vargas, R.; Pedreschi, R. Factors associated with postharvest ripening heterogeneity of ‘Hass’ avocados (Persea americana Mill). Fruits 2016, 79, 259–268. [Google Scholar] [CrossRef]
- FoodData Central. Avocados, Raw, California. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171706/nutrients (accessed on 1 March 2023).
- Smith, J.; Goldweber, S.; Lamberts, M.; Tyson, R.; Reynolds, J.S. Utilization potential of semi-tropical and tropical fruits and vegetables in therapeutic and family diets. Proc. Fla. State Hort. Soc. 1983, 96, 241–244. [Google Scholar]
- Slater, G.G.; Shankman, S.; Shepherd, J.S.; Alfin-Slater, R.B. Seasonal variation in the composition of California avocados. J. Agric. Food Chem. 1975, 23, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Australian Food Composition Database. F000162: Avocado, Raw. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/fooddetails.aspx?PFKID=F000162 (accessed on 1 March 2023).
- New Zealand Food Composition Data. Avocado, ‘Hass’, New Zealand. Available online: https://www.foodcomposition.co.nz/search/food/L1159/nip (accessed on 1 March 2023).
- New Zealand Food Composition Data. Avocado, Flesh, Fresh, Raw, late Season (April), ‘Hass’, New Zealand. Available online: https://www.foodcomposition.co.nz/search/food/L1157/nip (accessed on 1 March 2023).
- New Zealand Food Composition Data. Avocado, California, Flesh, Raw. Available online: https://www.foodcomposition.co.nz/search/food/L221/nip (accessed on 1 March 2023).
- Kant, A.K.; Graubard, B.I. Energy density of diets reported by American adults: Association with food group intake, nutrient intake, and body weight. Int. J. Obes. (Lond.) 2005, 29, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estevez, M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef] [PubMed]
- Viera, W.; Gaona, P.; Samaniego, I.; Sotomayor, A.; Viteri, P.; Noboa, M.; Merino, J.; Mejia, P.; Park, C.H. Mineral Content and Phytochemical Composition of Avocado var. Hass Grown Using Sustainable Agriculture Practices in Ecuador. Plants (Basel) 2023, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Terry, L.A. Fatty acid and sugar composition of avocado, cv. Hass, in response to treatment with an ethylene scavenger or 1-methylcyclopropene to extend storage life. Food Chem. 2010, 121, 1203–1210. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Saavedra, J.; Ortiz, J.; Zúñiga, C.; Troncoso, C.; Rivera, S.A.; González-Agüero, M.; Defilippi, B.G. Identification of pre-harvest factors that affect fatty acid profiles of avocado fruit (Persea americana Mill) cv. ‘Hass’ at harvest. S. Afr. J. Bot. 2016, 104, 15–20. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020. [Google Scholar]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Fats and Fatty Acids in Human Nutrition. Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.S.; Li, Y.; Rimm, E.B.; Manson, J.E.; Sun, Q.; Rexrode, K.; Hu, F.B.; Guasch-Ferre, M. Avocado Consumption and Risk of Cardiovascular Disease in US Adults. J. Am. Heart Assoc. 2022, 11, e024014. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Terry, L.A. Development of a rapid method for the sequential extraction and subsequent quantification of fatty acids and sugars from avocado mesocarp tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef]
- Plaza, L.; Sanchez-Moreno, C.; de Pascual-Teresa, S.; de Ancos, B.; Cano, M.P. Fatty acids, sterols, and antioxidant activity in minimally processed avocados during refrigerated storage. J. Agric. Food Chem. 2009, 57, 3204–3209. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 March 2023).
