The GRAS Salts of Na2SiO3 and EDTA-Na2 Control Citrus Postharvest Pathogens by Disrupting the Cell Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain, Citrus Fruit and GRAS Salts
2.2. High-Throughput Screening of GRAS Salts for Citrus Postharvest Diseases
2.3. Evaluation of Antifungal Activity In Vitro
2.4. Effect of Na2SiO3 and EDTA-Na2 on Spore and Hyphal Morphology
2.5. Effect of Na2SiO3 and EDTA-Na2 on Spore Germination
2.6. Cell Wall Integrity, Cell Membrane Integrity, and Lipid Droplet Accumulation Assays
2.7. Release of Cell Components
2.8. Fruit Decay Test
2.9. Fruit Quality Evaluation In Vivo
2.10. Statistical Analysis
3. Results
3.1. Inhibition Rates of 17 GRAS Salts (1%)
3.2. Digital Photography of Na2SiO3 and EDTA-Na2 against Four Postharvest Pathogens
3.3. Effect of Na2SiO3 and EDTA-Na2 on the Spore and Hyphal Morphology
3.4. Effect of Na2SiO3 and EDTA-Na2 on Spore Germination
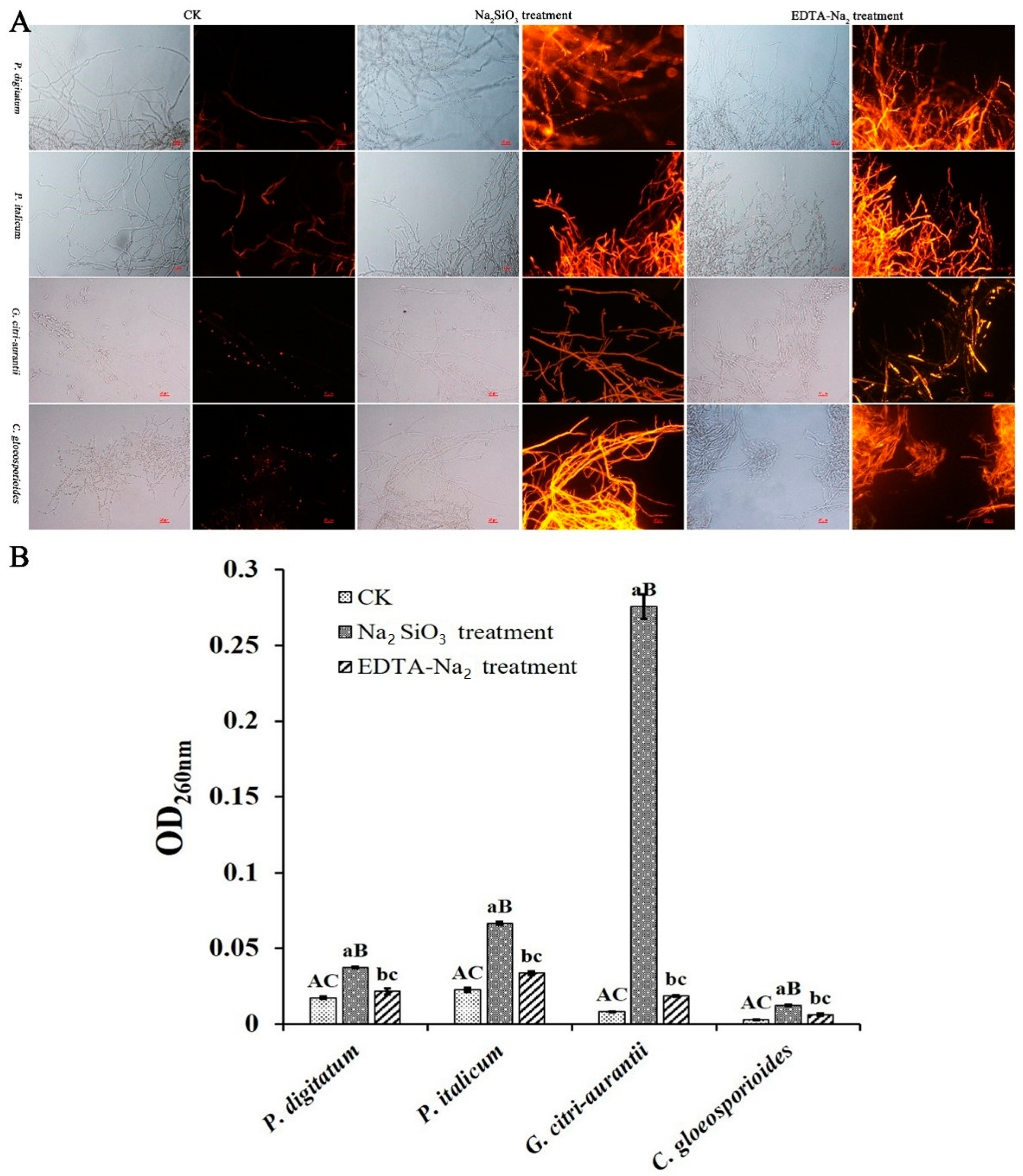
3.5. Effect of Na2SiO3 and EDTA-Na2 on Cell Wall Integrity and Lipid Droplet Accumulation
3.6. Effect of Na2SiO3 and EDTA-Na2 on Cell Membrane Integrity
3.7. Effect of Na2SiO3 and EDTA-Na2 on the Nucleic Acid Leakage
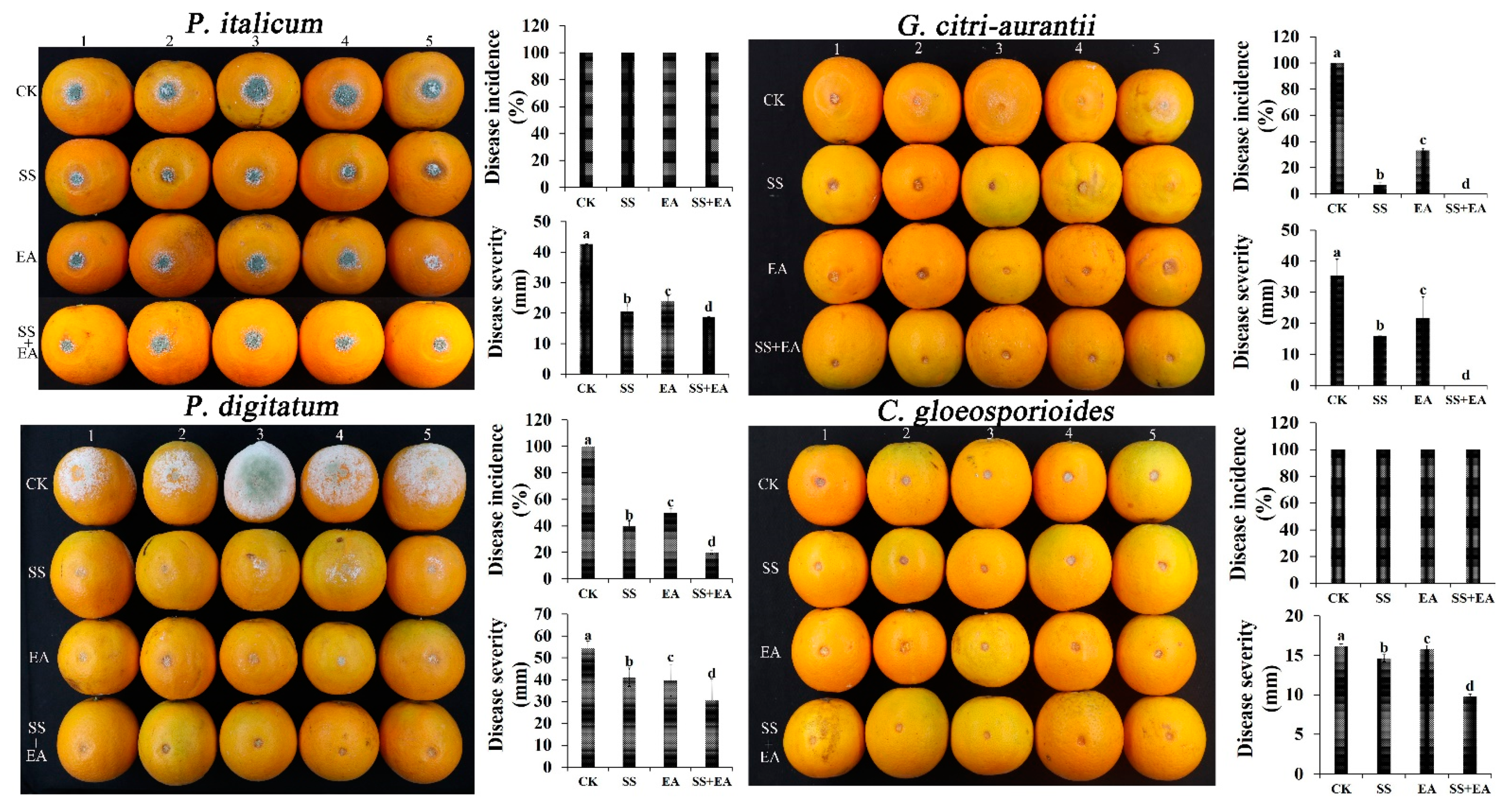
3.8. Pathogen Inhibition Ability of Na2SiO3 and EDTA-Na2 In Vivo
3.9. Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Nasrullah, K.F.; Nie, Z.; Wang, P.; Xu, J. Citrus genetic engineering for disease resistance: Past, present and future. Int. J. Mol. Sci. 2019, 20, 5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.P.; Ruan, C.Q.; Yi, L.H.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 2020, 87, 103375. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.H.; Li, H.; Li, H.; Liu, C.; Luo, H.J.; Liu, C.X.; Chen, J.F.; Zou, K.; Liu, S.H. Metabolite profiling reveals comprehensive effects of Chaetomium globosum on citrus preservation. Food Chem. 2022, 369, 130959. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Deng, L.; Ruan, C.; Yi, L.; Zeng, K. Pichia galeiformis induces resistance in postharvest citrus by activating the phenylpropanoid biosynthesis pathway. J. Agric. Food Chem. 2021, 69, 2619–2631. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with gras salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Silva, D.; Moslemi, A.; Edwards, J.; Taylor, P. Colletotrichum species causing anthracnose of citrus in australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef]
