Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Line and Culture Conditions
2.3. Preparation of the Gelatin Hydrolysate
2.4. Assays of Protein Content, Reactable -NH2 Amount, and Amino Acid Composition
2.5. Assay of Cell Apoptosis
2.6. Isolation of RNA and Oligonucleotide Microarray Assay
2.7. Real-Time RT-PCR Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Amino Acid Composition of Papain-Treated GH
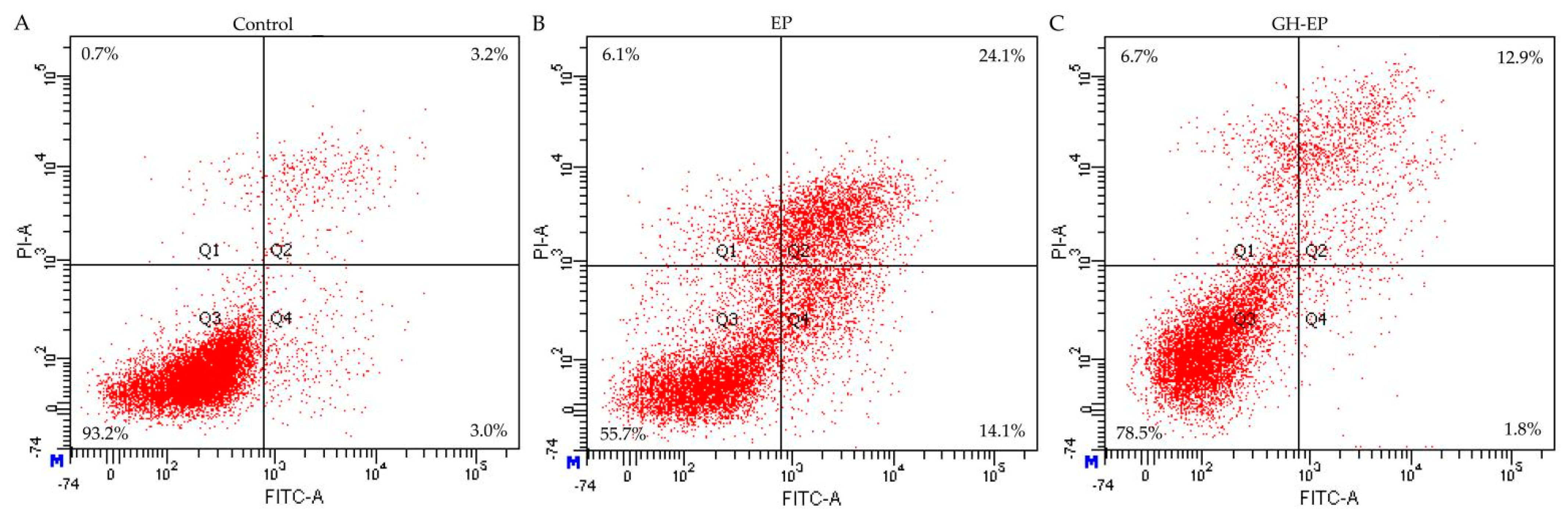
3.2. Apoptotic Prevention and Reversal of GH in the Osteoblasts
3.3. Downregulated Expression of Three Genes of JNK Family in the Osteoblasts
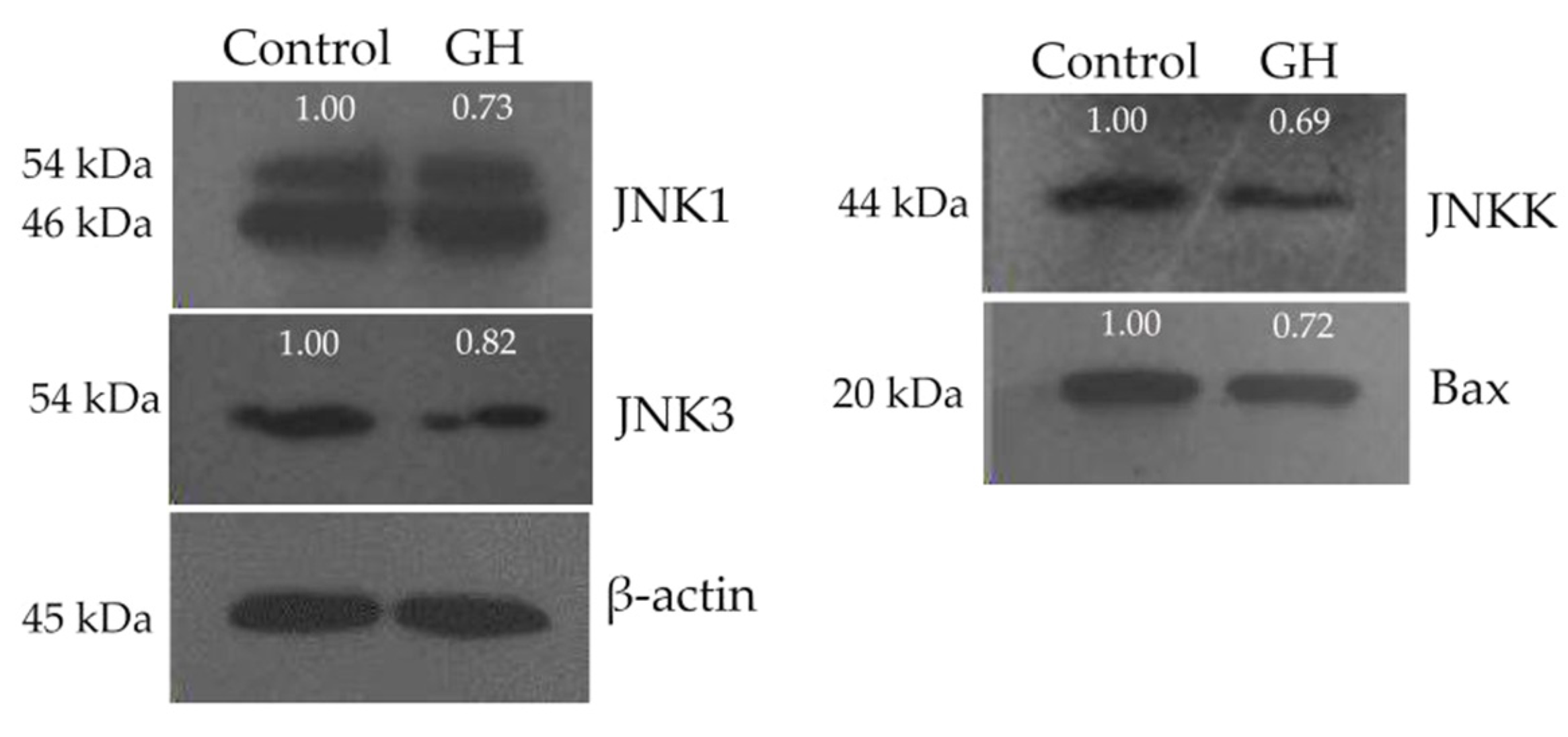
3.4. Down-Regulated Expression of Four Proteins in the Osteoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, J.E.; Kremer, R.; Goltzman, D. Chapter 60—Systemic factors in skeletal manifestations of malignancy. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 1079–1092. [Google Scholar]
- Ahuja, S.S.; Zhao, S.; Bellido, T.; Plotkin, L.I.; Jimenez, F.; Bonewald, L.F. CD40 ligand blocks apoptosis induced by tumor necrosis factor α, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology 2003, 144, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Ferreri, M.; Wang, Z.; Su, Y.; Han, B.; Su, J. Sodium fluoride affects proliferation and apoptosis through insulin-like growth factor I receptor in primary cultured mouse osteoblasts. Biol. Trace Elem. Res. 2011, 144, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Neuprez, A.; Reginster, J.Y. Bone-forming agents in the management of osteoporosis. Best Pract. Res. Clin. Haematol. 2008, 22, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Osteoporosis treatment: Focus on safety. Eur. J. Intern. Med. 2013, 24, 691–697. [Google Scholar] [CrossRef]
- Kondo, T.; Chiba, T.; Tousen, Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int. J. Food Sci. Nutr. 2022, 73, 800–808. [Google Scholar] [CrossRef]
- Zheng, H.; He, B.; Wu, T.X.; Cai, J.; Wei, J.S. Extraction, purification and anti-osteoporotic activity of a polysaccharide from Epimedium brevicornum Maxim. in vitro. Int. J. Biol. Macromol. 2020, 156, 1135–1145. [Google Scholar] [CrossRef]
- Yang, Q.H.; Yin, W.J.; Chen, Y.X.; Zhu, D.Y.; Yin, J.H.; Zhang, C.Q.; Gao, Y.S. Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed. Pharmacother. 2019, 120, 109486. [Google Scholar] [CrossRef]
