Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dielectric Measurements
2.3. Microwave Treatment of Lotus Seed Starch
2.4. Determination of Relative Degree of Complexation via the Quantification of Chlorogenic Acid with the Total Phenolic Content assay
2.5. X-ray Diffraction (XRD) Analysis
2.6. Thermogravimetric Analysis
2.7. Determination of Starch Swelling Power and Solubility
2.8. In Vitro Digestion
2.8.1. Digestibility Determination
2.8.2. Determination of Chlorogenic Acid (CA) Released during Digestion
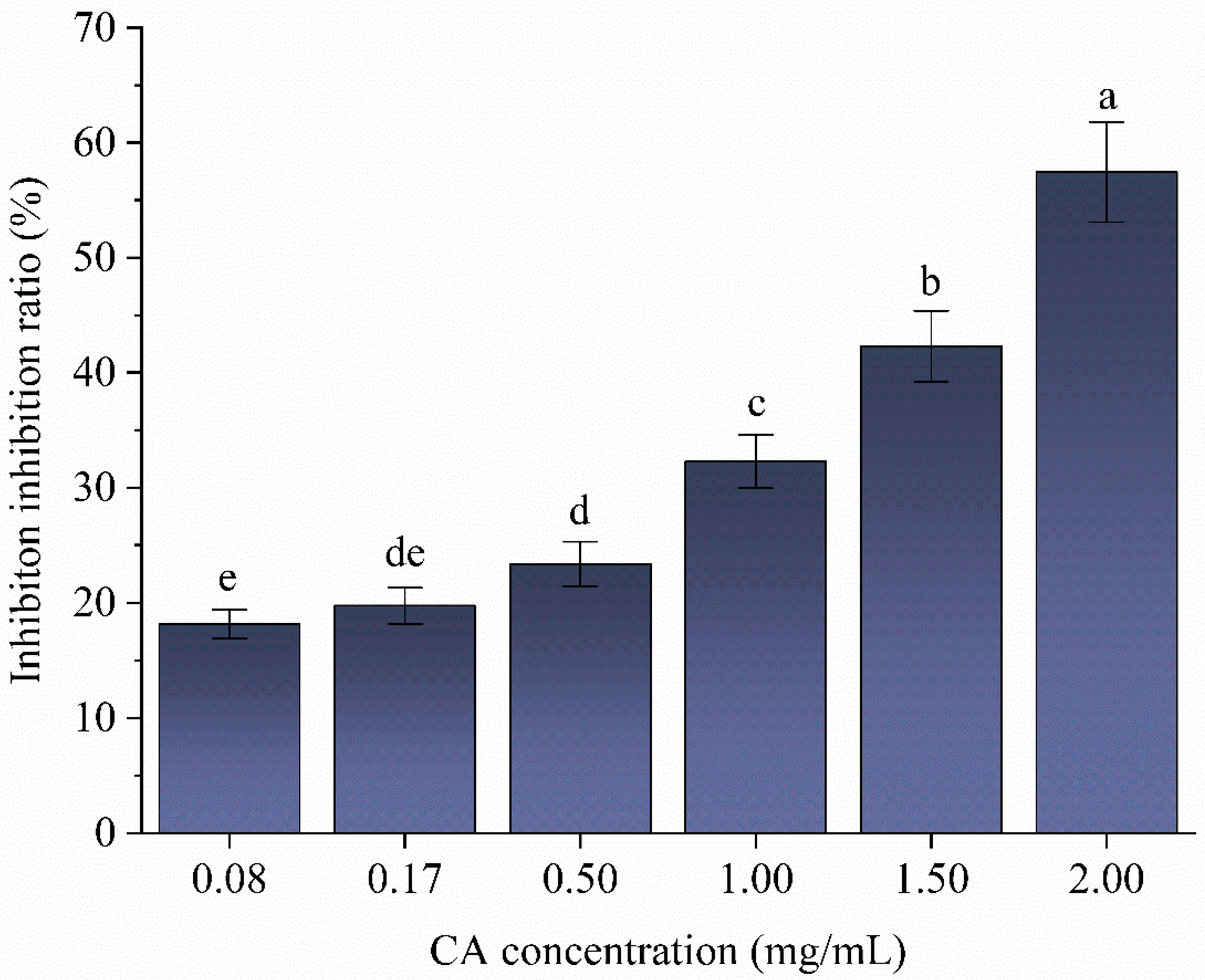
2.9. Determination of α-Amylase Inhibition and the Type of Inhibition by CA
2.10. Data Processing and Analysis
3. Results and Discussion
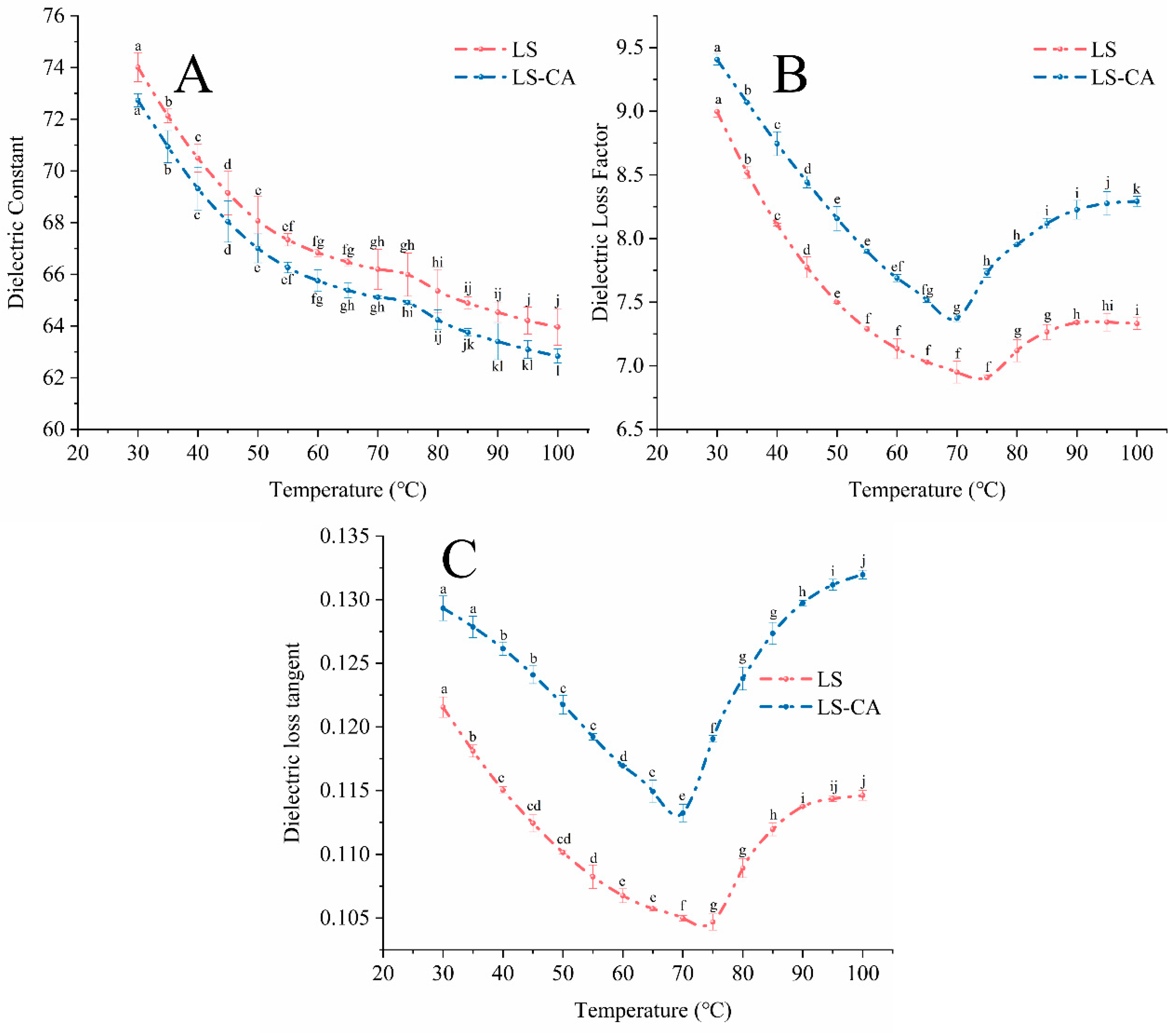
3.1. Effect of Chlorogenic Acid on the Dielectric Properties of Lotus Seed Starch
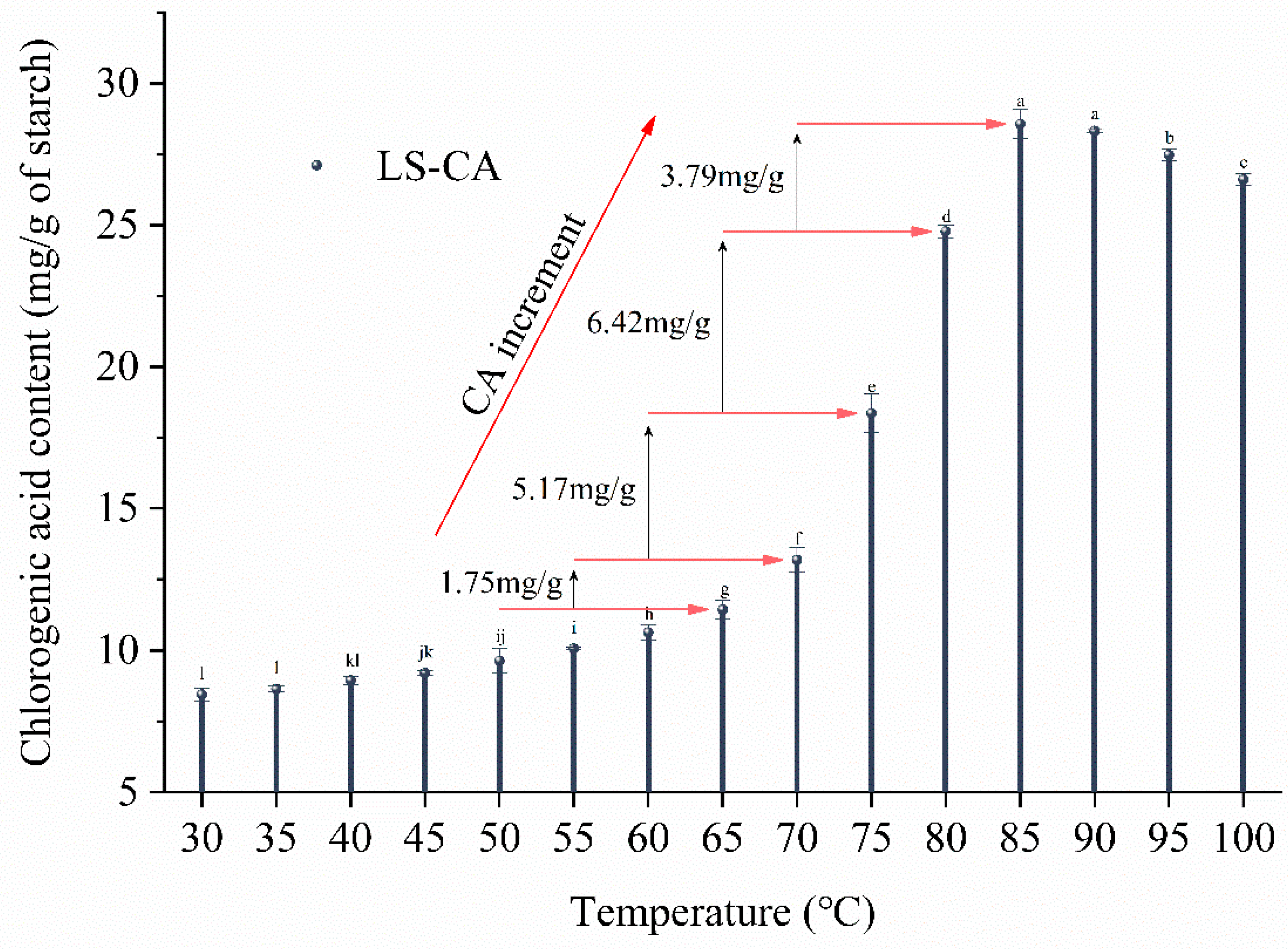
3.2. Extent of Complexation of Chlorogenic Acid in LS-CA with Temperature
3.3. Effect of Chlorogenic Acid on the Crystal Structure of Lotus Seed Starch
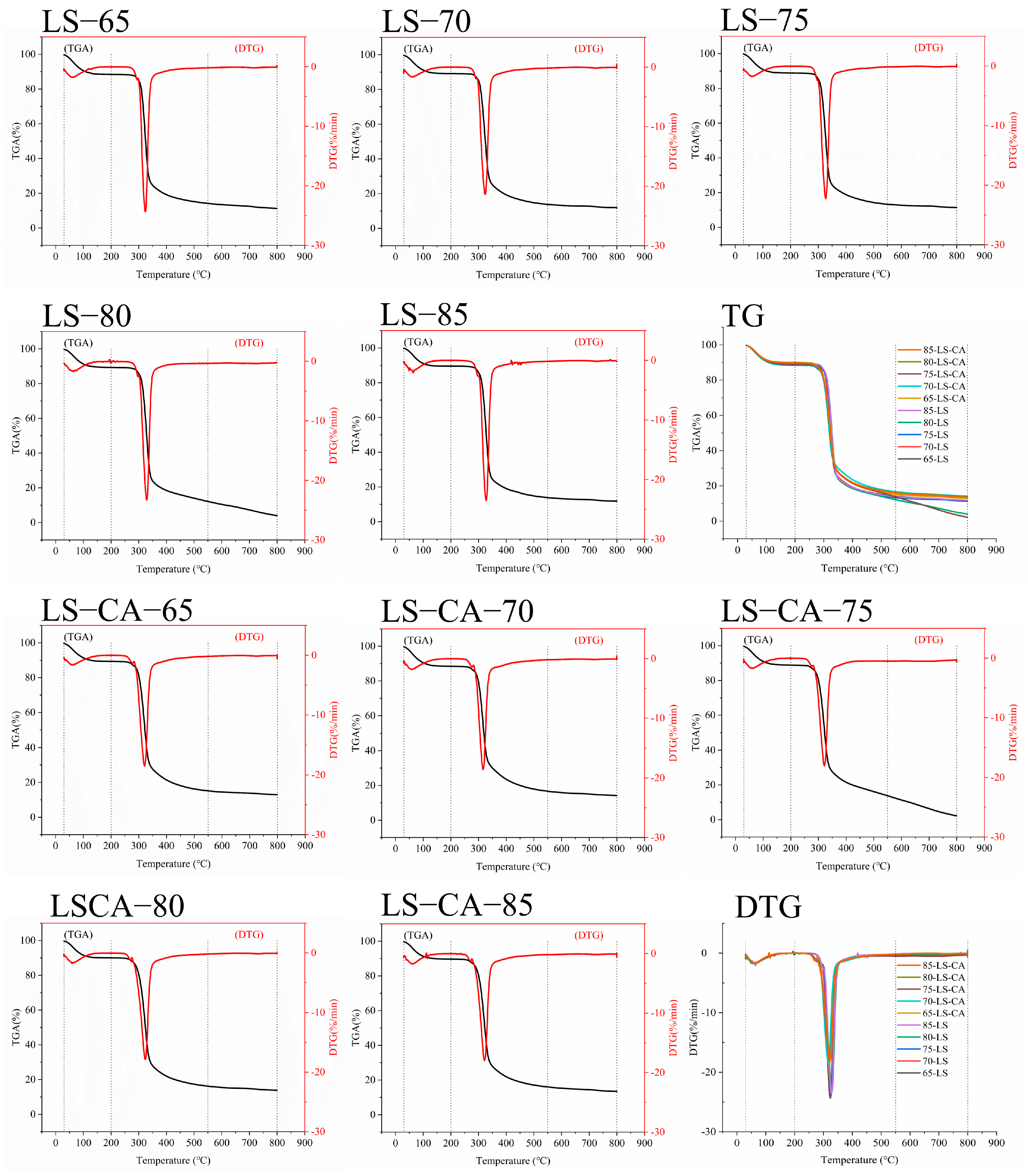
3.4. Effect of Chlorogenic Acid on the Thermal Stability of Lotus Seed Starch
3.5. Effect of Chlorogenic Acid on the Swelling Rate and Solubility of Lotus Seed Starch
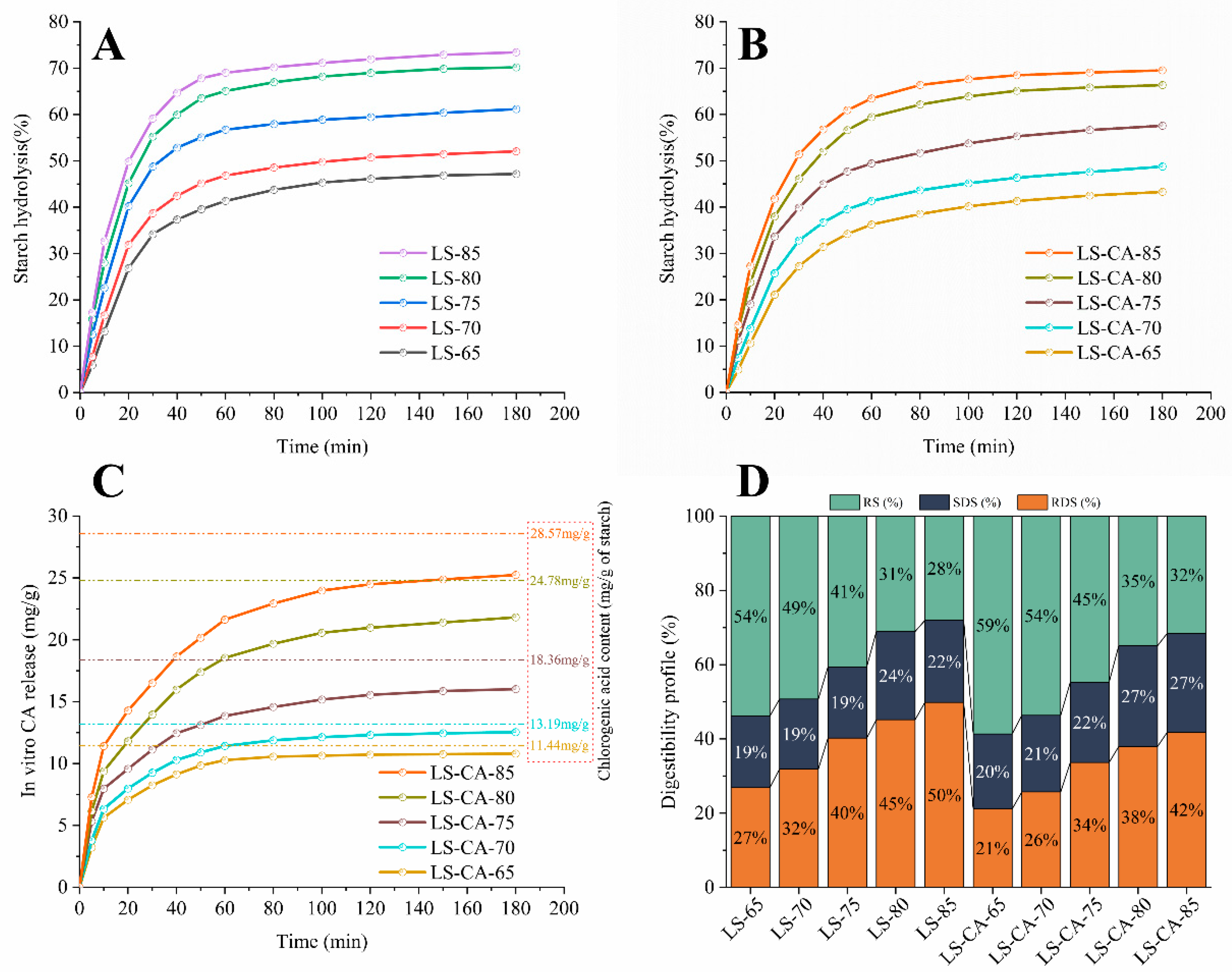
3.6. Effect of Chlorogenic Acid on the Digestive Properties of Lotus Seed Starch
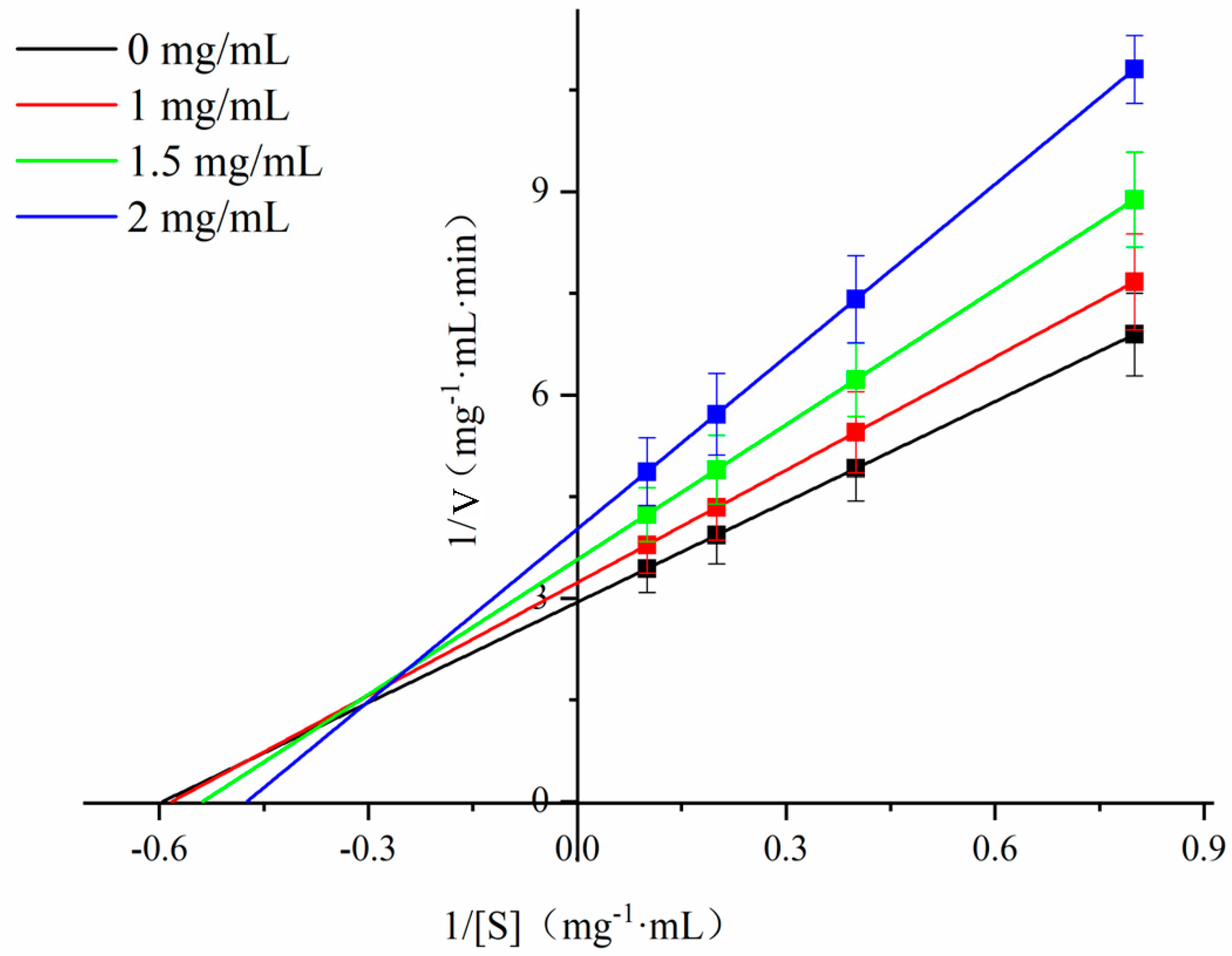
3.7. Effect of Chlorogenic Acid on α-Amylase Starch Hydrolysis Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Fan, D.; Ma, S.; Wang, L.; Zhao, J.; Zhang, H.; Chen, W. Effect of microwave heating on optical and thermal properties of rice starch. Starch Stärke 2012, 64, 740–744. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R. Behaviour of starch exposed to microwave radiation treatment. Starch Stärke 2014, 66, 3–14. [Google Scholar] [CrossRef]