- Martínez-Blay, V.; Pérez-Gago, M.B.; Fuente, B.; Carbó, R.; Palou, L. Edible coatings formulated with antifungal gras salts to control citrus anthracnose caused by colletotrichum gloeosporioides and preserve postharvest fruit quality. Coatings 2020, 10, 730. [Google Scholar] [CrossRef]
- Palou, L. Penicillium digitatum, Penicillium italicum (green mold, blue mold). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 25–102. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of sour rot in citrus fruit by three insect antimicrobial peptides. Postharvest Biol. Technol. 2019, 149, 200–208. [Google Scholar] [CrossRef]
- McKay, A.; Förster, H.; Adaskaveg, J. Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Soto-Muoz, L.; Taberner, V.; Fuente, B.; Jerbi, N.; Palou, L. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int. J. Food Microbiol. 2020, 335, 108860. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Diaz, M.A.; Soliz-Santander, F.F.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Screening methods for isolation of biocontrol epiphytic yeasts against Penicillium digitatum in lemons. J. Fungi 2021, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Hong, Y.; Ma, J.; Deng, L.; Zeng, K. Screening lactic acid bacteria from pickle and cured meat as biocontrol agents of Penicillium digitatum on citrus fruit. Biol. Control 2021, 158, 104606. [Google Scholar] [CrossRef]
- Klein, M.N.; Kupper, K.C. Biofilm production by aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018, 69, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, O.; Yi, L.; Deng, L.; Ruan, C.; Zeng, K. Screening antagonistic yeasts against citrus green mold and the possible biocontrol mechanisms of Pichia galeiformis (BAF03). J. Sci. Food Agric. 2020, 100, 3812–3821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, C.; Cheng, X.; Li, G.; Zhu, X. β-cyclodextrin inclusion complex containing litsea cubeba essential oil: Preparation, optimization, physicochemical, and antifungal characterization. Coatings 2020, 10, 850. [Google Scholar] [CrossRef]