- Evans, E.M.; Racette, S.B.; van Pelt, R.E.; Peterson, L.R.; Villareal, D.T. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 2007, 14, 481–488. [Google Scholar] [CrossRef]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef]
- Yu, Z.P.; Wang, Y.X.; Shuian, D.; Liu, J.B.; Zhao, W.Z. Identification and molecular mechanism of novel immunomodulatory peptides from gelatin hydrolysates: Molecular docking, dynamic simulation, and cell experiments. J. Agric. Food Chem. 2023, 71, 2924–2934. [Google Scholar] [CrossRef]
- Zhang, M.; He, J.L.; Pan, H.Y.; Sun, L.P.; Zhuang, Y.L. Phosphorylation modification of tilapia skin gelatin hydrolysate and identification and characterization of calcium-binding peptides. Process Biochem. 2023, 127, 1–9. [Google Scholar]
- Fawale, O.S.; Mudgil, P.; Abdelrahman, R.; Baba, W.N.; Nirmal, N.P.; Maqsood, S. Anti-hypercholesteraemic and antioxidative activities of camel skin gelatin hydrolysate: Effect of enzyme type, enzyme: Substrate ratio and time of hydrolysis. Int. J. Food Sci. Technol. 2023, 58, 2151–2160. [Google Scholar] [CrossRef]
- Park, J.E.; Ham, J.S.; Kim, H.K.; Lee, C.H.; Kim, D.W.; Seol, K.H.; Oh, M.H.; Kim, D.H.; Jang, A.R. Effect of pig skin gelatin hydrolysates on the bone mineral density of ovariectomized rats. Korean J. Food Sci. Anim. Resour. 2012, 32, 234–240. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Guillerminet, F.; Beaupied, H.; Fabien-Soule, V.; Tome, D.; Benhamou, C.L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Foods 2013, 5, 279–288. [Google Scholar] [CrossRef]
- Li, X.W.; Han, Y.F.; Guan, Y.; Zhang, L.; Bai, C.S.; Li, Y.F. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 502–508. [Google Scholar] [CrossRef]
- Huang, J.S.; Ye, Y.J.; Xiao, Y.S.; Ren, Q.; Zhou, Q.L.; Zhong, M.L.; Jiao, L.H.; Wu, L.H. Geniposide ameliorates glucocorticoid-induced osteoblast apoptosis by activating autophagy. Biomed. Pharmacother. 2022, 155, 113829. [Google Scholar] [CrossRef]
- Pan, X.W.; Zhao, X.H. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19). Int. J. Mol. Sci. 2015, 16, 13908–13920. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Nagy, A.; Marciniak-Darmochwa, K.; Krawczuk, S.; MierzeJewska, D.; Kostyra, H.; Gelencsér, É. Influence of glycation and pepsin hydrolysis on immunoreactivity of albumin/globulin fraction of herbicide resistant wheat line. Czech J. Food Sci. 2009, 27, 320–329. [Google Scholar] [CrossRef]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, Y.K.; Kuan, C.Y. Development of 4-hydroxyproline analysis kit and its application to collagen quantification. Food Chem. 2010, 119, 1271–1277. [Google Scholar] [CrossRef]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef]