- Matia-Merino, L.; Prieto, M.; Roman, L.; Gómez, M. The impact of basil seed gum on native and pregelatinized corn flour and starch gel properties. Food Hydrocoll. 2019, 89, 122–130. [Google Scholar] [CrossRef]
- Roebuck, B.D.; Goldblith, S.A.; Westphal, W.B. Dielectric properties of carbohydrate-water mixtures at microwave frequencies. J. Food Sci. 1972, 37, 199–204. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Wen, S.; Zhou, Y.; Ma, J.; Chen, S.; Jerrams, S. Fabrication of dielectric elastomers with improved electromechanical properties using silicone rubber and walnut polyphenols modified dielectric particles. Mater. Des. 2020, 192, 108674. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, B.; Li, C.; Wang, Y.; Wen, S.; Zhou, Y.; Jiang, L.; Zhou, F.; Betts, A.; Jerrams, S. Printable dielectric elastomers of high electromechanical properties based on SEBS ink incorporated with polyphenols modified dielectric particles. Eur. Polym. J. 2021, 159, 110730. [Google Scholar] [CrossRef]
- Najib, T.; Heydari, M.M.; Tu, K.; Meda, V. Modification in starch structure of soaked and germinated lentil seeds under various thermal processing methods, including conventional, microwave, and microwave-assisted techniques. Food Chem. Adv. 2023, 2, 100267. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Guo, Z.; Zheng, B.; Zhang, Y. Long-term retrogradation behavior of lotus seed starch-chlorogenic acid mixtures after microwave treatment. Food Hydrocoll. 2021, 121, 106994. [Google Scholar] [CrossRef]
- Cui, R.; Yeon Yoo, M.J.; Zhu, F. Comparison of microwave and conventional heating on physicochemical properties and phenolic profiles of purple sweetpotato and wheat flours. Food Biosci. 2022, 46, 101602. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Jiang, L.; Huang, H.; Sun, J.; Luo, Z. Improvement of phenolic compounds extraction from high-starch lotus (Nelumbo nucifera G.) seed kernels using glycerol: New insights to amylose/amylopectin—Phenolic relationships. Food Chem. 2019, 274, 933–941. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): Nutritional composition, health-related bioactive properties, and industrial applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Okumus, B.N.; Tacer-Caba, Z.; Kahraman, K.; Nilufer-Erdil, D. Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chem. 2018, 240, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Rahmat, A.R.; Rahman, W.A.W.A.; Sin, L.T.; Yussuf, A.A. Approaches to improve compatibility of starch filled polymer system: A review. Mater. Sci. Eng. C 2009, 29, 2370–2377. [Google Scholar] [CrossRef]
- Motwani, T.; Seetharaman, K.; Anantheswaran, R.C. Dielectric properties of starch slurries as influenced by starch concentration and gelatinization. Carbohydr. Polym. 2007, 67, 73–79. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Xie, F.; Li, L.; Bai, G. Modulating the in vitro digestibility and predicted glycemic index of rice starch gels by complexation with gallic acid. Food Hydrocoll. 2019, 89, 821–828. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Y.; Luo, Z.; Xiao, Z.; Ren, H.; Li, D.; Yu, J. Effects of Lecithin Addition on the Properties of Extruded Maize Starch. J. Food Process. Preserv. 2016, 40, 20–28. [Google Scholar] [CrossRef]
- Englyst, H.N.; Hudson, G.J. The classification and measurement of dietary carbohydrates. Food Chem. 1996, 57, 15–21. [Google Scholar] [CrossRef]
- Brena, B.M.; Pazos, C.; Franco-Fraguas, L.; Batista-Viera, F. Chromatographic methods for amylases. J. Chromatogr. B Biomed. Sci. Appl. 1996, 684, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Holmes, M.; Orfila, C. Inhibitory effect of chlorogenic acid on digestion of potato starch. Food Chem. 2017, 217, 498–504. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Peng, S.; Zhang, G. Effect of green tea catechins on the postprandial glycemic response to starches differing in amylose content. J. Agric Food Chem. 2011, 59, 4582–4588. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, B.; Fan, D.; Zhang, N.; Ma, S.; Wang, L.; Wu, Y.; Wang, M.; Zhao, J.; Zhang, H. Structural changes of starch subjected to microwave heating: A review from the perspective of dielectric properties. Trends Food Sci. Technol. 2020, 99, 593–607. [Google Scholar] [CrossRef]
- Soulintzis, A.; Kontos, G.; Karahaliou, P.; Psarras, G.C.; Georga, S.N.; Krontiras, C.A. Dielectric relaxation processes in epoxy resin—ZnO composites. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 445–454. [Google Scholar] [CrossRef]
- Piyasena, P.; Ramaswamy, H.S.; Awuah, G.B.; Defelice, C. Dielectric properties of starch solutions as influenced by temperature, concentration, frequency and salt. J. Food Process Eng. 2003, 26, 93–119. [Google Scholar] [CrossRef]
- Hamley, I.W. Liquid crystal phase formation by biopolymers. Soft Matter 2010, 6, 1863–1871. [Google Scholar] [CrossRef]
- Yu, K.; Huang, X.; He, W.; Wu, D.; Du, C. Kinetics of polyphenol losses during cooking of dried green tea noodles as influenced by microwave treatment of dough. LWT 2023, 180, 114675. [Google Scholar] [CrossRef]
- de Lima, A.C.S.; da Rocha Viana, J.D.; de Sousa Sabino, L.B.; da Silva, L.M.R.; da Silva, N.K.V.; de Sousa, P.H.M. Processing of three different cooking methods of cassava: Effects on in vitro bioaccessibility of phenolic compounds and antioxidant activity. LWT Food Sci. Technol. 2017, 76, 253–258. [Google Scholar] [CrossRef]
