Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Line and Culture Conditions
2.3. Preparation of the Gelatin Hydrolysate
2.4. Assays of Protein Content, Reactable -NH2 Amount, and Amino Acid Composition
2.5. Assay of Cell Apoptosis
2.6. Isolation of RNA and Oligonucleotide Microarray Assay
2.7. Real-Time RT-PCR Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Amino Acid Composition of Papain-Treated GH
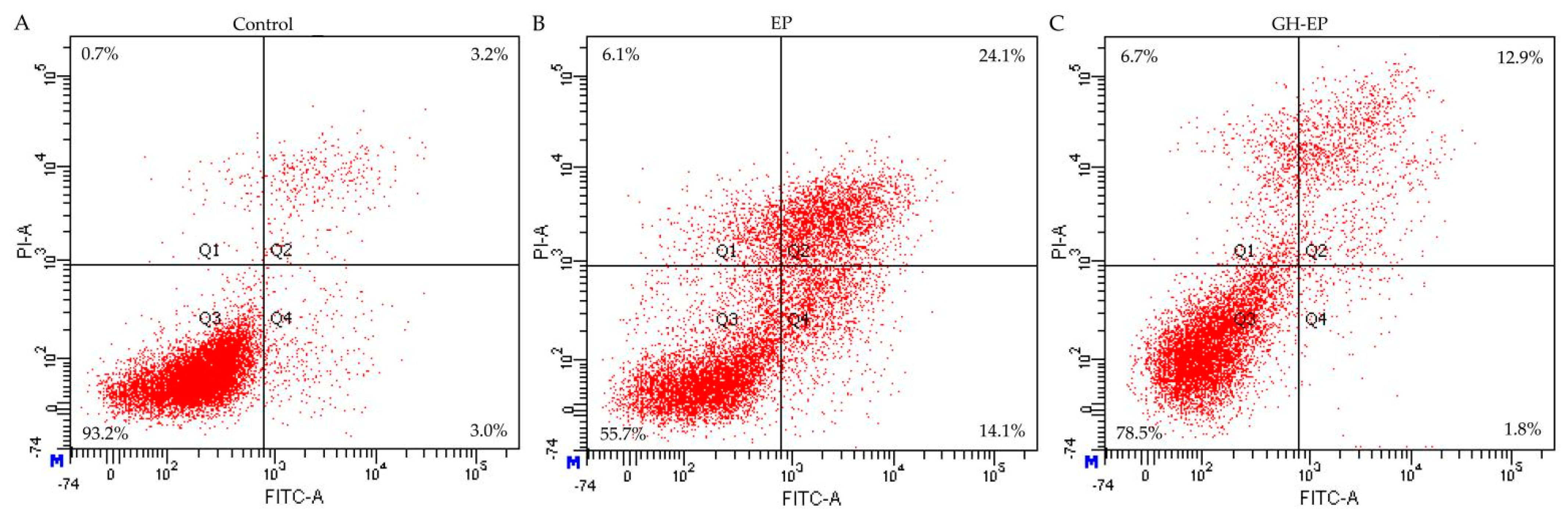
3.2. Apoptotic Prevention and Reversal of GH in the Osteoblasts
3.3. Downregulated Expression of Three Genes of JNK Family in the Osteoblasts
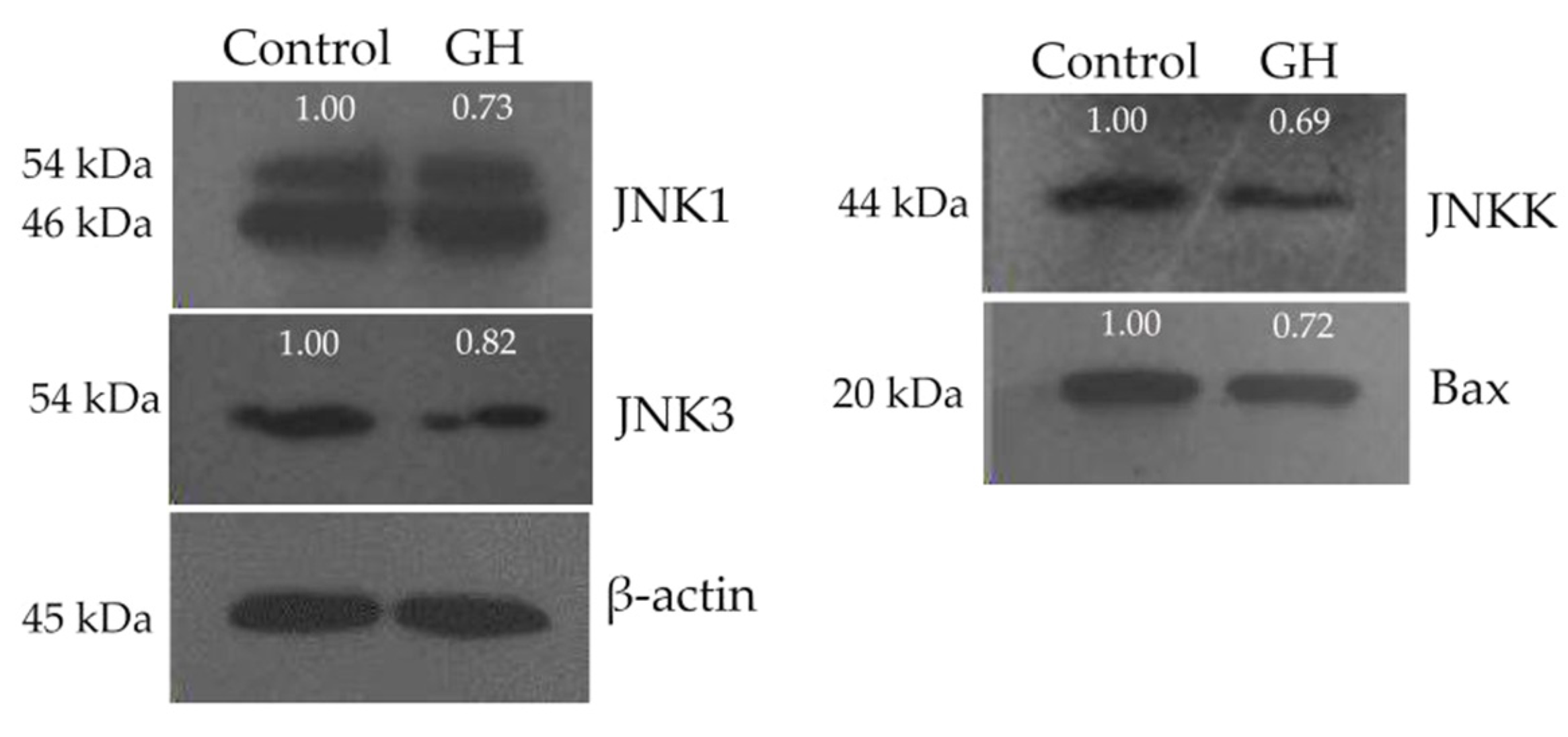
3.4. Down-Regulated Expression of Four Proteins in the Osteoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, J.E.; Kremer, R.; Goltzman, D. Chapter 60—Systemic factors in skeletal manifestations of malignancy. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 1079–1092. [Google Scholar]
- Ahuja, S.S.; Zhao, S.; Bellido, T.; Plotkin, L.I.; Jimenez, F.; Bonewald, L.F. CD40 ligand blocks apoptosis induced by tumor necrosis factor α, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology 2003, 144, 1761–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Ferreri, M.; Wang, Z.; Su, Y.; Han, B.; Su, J. Sodium fluoride affects proliferation and apoptosis through insulin-like growth factor I receptor in primary cultured mouse osteoblasts. Biol. Trace Elem. Res. 2011, 144, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Neuprez, A.; Reginster, J.Y. Bone-forming agents in the management of osteoporosis. Best Pract. Res. Clin. Haematol. 2008, 22, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Osteoporosis treatment: Focus on safety. Eur. J. Intern. Med. 2013, 24, 691–697. [Google Scholar] [CrossRef]
- Kondo, T.; Chiba, T.; Tousen, Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int. J. Food Sci. Nutr. 2022, 73, 800–808. [Google Scholar] [CrossRef]
- Zheng, H.; He, B.; Wu, T.X.; Cai, J.; Wei, J.S. Extraction, purification and anti-osteoporotic activity of a polysaccharide from Epimedium brevicornum Maxim. in vitro. Int. J. Biol. Macromol. 2020, 156, 1135–1145. [Google Scholar] [CrossRef]
- Yang, Q.H.; Yin, W.J.; Chen, Y.X.; Zhu, D.Y.; Yin, J.H.; Zhang, C.Q.; Gao, Y.S. Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed. Pharmacother. 2019, 120, 109486. [Google Scholar] [CrossRef]
- Evans, E.M.; Racette, S.B.; van Pelt, R.E.; Peterson, L.R.; Villareal, D.T. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 2007, 14, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef]