- McKeown, N.M.; Fahey, G.C., Jr.; Slavin, J.; van der Kamp, J.W. Fibre intake for optimal health: How can healthcare professionals support people to reach dietary recommendations? BMJ 2022, 378, e054370. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Blakey, R.J.; Tesfay, S.Z.; Bertling, I.; Bower, J.P. Changes in sugars, total protein, and oil in ‘Hass’ avocado (Persea americana Mill.) fruit during ripening. J. Hortic. Sci. Biotechnol. 2012, 87, 381–387. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Bejar, A.A.; Yahia, E.M.; Ornelas-Paz, J.J.; Perez-Martinez, J.D.; Rios-Velasco, C.; Escalante-Minakata, P. Metabolomic analysis and physical attributes of ripe fruits from Mexican Creole (Persea americana var. Drymifolia) and ‘Hass’ avocados. Food Chem. 2021, 354, 129571. [Google Scholar] [CrossRef] [PubMed]
- USDA Economic Research Service. Potatoes and tomatoes Are the Most Commonly Consumed Vegetables. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58340 (accessed on 12 April 2023).
- Jones, D.P.; Coates, R.J.; Flagg, E.W.; Eley, J.W.; Block, G.; Greenberg, R.S.; Gunter, E.W.; Jackson, B. Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr. Cancer 1992, 17, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.R.; Bellinge, J.W.; Dalgaard, F.; Sim, M.; Murray, K.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Croft, K.D.; Gislason, G.; et al. Association between vitamin K(1) intake and mortality in the Danish Diet, Cancer, and Health cohort. Eur. J. Epidemiol. 2021, 36, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- Bo, Y.; Zhu, Y.; Tao, Y.; Li, X.; Zhai, D.; Bu, Y.; Wan, Z.; Wang, L.; Wang, Y.; Yu, Z. Association Between Folate and Health Outcomes: An Umbrella Review of Meta-Analyses. Front. Public. Health 2020, 8, 550753. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.; Heber, D. Inhibition of prostate cancer cell growth by an avocado extract: Role of lipid-soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef]
- Morrell, A.; Tallino, S.; Yu, L.; Burkhead, J.L. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 2017, 69, 263–270. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Sodium and Potassium; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- D’Elia, L.; Masulli, M.; Cappuccio, F.P.; Zarrella, A.F.; Strazzullo, P.; Galletti, F. Dietary Potassium Intake and Risk of Diabetes: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2022, 14, 4785. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993, 328, 833–838. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Spiegelman, D.; Stampfer, M.J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997, 126, 497–504. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. FDA Total Diet Study (TDS): Results. Available online: https://www.fda.gov/food/fda-total-diet-study-tds/fda-total-diet-study-tds-results (accessed on 1 March 2023).
- Kim, O.K.; Murakami, A.; Nakamura, Y.; Takeda, N.; Yoshizumi, H.; Ohigashi, H. Novel nitric oxide and superoxide generation inhibitors, persenone A and B, from avocado fruit. J. Agric. Food Chem. 2000, 48, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Ruiz-Cruz, S.; Gardea-Béjar, A.A.; Yahia, E.M.; de Jesús Ornelas-Paz, J.; Pérez-Martínez, J.D.; Rios-Velasco, C.; Ibarra-Junquera, V. The importance of the bioactive compounds of avocado fruit (Persea americana Mill) on human health. Biotecnia 2019, 21, 154–162. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, D.G.; Flores-Garcia, M.; Silva-Platas, C.; Rizzo, S.; Torre-Amione, G.; De la Pena-Diaz, A.; Hernandez-Brenes, C.; Garcia-Rivas, G. Isolation and chemical identification of lipid derivatives from avocado (Persea americana) pulp with antiplatelet and antithrombotic activities. Food Funct. 2015, 6, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Carranza, M.J.; Alvizouri, M.M.; Herrera, J.E.; Chávez, F. Efectos del aguacate como fuente de ácidos grasos monoinsaturados en lípidos séricos, metabolismo de la glucosa y reología en pacientes con diabetes tipo 2. Med. Int. Mex. 2008, 24, 267–272. [Google Scholar]
