Color Characterization of Bordeaux Red Wines Produced without Added Sulfites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Red Wine Colorimetric Analysis
2.2.1. CIELab Analysis
2.2.2. Color Intensity Analysis
2.3. Total Phenolics Index
2.4. Polymeric Pigments Analyses by UPLC-DAD/ESI-QTof
2.4.1. Fractionation
2.4.2. Acidic Depolymerization
2.4.3. UPLC-DAD/ESI-QTof
2.5. Statistical Analysis
3. Results and Discussion
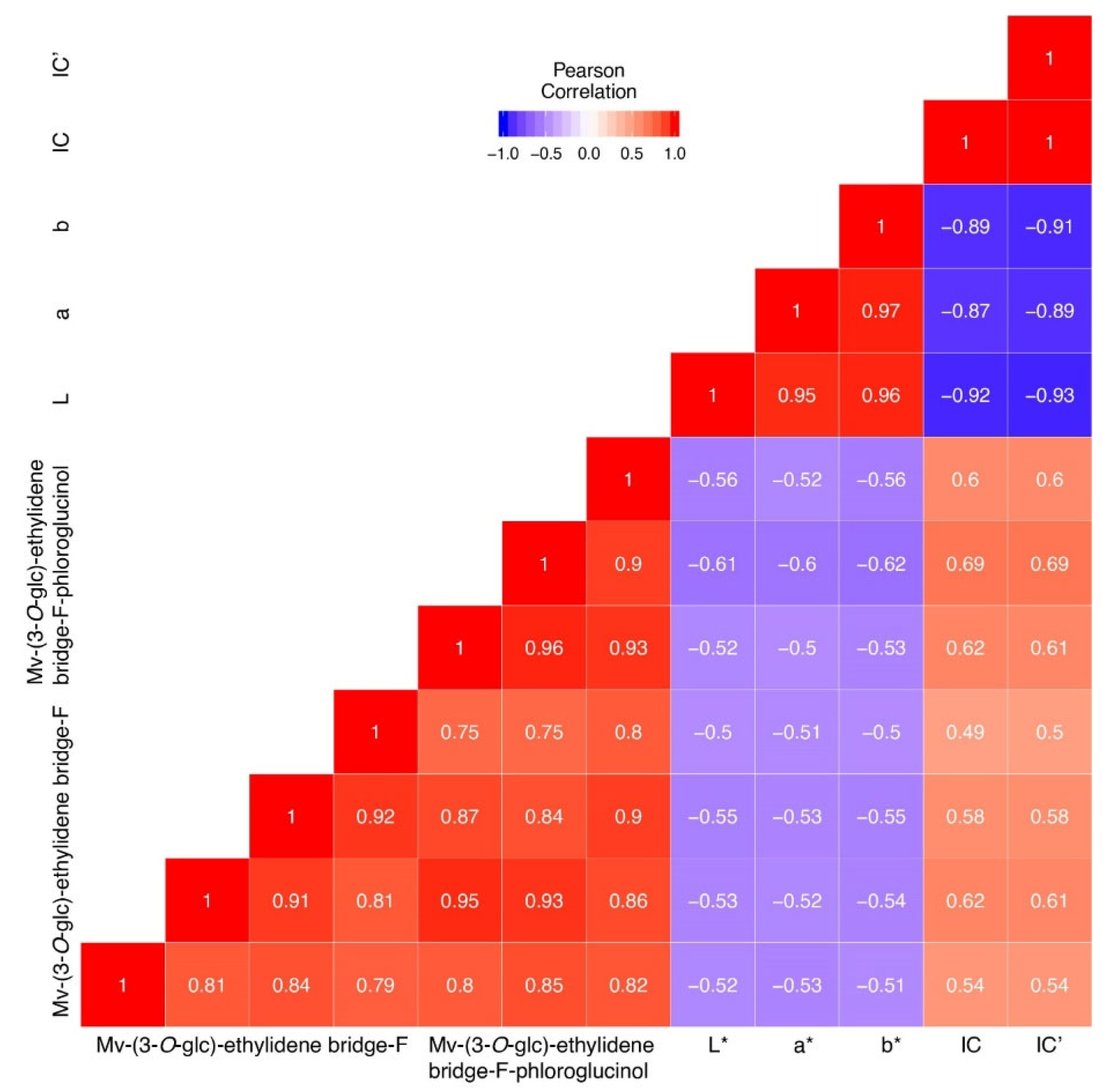