- Yu, Z.P.; Wang, Y.X.; Shuian, D.; Liu, J.B.; Zhao, W.Z. Identification and molecular mechanism of novel immunomodulatory peptides from gelatin hydrolysates: Molecular docking, dynamic simulation, and cell experiments. J. Agric. Food Chem. 2023, 71, 2924–2934. [Google Scholar] [CrossRef]
- Zhang, M.; He, J.L.; Pan, H.Y.; Sun, L.P.; Zhuang, Y.L. Phosphorylation modification of tilapia skin gelatin hydrolysate and identification and characterization of calcium-binding peptides. Process Biochem. 2023, 127, 1–9. [Google Scholar]
- Fawale, O.S.; Mudgil, P.; Abdelrahman, R.; Baba, W.N.; Nirmal, N.P.; Maqsood, S. Anti-hypercholesteraemic and antioxidative activities of camel skin gelatin hydrolysate: Effect of enzyme type, enzyme: Substrate ratio and time of hydrolysis. Int. J. Food Sci. Technol. 2023, 58, 2151–2160. [Google Scholar] [CrossRef]
- Park, J.E.; Ham, J.S.; Kim, H.K.; Lee, C.H.; Kim, D.W.; Seol, K.H.; Oh, M.H.; Kim, D.H.; Jang, A.R. Effect of pig skin gelatin hydrolysates on the bone mineral density of ovariectomized rats. Korean J. Food Sci. Anim. Resour. 2012, 32, 234–240. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Guillerminet, F.; Beaupied, H.; Fabien-Soule, V.; Tome, D.; Benhamou, C.L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Foods 2013, 5, 279–288. [Google Scholar] [CrossRef]
- Li, X.W.; Han, Y.F.; Guan, Y.; Zhang, L.; Bai, C.S.; Li, Y.F. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 502–508. [Google Scholar] [CrossRef]
- Huang, J.S.; Ye, Y.J.; Xiao, Y.S.; Ren, Q.; Zhou, Q.L.; Zhong, M.L.; Jiao, L.H.; Wu, L.H. Geniposide ameliorates glucocorticoid-induced osteoblast apoptosis by activating autophagy. Biomed. Pharmacother. 2022, 155, 113829. [Google Scholar] [CrossRef]
- Pan, X.W.; Zhao, X.H. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19). Int. J. Mol. Sci. 2015, 16, 13908–13920. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Nagy, A.; Marciniak-Darmochwa, K.; Krawczuk, S.; MierzeJewska, D.; Kostyra, H.; Gelencsér, É. Influence of glycation and pepsin hydrolysis on immunoreactivity of albumin/globulin fraction of herbicide resistant wheat line. Czech J. Food Sci. 2009, 27, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, Y.K.; Kuan, C.Y. Development of 4-hydroxyproline analysis kit and its application to collagen quantification. Food Chem. 2010, 119, 1271–1277. [Google Scholar] [CrossRef]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef] [Green Version]