- Salinas-Salazar, C.; Hernandez-Brenes, C.; Rodriguez-Sanchez, D.G.; Castillo, E.C.; Navarro-Silva, J.M.; Pacheco, A. Inhibitory Activity of Avocado Seed Fatty Acid Derivatives (Acetogenins) Against Listeria Monocytogenes. J. Food Sci. 2017, 82, 134–144. [Google Scholar] [CrossRef]
- Tcheng, M.; Roma, A.; Ahmed, N.; Smith, R.; Jayanth, P.; Minden, M.D.; Hurren, R.; Schimmer, A.D.; Bozzo, G.; Hess, D.; et al. Inhibiting Very Long Chain Acyl-CoA Dehydrogenase (VLCAD) Induces Selective Leukemia Cell Death. Blood 2019, 134, 3922. [Google Scholar] [CrossRef]
- Tcheng, M.; Roma, A.; Ahmed, N.; Smith, R.W.; Jayanth, P.; Minden, M.D.; Schimmer, A.D.; Hess, D.A.; Hope, K.; Rea, K.A.; et al. Very long chain fatty acid metabolism is required in acute myeloid leukemia. Blood 2021, 137, 3518–3532. [Google Scholar] [CrossRef]
- Lee, E.A.; Angka, L.; Rota, S.G.; Hanlon, T.; Mitchell, A.; Hurren, R.; Wang, X.M.; Gronda, M.; Boyaci, E.; Bojko, B.; et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer Res. 2015, 75, 2478–2488. [Google Scholar] [CrossRef]
- Ahmed, N.; Tcheng, M.; Roma, A.; Buraczynski, M.; Jayanth, P.; Rea, K.; Akhtar, T.A.; Spagnuolo, P.A. Avocatin B Protects Against Lipotoxicity and Improves Insulin Sensitivity in Diet-Induced Obesity. Mol. Nutr. Food Res. 2019, 63, e1900688. [Google Scholar] [CrossRef]
- Ahmed, N.; Smith, R.W.; Henao, J.J.A.; Stark, K.D.; Spagnuolo, P.A. Analytical Method To Detect and Quantify Avocatin B in Hass Avocado Seed and Pulp Matter. J. Nat. Prod. 2018, 81, 818–824. [Google Scholar] [CrossRef]
- Rodríguez-López, C.E.; Hernández-Brenes, C.; Díaz de la Garza, R.I. A targeted metabolomics approach to characterize acetogenin profiles in avocado fruit (Persea americana Mill. ) RSC Adv. 2015, 5, 106019. [Google Scholar] [CrossRef]
- Arita, M.; Fuchino, H.; Kawakami, H.; Ezaki, M.; Kawahara, N. Characterization of a New Antienterovirus D68 Compound Purified from Avocado. ACS Infect. Dis. 2020, 6, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Absorption and effect of ingested mannoheptulose. Nutr. Rev. 1969, 27, 206–208. [CrossRef]
- Ingram, D.K.; Roth, G.S. Glycolytic inhibition: An effective strategy for developing calorie restriction mimetics. Geroscience 2021, 43, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Pistell, P.J.; Wang, Z.Q.; Yu, Y.; Massimino, S.; Davenport, G.M.; Hayek, M.; Roth, G.S. Characterization and Mechanisms of Action of Avocado Extract Enriched in Mannoheptulose as a Candidate Calorie Restriction Mimetic. J. Agric. Food Chem. 2021, 69, 7367–7376. [Google Scholar] [CrossRef] [PubMed]
- Viktora, J.K.; Johnson, B.F.; Penhos, J.C.; Rosenberg, C.A.; Wolff, F.W. Effect of ingested mannoheptulose in animals and man. Metabolism 1969, 18, 87–102. [Google Scholar] [CrossRef]
- Pistell, P.J.; Utsuki, T.; Francis, J.; Ebenezer, P.J.; Terrebonne, J.; Roth, G.S.; Ingram, D.K. An Avocado Extract Enriched in Mannoheptulose Prevents the Negative Effects of a High-Fat Diet in Mice. Nutrients 2021, 14, 155. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell. Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Lyu, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic Compounds Profiling and Their Antioxidant Capacity in the Peel, Pulp, and Seed of Australian Grown Avocado. Antioxidants 2023, 12, 185. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Lo Bianco, R. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2017, 20, 1302–1312. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Wasswa-Kintu, S. USDA’s Expanded Flavonoid Database for the Assessment of Dietary Intakes. Available online: https://data.nal.usda.gov/dataset/usda-special-interest-databases-flavonoids (accessed on 1 March 2023).