- Gan, Z.; Huang, J.; Chen, J.; Nisar, M.F.; Qi, W. Synthesis and antifungal activities of cinnamaldehyde derivatives against penicillium digitatum causing citrus green mold. J. Food Qual. 2020, 2020, 8898692. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Serrano, M.; Valero, D.; Rodríguez-López, M.I.; Gabaldón, J.A.; Castillo, S.; Guillén, F.; Zapata, P.J.; Martínez-Romero, D. Effect of thymol and carvacrol encapsulated in hp-Β-cyclodextrin by two inclusion methods against Geotrichum citri-aurantii. J. Food Sci. 2019, 84, 1513–1521. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; He, J.; Wang, Z.; Li, Z. Anthocyanins contribute to fruit defense against postharvest green mold. Postharvest Biol. Technol. 2021, 181, 111661. [Google Scholar] [CrossRef]
- Karim, H.; Boubaker, H.; Askarne, L.; Talibi, I.; Msanda, F.; Boudyach, E.H.; Saadi, B.; Ait, B.A.A. Antifungal properties of organic extracts of eight cistus l. species against postharvest citrus sour rot. Lett. Appl. Microbiol. 2016, 62, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue molds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. Gras, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A. Reducing or Replacing Conventional Postharvest Fungicides with Low Toxicity Acids and Salts. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 595–632. [Google Scholar] [CrossRef]
- Khamis, Y.; Hussien, A. Electrolysed water and salt solutions can reduce green and blue molds while maintain the quality properties of ‘valencia’ late oranges. Postharvest Biol. Technol. 2019, 159, 111025. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.A.; Palou, L. Preventive and curative activity of postharvest potassium silicate treatments to control green and blue molds on orange fruit. Eur. J. Plant Pathol. 2014, 138, 721–732. [Google Scholar] [CrossRef]
- Montesinos-Herrero, C.; Moscoso-Ramírez, P.A.; Palou, L. Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 2016, 115, 72–80. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.A.; Montesinos-Herrero, C.; Palou, L. Characterization of postharvest treatments with sodium methylparaben to control citrus green and blue molds. Postharvest Biol. Technol. 2013, 77, 128–137. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Q.Y.; Wang, Y.Q.; Lu, X.J.; Pan, H.; Long, C.A. Curative activity of KCl treatments to control citrus sour rot. LWT-Food Sci. Technol. 2022, 168, 113853. [Google Scholar] [CrossRef]