- Terajima, M.; Perdivara, I.; Sricholpech, M.; Deguchi, Y.; Pleshko, N.; Tomer, K.B.; Yamauchi, M. Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 2014, 289, 22636–22647. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Zhang, B.; Song, S.J.; Ma, M.; Si, S.Y.; Wang, Y.H.; Xu, B.X.; Feng, K.; Wu, J.G.; Guo, Y.C. Bovine collagen peptides compounds promote the proliferation and differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE 2014, 9, e99920. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Oohashi, K.; Watanabe, M.; Kasugai, S. Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 2005, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Sun, L.P.; Zhuang, Y.L. Protective effects of tilapia (Oreochromis niloticus) skin gelatin hydrolysates on osteoporosis rats induced by retinoic acid. Food Sci. Hum. Wellness 2022, 11, 1500–1507. [Google Scholar] [CrossRef]
- Wang, J.N.; Zhang, B.; Lu, W.P.; Liu, J.L.; Zhang, W.J.; Wang, Y.H.; Ma, M.; Cao, X.F.; Guo, Y.C. Cell proliferation stimulation ability and osteogenic activity of low molecular weight peptides derived from bovine gelatin hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Yuce, H.B.; Karatas, O.; Gevrek, F.; Toker, H. Evaluation of the effect of oleuropein on alveolar bone loss, inflammation, and apoptosis in experimental periodontitis. J. Periodontal Res. 2019, 54, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Geng, X.; Jiang, X.P.; Gao, X.; Liu, K.X.; Li, Y.F. Melatonin attenuates AlCl3-induced apoptosis and osteoblastic differentiation suppression by inhibiting oxidative stress in MC3T3-E1 cells. Biol. Trace Elem. Res. 2020, 196, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Lin, A.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Lin, A. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. Bioessays 2003, 25, 17–24. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef]
- Yang, X.C.; Xiang, X.; Xia, M.; Su, J.; Wu, Y.; Shen, L.Y.; Xu, Y.; Sun, L.K. Inhibition of JNK3 promotes apoptosis induced by BH3 mimetic S1 in chemoresistant human ovarian cancer cells. Anat. Rec. 2015, 298, 386–395. [Google Scholar] [CrossRef]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liang, X.C.; Yin, C.; Huai, Y.; Zhao, Y.P.; Huang, Q.; Chu, X.H.; Wang, W.S.; Qian, A.R. Bergamottin promotes osteoblast differentiation and bone formation via activating the Wnt/beta-catenin signaling pathway. Food Funct. 