- Terajima, M.; Perdivara, I.; Sricholpech, M.; Deguchi, Y.; Pleshko, N.; Tomer, K.B.; Yamauchi, M. Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 2014, 289, 22636–22647. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, B.; Song, S.J.; Ma, M.; Si, S.Y.; Wang, Y.H.; Xu, B.X.; Feng, K.; Wu, J.G.; Guo, Y.C. Bovine collagen peptides compounds promote the proliferation and differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE 2014, 9, e99920. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Oohashi, K.; Watanabe, M.; Kasugai, S. Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 2005, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Sun, L.P.; Zhuang, Y.L. Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid. Food Sci. Hum. Wellness 2022, 11, 1500–1507. [Google Scholar] [CrossRef]
- Wang, J.N.; Zhang, B.; Lu, W.P.; Liu, J.L.; Zhang, W.J.; Wang, Y.H.; Ma, M.; Cao, X.F.; Guo, Y.C. Cell proliferation stimulation ability and osteogenic activity of low molecular weight peptides derived from bovine gelatin hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Yuce, H.B.; Karatas, O.; Gevrek, F.; Toker, H. Evaluation of the effect of oleuropein on alveolar bone loss, inflammation, and apoptosis in experimental periodontitis. J. Periodontal Res. 2019, 54, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Geng, X.; Jiang, X.P.; Gao, X.; Liu, K.X.; Li, Y.F. Melatonin attenuates AlCl3-induced apoptosis and osteoblastic differentiation suppression by inhibiting oxidative stress in MC3T3-E1 cells. Biol. Trace Elem. Res. 2020, 196, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Lin, A.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Lin, A. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. Bioessays 2003, 25, 17–24. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef]
- Yang, X.C.; Xiang, X.; Xia, M.; Su, J.; Wu, Y.; Shen, L.Y.; Xu, Y.; Sun, L.K. Inhibition of JNK3 promotes apoptosis induced by BH3 mimetic S1 in chemoresistant human ovarian cancer cells. Anat. Rec. 2015, 298, 386–395. [Google Scholar] [CrossRef]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liang, X.C.; Yin, C.; Huai, Y.; Zhao, Y.P.; Huang, Q.; Chu, X.H.; Wang, W.S.; Qian, A.R. Bergamottin promotes osteoblast differentiation and bone formation via activating the Wnt/beta-catenin signaling pathway. Food Funct. 