- Zhang, H.; Huangfu, H.P.; Wang, X.; Zhao, S.S.; Tan, Z. Antibacterial activity of lactic acid producing leuconostoc mesenteroides qz1178 against pathogenic Gallibacterium anatis. Front. Vet. Sci. 2021, 8, 630294. [Google Scholar] [CrossRef]
- Liu, N.; Yun, Y.Z.; Yin, Y.N.; Matthias, H.; Ma, Z.H. Lipid droplet biogenesis regulated by the fgnem1/spo7-fgpah1 phosphatase cascade plays critical roles in fungal development and virulence in Fusarium graminearum. New Phytol. 2019, 223, 412–429. [Google Scholar] [CrossRef]
- Jurick, W.M.; Macarisin, O.; Gaskins, V.L.; Janisiewicz, W.J.; Peter, K.A.; Cox, K.D. Baseline sensitivity of Penicillium spp. to difenoconazole. Plant Dis. 2019, 103, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yang, Y.; Zhu, X.; Yuan, P.; Shan, Y. Inhibitory mechanisms of cinnamic acid on the growth of Geotrichum citri-aurantii. Food Control 2021, 131, 108459. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Zhang, D.; Yang, F.; He, X.; Long, C.A. Aluminum sulfate inhibits green mold by inducing chitinase activity of Penicillium digitatum and enzyme activity of citrus fruit. Food Control 2022, 136, 108854. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Liu, S.; Ruan, C.; Zeng, K. Effects of the peptide h-ooww-nh2 and its derived lipopeptide c12-ooww-nh2 on controlling of citrus postharvest green mold. Postharvest Biol. Technol. 2019, 158, 110979. [Google Scholar] [CrossRef]
- Li, L.; Tang, X.; Ouyang, Q.; Tao, N.G. Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit. Postharvest Biol. Technol. 2019, 151, 19–25. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Jing, G.; OuYang, Q.; Tao, N.G. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G.Z. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- Wang, Y.F.; Bi, Y.; Ren, Y.L.; Wang, Y.; Fan, C.F.; Li, D.Q.; Yang, Z.M. Control of postharvest diseases and potentiation of reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruits treated by sodium silicate. Sci. Agric. Sin. 2012, 45, 2242–2248. Available online: https://211.155.251.135:81/jwk_zgnykx/en/y2012/v45/i11/2242 (accessed on 1 May 2023).