2022, 13, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Sharma, G.; Lee, Y.H.; Chakraborty, C.; Lee, S.S.; Seo, E.M. Sodium selenite promotes osteoblast differentiation via the WNT/ss-catenin signaling pathway. Cell J. 2022, 24, 309–315. [Google Scholar] [PubMed]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Cuo, C.; Wang, S.L.; Xu, S.T.; Wang, J.G.; Song, G.H. SP600125 reduces lipopolysaccharide-induced apoptosis and restores the early-stage differentiation of osteoblasts inhibited by LPS through the MAPK pathway in MC3T3-E1 cells. Int. J. Mol. Med. 2015, 35, 1427–1434. [Google Scholar]
- Shi, Y.; Guo, B.D.; Shi, Y.L.; Zhang, T.L.; Wang, K. Lanthanum chloride suppresses hydrogen peroxide-enhanced calcification in rat calcifying vascular cells. Biometals 2009, 22, 317–327. [Google Scholar] [CrossRef]
- Li, Q.M.; Tep, C.; Yune, T.Y.; Zhou, X.Z.; Uchida, T.; Lu, K.P.; Yoon, S.O. Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury. J. Neurosci. 2007, 27, 8395–8404. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, S.; Puyal, J.; Bielmann, C.; Buchillier, V.; Abderrahmani, A.; Clarke, P.G.H.; Beckmann, J.S.; Bonny, C. JNK3 is abundant in insulin-secreting cells and protects against cytokine-induced apoptosis. Diabetologia 2009, 52, 1871–1880. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.D.; Kuan, C.Y.; Whitmarsh, A.J.; Rincon, M.; Zheng, T.S.; Davis, R.J.; Rakic, P.; Flavell, R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 1997, 389, 865–870. [Google Scholar] [CrossRef]
- Ries, V.; Silva, R.M.; Oo, T.F.; Cheng, H.C.; Rzhetskaya, M.; Kholodilov, N.; Flavell, R.A.; Kuan, C.Y.; Rakic, P.; Burke, R.E. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J. Neurochem. 2008, 107, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.Y.; Lee, J.Y.; Lee, S.T.; Jun, D.Y.; Kim, Y.H. Inhibition of JNK2 and JNK3 by JNK inhibitor IX induces prometaphase arrest-dependent apoptotic cell death in human Jurkat T cells. Biochem. Biophys. Res. Commun. 2014, 452, 845–851. [Google Scholar] [CrossRef]
- Ham, Y.M.; Lim, J.H.; Lee, S.K. Distinct roles for JNK1 and JNK3 during TNF-α- or etoposide-induced apoptosis in HeLa cells. Mol. Cell 2009, 28, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Yousefi, R.; Eun, J.B. Bio/multi-functional peptides derived from fish gelatin hydrolysates: Technological and functional properties. Biocatal. Agric. Biotechnol. 2021, 36, 102152. [Google Scholar] [CrossRef]

| Gene | Sequence (5′-3′) | |