2022, 13, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Sharma, G.; Lee, Y.H.; Chakraborty, C.; Lee, S.S.; Seo, E.M. Sodium selenite promotes osteoblast differentiation via the WNT/ss-catenin signaling pathway. Cell J. 2022, 24, 309–315. [Google Scholar] [PubMed]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Cuo, C.; Wang, S.L.; Xu, S.T.; Wang, J.G.; Song, G.H. SP600125 reduces lipopolysaccharide-induced apoptosis and restores the early-stage differentiation of osteoblasts inhibited by LPS through the MAPK pathway in MC3T3-E1 cells. Int. J. Mol. Med. 2015, 35, 1427–1434. [Google Scholar]
- Shi, Y.; Guo, B.D.; Shi, Y.L.; Zhang, T.L.; Wang, K. Lanthanum chloride suppresses hydrogen peroxide-enhanced calcification in rat calcifying vascular cells. Biometals 2009, 22, 317–327. [Google Scholar] [CrossRef]
- Li, Q.M.; Tep, C.; Yune, T.Y.; Zhou, X.Z.; Uchida, T.; Lu, K.P.; Yoon, S.O. Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury. J. Neurosci. 2007, 27, 8395–8404. [Google Scholar] [CrossRef]
- Abdelli, S.; Puyal, J.; Bielmann, C.; Buchillier, V.; Abderrahmani, A.; Clarke, P.G.H.; Beckmann, J.S.; Bonny, C. JNK3 is abundant in insulin-secreting cells and protects against cytokine-induced apoptosis. Diabetologia 2009, 52, 1871–1880. [Google Scholar] [CrossRef]
- Yang, D.D.; Kuan, C.Y.; Whitmarsh, A.J.; Rincon, M.; Zheng, T.S.; Davis, R.J.; Rakic, P.; Flavell, R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 1997, 389, 865–870. [Google Scholar] [CrossRef]
- Ries, V.; Silva, R.M.; Oo, T.F.; Cheng, H.C.; Rzhetskaya, M.; Kholodilov, N.; Flavell, R.A.; Kuan, C.Y.; Rakic, P.; Burke, R.E. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J. Neurochem. 2008, 107, 1578–1588. [Google Scholar] [CrossRef]
- Jang, W.Y.; Lee, J.Y.; Lee, S.T.; Jun, D.Y.; Kim, Y.H. Inhibition of JNK2 and JNK3 by JNK inhibitor IX induces prometaphase arrest-dependent apoptotic cell death in human Jurkat T cells. Biochem. Biophys. Res. Commun. 2014, 452, 845–851. [Google Scholar] [CrossRef]
- Ham, Y.M.; Lim, J.H.; Lee, S.K. Distinct roles for JNK1 and JNK3 during TNF-α- or etoposide-induced apoptosis in HeLa cells. Mol. Cell 2009, 28, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Yousefi, R.; Eun, J.B. Bio/multi-functional peptides derived from fish gelatin hydrolysates: Technological and functional properties. Biocatal. Agric. Biotechnol. 2021, 36, 102152. [Google Scholar] [CrossRef]