- Hurtado-Fernández, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Quantitative characterization of important metabolites of avocado fruit by gas chromatography coupled to different detectors (APCI-TOF MS and FID). Food Res. Int. 2014, 62, 801–811. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (-)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Ashton, O.B.; Wong, M.; McGhie, T.K.; Vather, R.; Wang, Y.; Requejo-Jackman, C.; Ramankutty, P.; Woolf, A.B. Pigments in avocado tissue and oil. J. Agric. Food Chem. 2006, 54, 10151–10158. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.P.; Gao, K.; Byrns, R.; Heber, D. California Hass avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M.; Ornelas-Paz, J.J.; Victoria-Campos, C.I.; Perez-Martinez, J.D.; Reyes-Hernandez, J. Bioaccessibility of fat-soluble bioactive compounds (FSBC) from avocado fruit as affected by ripening and FSBC composition in the food matrix. Food Res. Int. 2021, 139, 109960. [Google Scholar] [CrossRef]
- Noakes, M.; Clifton, P.; Ntanios, F.; Shrapnel, W.; Record, I.; McInerney, J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. Am. J. Clin. Nutr. 2002, 75, 79–86. [Google Scholar] [CrossRef]
- van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Cooperstone, J.L.; Schweiggert, R.M.; Young, G.S.; Harrison, E.H.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-beta-carotene tomato sauce and from carrots. J. Nutr. 2014, 144, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Giossi, C.; Cartaxana, P.; Cruz, S. Photoprotective Role of Neoxanthin in Plants and Algae. Molecules 2020, 25, 4617. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Asai, A.; Zhang, H.; Nagao, A. A highly polar xanthophyll of 9’-cis-neoxanthin induces apoptosis in HCT116 human colon cancer cells through mitochondrial dysfunction. Mol. Cell. Biochem. 2007, 300, 227–237. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef]
- Chang, J.M.; WC, C.H.; Hong, D.; Lin, J.K. The inhibition of DMBA-induced carcinogenesis by neoxanthin in hamster buccal pouch. Nutr. Cancer 1995, 24, 325–333. [Google Scholar] [CrossRef]
- Sekiya, M.; Suzuki, S.; Ushida, Y.; Suganuma, H. Neoxanthin in young vegetable leaves prevents fat accumulation in differentiated adipocytes. Biosci. Biotechnol. Biochem. 2021, 85, 2145–2152. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Yin, W.; Zhang, L.; Li, G.; Ma, J.; Xu, L.; Xiong, Y.; Liu, L.; Zhang, W.; et al. Neoxanthin alleviates the chronic renal failure-induced aging and fibrosis by regulating inflammatory process. Int. Immunopharmacol. 2023, 114, 109429. [Google Scholar] [CrossRef]
- Gyemant, N.; Tanaka, M.; Molnar, P.; Deli, J.; Mandoky, L.; Molnar, J. Reversal of multidrug resistance of cancer cells in vitro: Modification of drug resistance by selected carotenoids. Anticancer. Res. 2006, 26, 367–374. [Google Scholar] [PubMed]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the Lifespan: From Childhood Cognitive Performance to the Aging Eye and Brain. Curr. Dev. Nutr. 2019, 3, nzz066. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of beta-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef]
- Hammond, B.R., Jr.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin. Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2020, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Duester, K.C. Avocado fruit is a rich source of beta-sitosterol. J. Am. Diet. Assoc. 2001, 101, 404–405. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.83 (accessed on 1 March 2023).