|---|---|---|
| JNKK | Forward | 5′-AAGCGCATCACGACAAGGAT-3′ |
| Reverse | 5′-TGCGGAGGATCTCCTTTCAC-3′ | |
| JNK1 | Forward | 5′-CAGTCAGGCAAGGGATTTGTTAT-3′ |
| Reverse | 5′-TGCTTGTCAGGGATCTTTGGT-3′ | |
| JNK2 | Forward | 5′-AACTCATGCAAAGAGAGCTTATCGT-3′ |
| Reverse | 5′-TTAGCATCCATTAATTCCATAACCAA-3′ | |
| JNK3 | Forward | 5′-GGGACTCACCTTCCCCAAAC-3′ |
| Reverse | 5′-TGTAAGGCGTCGTCCACTGA-3′ | |
| Beta-actin | Forward | 5′-TTGCCGACAGGATGCAGAA-3′ |
| Reverse | 5′-CTCCTGCTTGCTGATCCACAT-3′ |
| Amino Acid | Molar Percentage (%) | Amino Acid | Molar Percentage (%) |
|---|---|---|---|
| Ala | 11.2 | Leu | 2.8 |
| Arg | 5.2 | Lys | 2.6 |
| Asp | 4.1 | Met | 0.5 |
| Cys | Not detectable | Phe | 1.4 |
| Glu | 7.7 | Pro | 12.7 |
| Gly | 35.4 | Ser | 3.3 |
| His | 0.4 | Thr | 1.6 |
| 4-Hyp | 7.2 | Tyr | Not detectable |
| Ile | 1.2 | Val | 2.7 |
| Action Mode | EP-Treated Cells | GH/EP-Treated Cells |
|---|---|---|
| Q2 + Q4 (%) | Q2 + Q4 (%) | |
| Apoptotic prevention | 31.6 ± 8.0 | 13.3 ± 0.7 |
| Apoptotic reversal | 13.6 ± 1.8 | 11.8 ± 0.3 |
| Gene | Description | Fold Change | |
|---|---|---|---|
| Microarray Assay | RT-PCR Assay | ||
| JNKK | c-Jun-N-terminal kinase kinase 1 | ↓ 1.7 | ↓ 2.1 |
| JNK1 | Mitogen-activated protein kinase 8 | ↓ 3.3 | ↓ 2.7 |
| JNK2 | Mitogen-activated protein kinase 9 | Not expressed | Not expressed |
| JNK3 | Mitogen-activated protein kinase 10 | ↓ 1.6 | ↓ 1.5 |
| Protein | Fold Change | Protein | Fold Change |
|---|---|---|---|
| JNK1 | ↓ 1.33 ± 0.09 | JNKK | ↓ 1.37 ± 0.15 |
| JNK2 | Not expressed | Bax | ↓ 1.41 ± 0.16 |
| JNK3 | ↓ 1.25 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-F.; Pan, X.-W.; Li, H.-Q.; Zhang, X.-N.; Zhao, X.-H. Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods 2023, 12, 2419. https://doi.org/10.3390/foods12122419
Liu H-F, Pan X-W, Li H-Q, Zhang X-N, Zhao X-H. Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods. 2023; 12(12):2419. https://doi.org/10.3390/foods12122419
Chicago/Turabian StyleLiu, Hong-Fang, Xiao-Wen Pan, Hua-Qiang Li, Xiao-Nan Zhang, and Xin-Huai Zhao. 2023. "Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts" Foods 12, no. 12: 2419. https://doi.org/10.3390/foods12122419
APA StyleLiu, H.-F., Pan, X.-W., Li, H.-Q., Zhang, X.-N., & Zhao, X.-H. (2023). Amino Acid Composition of a Chum Salmon (Oncorhynchus keta) Skin Gelatin Hydrolysate and Its Antiapoptotic Effects on Etoposide-Induced Osteoblasts. Foods, 12(12), 2419. https://doi.org/10.3390/foods12122419