| Gene | Sequence (5′-3′) | |
|---|---|---|
| JNKK | Forward | 5′-AAGCGCATCACGACAAGGAT-3′ |
| Reverse | 5′-TGCGGAGGATCTCCTTTCAC-3′ | |
| JNK1 | Forward | 5′-CAGTCAGGCAAGGGATTTGTTAT-3′ |
| Reverse | 5′-TGCTTGTCAGGGATCTTTGGT-3′ | |
| JNK2 | Forward | 5′-AACTCATGCAAAGAGAGCTTATCGT-3′ |
| Reverse | 5′-TTAGCATCCATTAATTCCATAACCAA-3′ | |
| JNK3 | Forward | 5′-GGGACTCACCTTCCCCAAAC-3′ |
| Reverse | 5′-TGTAAGGCGTCGTCCACTGA-3′ | |
| Beta-actin | Forward | 5′-TTGCCGACAGGATGCAGAA-3′ |
| Reverse | 5′-CTCCTGCTTGCTGATCCACAT-3′ |
| Amino Acid | Molar Percentage (%) | Amino Acid | Molar Percentage (%) |
|---|---|---|---|
| Ala | 11.2 | Leu | 2.8 |
| Arg | 5.2 | Lys | 2.6 |
| Asp | 4.1 | Met | 0.5 |
| Cys | Not detectable | Phe | 1.4 |
| Glu | 7.7 | Pro | 12.7 |
| Gly | 35.4 | Ser | 3.3 |
| His | 0.4 | Thr | 1.6 |
| 4-Hyp | 7.2 | Tyr | Not detectable |
| Ile | 1.2 | Val | 2.7 |
| Action Mode | EP-Treated Cells | GH/EP-Treated Cells |
|---|---|---|
| Q2 + Q4 (%) | Q2 + Q4 (%) | |
| Apoptotic prevention | 31.6 ± 8.0 | 13.3 ± 0.7 |
| Apoptotic reversal | 13.6 ± 1.8 | 11.8 ± 0.3 |
| Gene | Description | Fold Change | |
|---|---|---|---|
| Microarray Assay | RT-PCR Assay | ||
| JNKK | c-Jun-N-terminal kinase kinase 1 | ↓ 1.7 | ↓ 2.1 |
| JNK1 | Mitogen-activated protein kinase 8 | ↓ 3.3 | ↓ 2.7 |
| JNK2 | Mitogen-activated protein kinase 9 | Not expressed | Not expressed |
| JNK3 | Mitogen-activated protein kinase 10 | ↓ 1.6 | ↓ 1.5 |
| Protein | Fold Change | Protein | Fold Change |
|---|---|---|---|
| JNK1 | ↓ 1.33 ± 0.09 | JNKK | ↓ 1.37 ± 0.15 |
| JNK2 | Not expressed | Bax | ↓ 1.41 ± 0.16 |
| JNK3 | ↓ 1.25 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-F.; Pan, X.-W.; Li, H.-Q.; Zhang, X.-N.; Zhao, X.-H. Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods 2023, 12, 2419. https://doi.org/10.3390/foods12122419
Liu H-F, Pan X-W, Li H-Q, Zhang X-N, Zhao X-H. Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods. 2023; 12(12):2419. https://doi.org/10.3390/foods12122419
Chicago/Turabian StyleLiu, Hong-Fang, Xiao-Wen Pan, Hua-Qiang Li, Xiao-Nan Zhang, and Xin-Huai Zhao. 2023. "Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts" Foods 12, no. 12: 2419. https://doi.org/10.3390/foods12122419
APA StyleLiu, H.-F., Pan, X.-W., Li, H.-Q., Zhang, X.-N., & Zhao, X.-H. (2023). Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods, 12(12), 2419. https://doi.org/10.3390/foods12122419