- Ouyang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Fan, J. Links between autophagy and lipid droplet dynamics. J. Exp. Bot. 2022, 73, 2848–2858. [Google Scholar] [CrossRef]
- Robenek, H.; Hofnagel, O.; Buers, I.; Robenek, M.J.; Severs, N.J. Adipophilin-enriched domains in the er membrane are sites of lipid droplet biogenesis. J. Cell Sci. 2006, 119, 4215–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Ouyang, Q.; Jing, G.; Tao, N. Effect of sodium dehydroacetate on the development of sour rot on satsuma mandarin. Food Control 2016, 65, 8–13. [Google Scholar] [CrossRef]





| Number | GRAS Salts (1%) | P. digitatum (B3, %) | P. italicum (P44, %) | G.citri-aurantii (AY-1, %) | C. gloeosporioides (NF28, %) |
|---|---|---|---|---|---|
| 1 | Sodium silicate | 100 | 100 | 100 | 100 |
| 2 | Ethylenediaminetetraacetic acid disodium salt | 87.90 ± 0.19 | 100 | 100 | 100 |
| 3 | Sodium benzoate | 100 | 86.04 ± 0.45 | 100 | 100 |
| 4 | Sodium diacetate | 100 | 88.09 ± 1.01 | 0 | 100 |
| 5 | Succinic acid | 0 | 79.08 ± 0.52 | 89.47 ± 0.47 | 85.09 ± 0.19 |
| 6 | Maleic acid | 0 | 79.66 ± 0.90 | 90.36 ± 0.42 | 84.61 ± 1.03 |
| 7 | Sodium carbonate | 100 | 100 | 0 | 0 |
| 8 | Sodium sesquicarbonate | 0 | 0 | 100 | 89.58 ± 0.69 |
| 9 | Sodium stearyl lactate | 0 | 27.18 ± 1.66 | 18.63 ± 2.49 | 0 |
| 10 | Adipic acid | 0 | 0 | 86.34 ± 0.38 | 100 |
| 11 | Aconitic acid | 0 | 0 | 89.21 ± 0.66 | 83.46 ± 1.09 |
| 12 | Fumaric acid | 0 | 0 | 100 | 0 |
| 13 | Calcium glycerophosphate | 0 | 0 | 0 | 7.01 ± 1.78 |
| 14 | Aspartame | 0 | 20.33 ± 3.70 | 0 | 0 |
| 15 | DL-Malic acid | 0 | 0 | 89.69 ± 0.33 | 0 |
| 16 | Citric acid | 0 | 38.38 ± 1.78 | 0 | 0 |
| 17 | Ferrous gluconate hydrate | 0 | 91.26 ± 0.28 | 0 | 0 |
| GRAS Salts | Citrus Postharvest Disease | Virulence Equation (Y = ax + b) | EC50 (%) | EC95 (%) |
|---|---|---|---|---|
| Sodium silicate | G. citri-aurantii | y = 3.4498x + 16.216 | 0.06 | 0.17 |
| P. digitatum | y = 3.6899x + 17.2108 | 0.05 | 0.14 | |
| P. italicum | y = 4.1557x + 18.0528 | 0.07 | 0.14 | |
| C. gloeosporioides | y = 1.0819x + 7.2617 | 0.08 | 0.19 | |