- Tang, H.; Mitsunaga, T.; Kawamura, Y. Molecular arrangement in blocklets and starch granule architecture. Carbohydr. Polym. 2006, 63, 555–560. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Borah, P.K.; Rappolt, M.; Duary, R.K.; Sarkar, A. Effects of folic acid esterification on the hierarchical structure of amylopectin corn starch. Food Hydrocoll. 2019, 86, 162–171. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef]
- Paris, M.; Bizot, H.; Emery, J.; Buzaré, J.Y.; Buléon, A. Crystallinity and structuring role of water in native and recrystallized starches by 13C CP-MAS NMR spectroscopy: 1: Spectral decomposition. Carbohydr. Polym. 1999, 39, 327–339. [Google Scholar] [CrossRef]
- Fan, D.; Ma, S.; Wang, L.; Zhao, H.; Zhao, J.; Zhang, H.; Chen, W. 1H NMR studies of starch–water interactions during microwave heating. Carbohydr. Polym. 2013, 97, 406–412. [Google Scholar] [CrossRef]
- Li, Y.; Hu, A.; Wang, X.; Zheng, J. Physicochemical and in vitro digestion of millet starch: Effect of moisture content in microwave. Int. J. Biol. Macromol. 2019, 134, 308–315. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Guo, Z.; Zheng, B.; Zhang, Y. Insights into the multi-scale structural properties and digestibility of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. Food Chem 2021, 361, 130171. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Xu, X.; Jin, Z. Starch retrogradation studied by thermogravimetric analysis (TGA). Carbohydr. Polym. 2011, 84, 1165–1168. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, H.-Q.; Xu, G.-X.; Cheng, J.-S.; Zhang, B. Preparation, characterization, and encapsulation capability of the hydrogel cross-linked by esterified tapioca starch. Int. J. Biol. Macromol. 2020, 155, 1–5. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Effect of modified resistant starch of culinary banana on physicochemical, functional, morphological, diffraction, and thermal properties. Int. J. Food Prop. 2017, 20, 133–150. [Google Scholar] [CrossRef]