3.1. Color Parameters
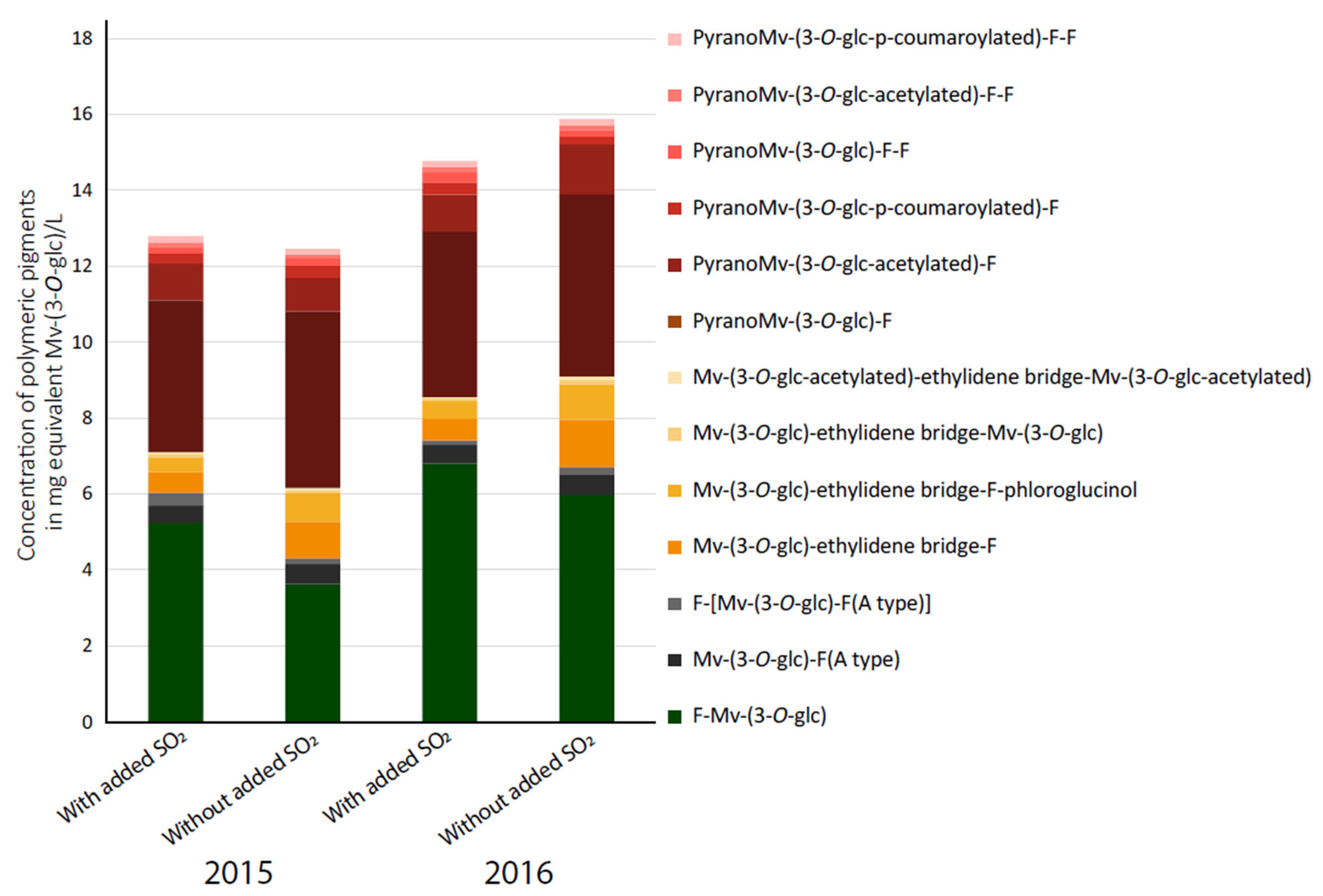
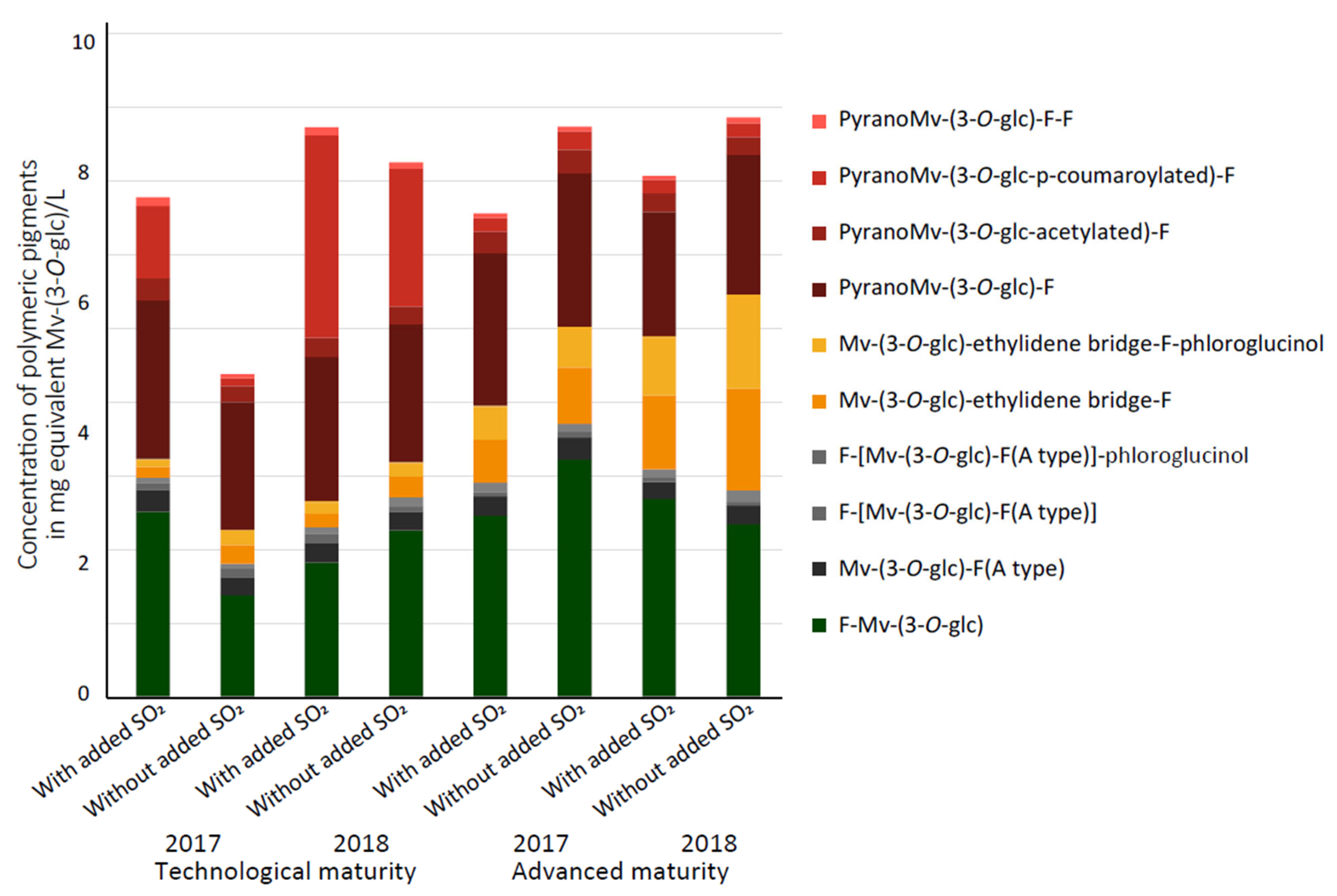
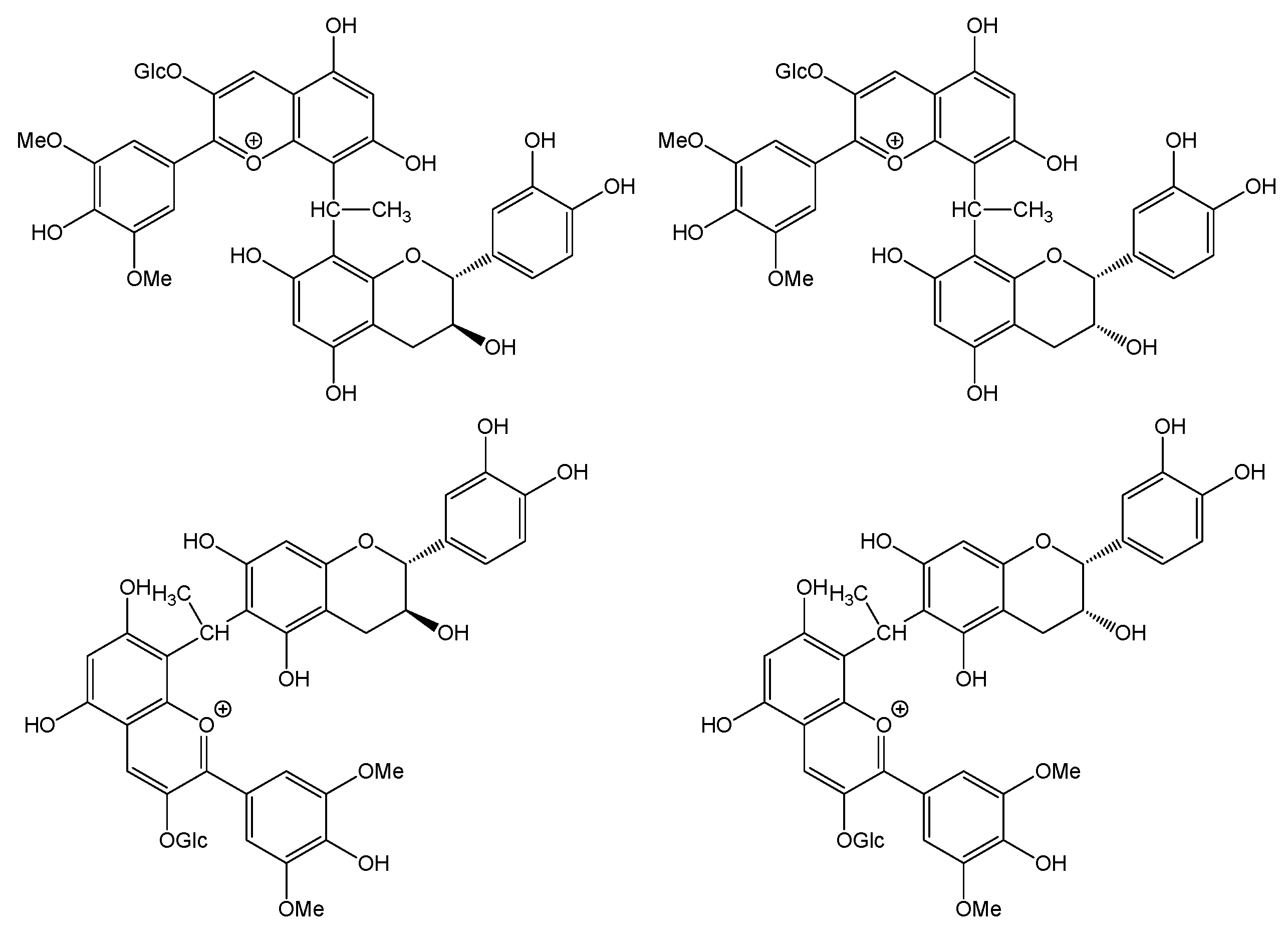
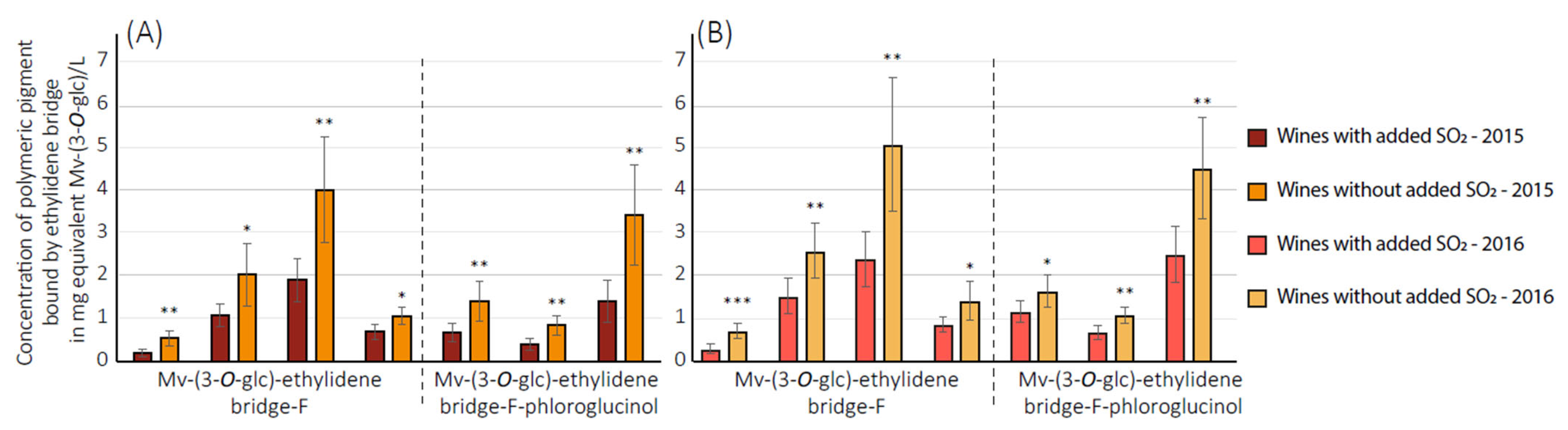