| Ethylenediaminetetraacetic acid disodium salt | G.citri-aurantii | y = 2.9653x + 13.7919 | 0.11 | 0.39 |
| P. digitatum | y = 1.7099x + 10.2781 | 0.08 | 0.75 | |
| P. italicum | y = 1.9901x + 9.5804 | 0.5 | 3.35 | |
| C. gloeosporioides | y = 2.0019x + 11.3364 | 0.07 | 0.45 |
| Time | Samples | Weight Loss Rate (%) | Soluble Solid | Titratable Acid | VC (mg/100 g) |
|---|---|---|---|---|---|
| 30 d | CK | 0.64 ± 0.06 AC | 14.00 ± 0.00 AC | 0.83 ± 0.00 AC | 0.66 ± 0.01 AC |
| Na2SiO3 | 0.56 ± 0.04 aB | 13.50 ± 0.00 aB | 0.98 ± 0.02 aB | 0.64 ± 0.04 AB | |
| EDTA-Na2 | 0.52 ± 0.03 bc | 13.43 ± 0.06 Bc | 0.76 ± 0.01 bc | 0.65 ± 0.02 Bc | |
| 60 d | CK | 2.41 ± 0.20 AC | 13.67 ± 0.06 AC | 0.84 ± 0.06 AC | 0.62 ± 0.01 AC |
| Na2SiO3 | 2.29 ± 0.17 aB | 15.20 ± 0.10 aB | 1.06 ± 0.18 AB | 0.74 ± 0.05 aB | |
| EDTA-Na2 | 2.13 ± 0.15 bc | 13.90 ± 0.00 bc | 0.79 ± 0.04 bC | 0.67 ± 0.01 bc | |
| 80 d | CK | 4.99 ± 0.42 AC | 13.45 ± 0.25 AC | 0.75 ± 0.07 AC | 0.60 ± 0.02 AC |
| Na2SiO3 | 4.88 ± 0.39 aB | 14.67 ± 0.06 aB | 0.85 ± 0.03 aB | 0.70 ± 0.01 aB | |
| EDTA-Na2 | 4.49 ± 0.33 bc | 13.57 ± 0.21 bC | 0.67 ± 0.01 bC | 0.64 ± 0.01 bc | |
| 90 d | CK | 6.19 ± 0.53 AC | 14.07 ± 0.06 AC | 0.77 ± 0.03 AC | 0.58 ± 0.01 AC |
| Na2SiO3 | 6.10 ± 0.19 AB | 14.57 ± 0.06 aB | 0.89 ± 0.01 aB | 0.69 ± 0.01 aB | |
| EDTA-Na2 | 5.65 ± 0.43 bc | 14.17 ± 0.15 bC | 0.75 ± 0.01 bc | 0.62 ± 0.01 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, Y.; Liu, Q.; Liu, S.; Pan, H.; Cheng, Y.; Long, C. The GRAS Salts of Na2SiO3 and EDTA-Na2 Control Citrus Postharvest Pathogens by Disrupting the Cell Membrane. Foods 2023, 12, 2368. https://doi.org/10.3390/foods12122368
Zhao J, Wang Y, Liu Q, Liu S, Pan H, Cheng Y, Long C. The GRAS Salts of Na2SiO3 and EDTA-Na2 Control Citrus Postharvest Pathogens by Disrupting the Cell Membrane. Foods. 2023; 12(12):2368. https://doi.org/10.3390/foods12122368
Chicago/Turabian StyleZhao, Juan, Yuqing Wang, Qianyi Liu, Shuqi Liu, Hui Pan, Yunjiang Cheng, and Chaoan Long. 2023. "The GRAS Salts of Na2SiO3 and EDTA-Na2 Control Citrus Postharvest Pathogens by Disrupting the Cell Membrane" Foods 12, no. 12: 2368. https://doi.org/10.3390/foods12122368