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.; Ishani, A.; MacDonald, R.; Stark, G.; Mulrow, C.; Lau, J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2000, 1999, CD001043. [Google Scholar] [CrossRef] [PubMed]
- Berges, R.R.; Windeler, J.; Trampisch, H.J.; Senge, T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet 1995, 345, 1529–1532. [Google Scholar] [CrossRef]
- Berges, R.R.; Kassen, A.; Senge, T. Treatment of symptomatic benign prostatic hyperplasia with beta-sitosterol: An 18-month follow-up. BJU Int. 2000, 85, 842–846. [Google Scholar] [CrossRef]
- Klippel, K.F.; Hiltl, D.M.; Schipp, B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study group. Br. J. Urol. 1997, 80, 427–432. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Puupponen-Pimiä, R.; Lampi, A.M. Plant sterols in vegetables, fruits and berries. J. Sci. Food Agric. 2003, 83, 330–337. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Ziqubu, K.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Nkambule, B.B.; Basson, A.K.; Pheiffer, C.; Tiano, L.; Kengne, A.P. Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Nutrients 2023, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, S.; Noda, Y.; Tarumi, R.; Mimura, Y.; Yoshida, K.; Iwata, Y.; Elsalhy, M.; Kuromiya, M.; Kurose, S.; Masuda, F.; et al. Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: A systematic review and meta-analysis. J. Psychopharmacol. 2019, 33, 1199–1214. [Google Scholar] [CrossRef] [PubMed]
- Sydnor, V.J.; Roalf, D.R. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: Implications for studies of psychosis risk. Schizophr. Res. 2020, 226, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, B.; Bast, A.; Diliën, H.; de Boer, A. Nutrient composition of fresh produce—Assessing variability between European countries to substantiate nutrition and health claims. J. Food Compost. Ana 2023, 118, 105201. [Google Scholar] [CrossRef]
- Ozdemir, F.; Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004, 86, 79–83. [Google Scholar] [CrossRef]
- Bower, J.P.; Cutting, J.G. Avocado fruit development and ripening physiology. Hortic. Rev. 1988, 10, 229–271. [Google Scholar]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Kris-Etherton, P.M.; Petersen, K.S.; Matthan, N.R.; Barnes, S.; Vitolins, M.Z.; Li, Z.; Sabate, J.; Rajaram, S.; Chowdhury, S.; et al. Effect of Incorporating 1 Avocado Per Day Versus Habitual Diet on Visceral Adiposity: A Randomized Trial. J. Am. Heart Assoc. 2022, 11, e025657. [Google Scholar] [CrossRef]
- Wang, L.; Bordi, P.L.; Fleming, J.A.; Hill, A.M.; Kris-Etherton, P.M. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: A randomized, controlled trial. J. Am. Heart Assoc. 2015, 4, e001355. [Google Scholar] [CrossRef]
- James-Martin, G.; Brooker, P.G.; Hendrie, G.A.; Stonehouse, W. Avocado Consumption and Cardiometabolic Health: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, N.A.; Spagnuolo, P.; Kraft, J.; Bauer, E. Nutritional Composition of Hass Avocado Pulp. Foods 2023, 12, 2516. https://doi.org/10.3390/foods12132516
Ford NA, Spagnuolo P, Kraft J, Bauer E. Nutritional Composition of Hass Avocado Pulp. Foods. 2023; 12(13):2516. https://doi.org/10.3390/foods12132516
Chicago/Turabian StyleFord, Nikki A., Paul Spagnuolo, Jana Kraft, and Ella Bauer. 2023. "Nutritional Composition of Hass Avocado Pulp" Foods 12, no. 13: 2516. https://doi.org/10.3390/foods12132516
APA StyleFord, N. A., Spagnuolo, P., Kraft, J., & Bauer, E. (2023). Nutritional Composition of Hass Avocado Pulp. Foods, 12(13), 2516. https://doi.org/10.3390/foods12132516