- Convention, U. S. P. U.S. Pharmacopeia and national formulary. Rockville: United States Pharmacopeial Convention. 2007, USP 30–NF–25. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218324/ (accessed on 6 June 2023).
- Sun, Q.; Dai, L.; Nan, C.; Xiong, L. Effect of heat moisture treatment on physicochemical and morphological properties of wheat starch and xylitol mixture. Food Chem. 2014, 143, 54–59. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-Based Hydrogels: Present Status and Applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Miles, M.J.; Morris, V.J.; Orford, P.D.; Ring, S.G. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr. Res. 1985, 135, 271–281. [Google Scholar] [CrossRef]
- Qadir, N.; Wani, I.A. In-vitro digestibility of rice starch and factors regulating its digestion process: A review. Carbohydr. Polym. 2022, 291, 119600. [Google Scholar] [CrossRef]
- Cai, L.; Shi, Y.-C.; Rong, L.; Hsiao, B.S. Debranching and crystallization of waxy maize starch in relation to enzyme digestibility. Carbohydr. Polym. 2010, 81, 385–393. [Google Scholar] [CrossRef]
- Hou, Z.W.; Chen, C.H.; Ke, J.P.; Zhang, Y.Y.; Qi, Y.; Liu, S.Y.; Yang, Z.; Ning, J.M.; Bao, G.H. α-Glucosidase Inhibitory Activities and the Interaction Mechanism of Novel Spiro-Flavoalkaloids from YingDe Green Tea. J. Agric. Food Chem. 2022, 70, 136–148. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics of Allmania nodiflora (L.) R.Br. ex Wight polyphenols during in vitro simulated digestion. Food Chem. 2022, 372, 131294. [Google Scholar] [CrossRef]
- Lorentz, C.; Pencreac’h, G.; Soultani-Vigneron, S.; Rondeau-Mouro, C.; de Carvalho, M.; Pontoire, B.; Ergan, F.; Le Bail, P. Coupling lipophilization and amylose complexation to encapsulate chlorogenic acid. Carbohydr. Polym. 2012, 90, 152–158. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Wang, S.; Luo, H.; Zhang, J.; Zhang, Y.; He, Z.; Wang, S. Alkali-Induced Changes in Functional Properties and in Vitro Digestibility of Wheat Starch: The Role of Surface Proteins and Lipids. J. Agric. Food Chem. 2014, 62, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, M.; Tang, A.; Jane, J.L.; Dhital, S.; Guo, B. RS Content and eGI Value of Cooked Noodles (I): Effect of Cooking Methods. Foods 2020, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Mol. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Inhibitory effects of chlorogenic acids from green coffee beans and cinnamate derivatives on the activity of porcine pancreas α-amylase isozyme I. Food Chem. 2011, 127, 1532–1539. [Google Scholar] [CrossRef]
- Ismail, B.B.; Guo, M.; Pu, Y.; Çavuş, O.; Ayub, K.A.; Watharkar, R.B.; Ding, T.; Chen, J.; Liu, D. Investigating the effect of in vitro gastrointestinal digestion on the stability, bioaccessibility, and biological activities of baobab (Adansonia digitata) fruit polyphenolics. LWT 2021, 145, 111348. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and a-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]

| CA Concentration (%) | ε′ | ε″ | tanδε |
|---|---|---|---|
| 0 | 76.7812 ± 0.1675 a | 9.5831 ± 0.0407 c | 0.1248 ± 0.0008 d |
| 1 | 75.3462 ± 0.1230 b | 9.7368 ± 0.0887 bc | 0.1292 ± 0.0012 c |
| 3 | 75.1475 ± 0.1191 bc | 9.7785 ± 0.0611 ab | 0.1301 ± 0.0008 bc |
| 5 | 74.9954 ± 0.0404 cd | 9.8868 ± 0.0403 ab | 0.1318 ± 0.0005 ab |
| 7 | 74.8238 ± 0.0299 d | 9.9050 ± 0.0526 ab | 0.1324 ± 0.0007 a |
| 9 | 74.7776 ± 0.2069 d | 9.9354 ± 0.1779 a | 0.1329 ± 0.0020 a |
| Samples | 30–200 °C Weight Loss (%) | 200–550 °C Weight Loss (%) | 550–800 °C Weight Loss (%) |
|---|---|---|---|
| 65-LS | −11.38 | −74.25 | −2.89 |
| 70-LS | −10.54 | −75.33 | −1.94 |
| 75-LS | −11.03 | −75.51 | −1.87 |
| 80-LS | −10.41 | −77.21 | −10.04 |
| 85-LS | −10.24 | −75.65 | −2.04 |
| 65-LS-CA | −10.43 | −74.15 | −2.18 |
| 70-LS-CA | −11.28 | −71.78 | −2.49 |
| 75-LS-CA | −10.79 | −75.11 | −11.55 |
| 80-LS-CA | −9.75 | −73.81 | −2.39 |
| 85-LS-CA | −9.98 | −73.68 | −2.61 |
| Samples | Swelling Power (SP) | Solubility (%) |
|---|---|---|
| LS-65 | 2.083 ± 0.037 h | 0.093 ± 0.018 f |
| LS-70 | 2.571 ± 0.015 g | 0.152 ± 0.017 def |
| LS-75 | 3.617 ± 0.076 f | 0.216 ± 0.029 d |
| LS-80 | 5.832 ± 0.269 c | 0.360 ± 0.061 bc |
| LS-85 | 7.146 ± 0.148 a | 0.459 ± 0.056 a |
| LS-CA-65 | 2.034 ± 0.129 h | 0.081 ± 0.011 f |
| LS-CA-70 | 2.751 ± 0.083 g | 0.137 ± 0.023 ef |
| LS-CA-75 | 4.219 ± 0.120 e | 0.192 ± 0.037 de |
| LS-CA-80 | 5.507 ± 0.274 d | 0.314 ± 0.040 c |
| LS-CA-85 | 6.403 ± 0.258 b | 0.411 ± 0.059 ab |
| CA (mg·mL−1) | Km (mg·mL−1) | Vmax (mg·mL−1·min−1) |
|---|---|---|
| 0.00 | 1.68 | 0.34 |
| 1.00 | 1.72 | 0.31 |
| 1.50 | 1.86 | 0.28 |
| 2.00 | 2.12 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wang, J.; Li, L.; Zheng, B.; Zheng, S.; Lu, X. Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex. Foods 2023, 12, 2506. https://doi.org/10.3390/foods12132506
Jiang X, Wang J, Li L, Zheng B, Zheng S, Lu X. Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex. Foods. 2023; 12(13):2506. https://doi.org/10.3390/foods12132506
Chicago/Turabian StyleJiang, Xiangfu, Jianyi Wang, Lanxin Li, Baodong Zheng, Shuyi Zheng, and Xu Lu. 2023. "Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex" Foods 12, no. 13: 2506. https://doi.org/10.3390/foods12132506
APA StyleJiang, X., Wang, J., Li, L., Zheng, B., Zheng, S., & Lu, X. (2023). Microwave-Induced Behavior and Digestive Properties of the Lotus Seed Starch—Chlorogenic Acid Complex. Foods, 12(13), 2506. https://doi.org/10.3390/foods12132506