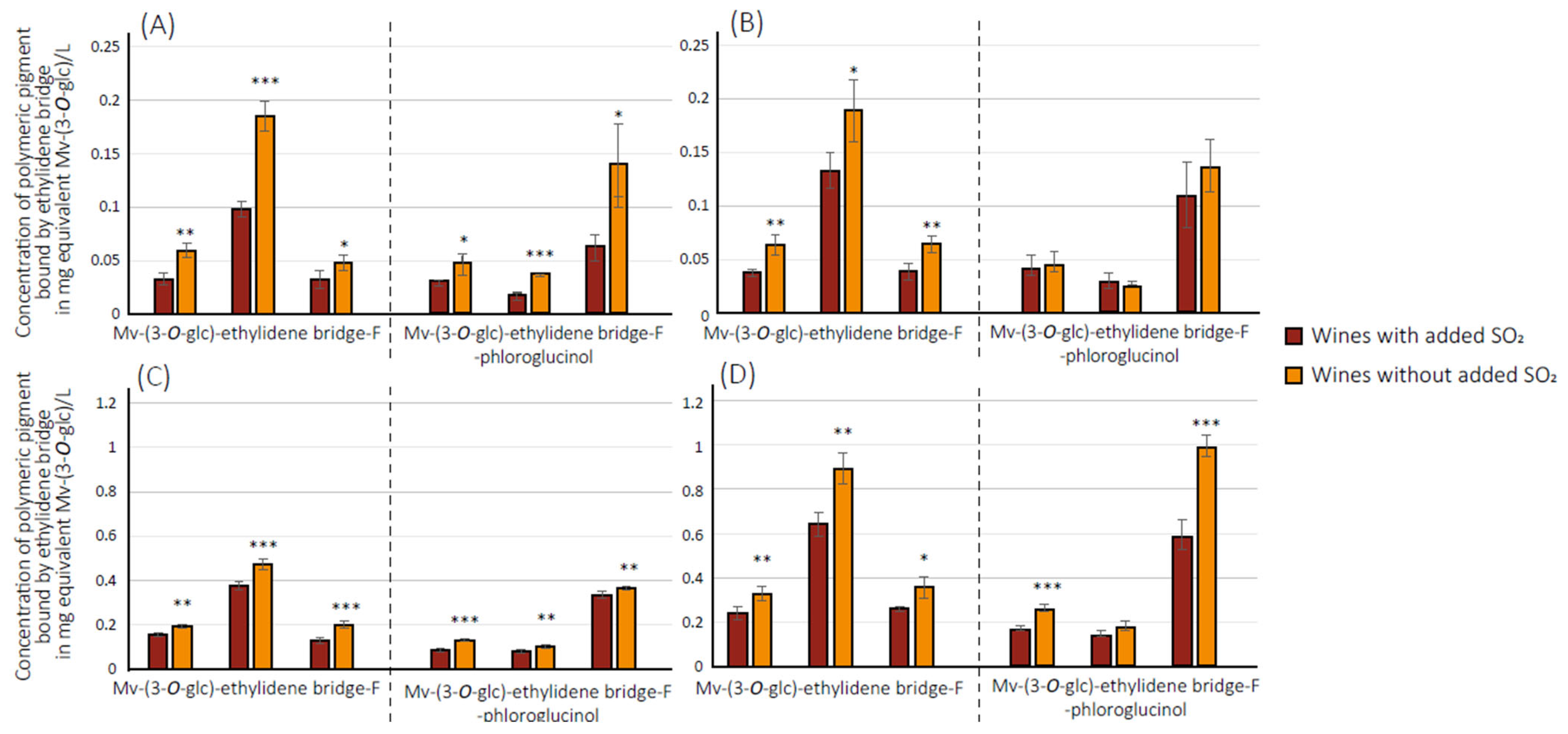
3.2. Quantification of Polymeric Pigments in Wines
3.3. Quantification of 2,2′-Ethylidenediphloroglucinol (EDP) in Wines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
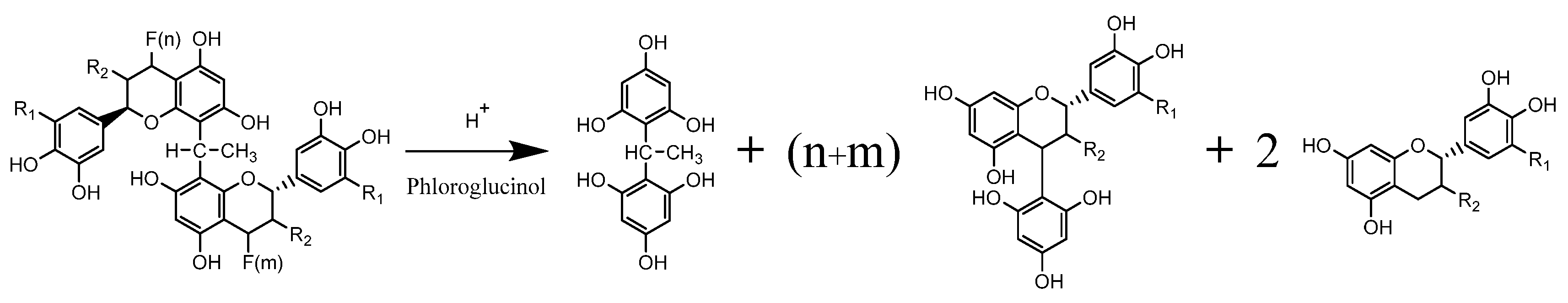
Appendix B
| Sugar (g/L) | Total Acidity (g/L H2SO4) | pH | |
|---|---|---|---|
| Without SO2 Technological maturity 2017 | 222 | 3.10 | 3.45 |
| With SO2 Technological maturity 2017 | 222 | 3.10 | 3.45 |
| Without SO2 Advanced maturity 2017 | 234 | 2.70 | 3.58 |
| With SO2 Advanced maturity 2017 | 234 | 2.70 | 3.58 |
| Without SO2 Technological maturity 2018 | 216 | 2.80 | 3.57 |
| With SO2 Technological maturity 2018 | 216 | 2.80 | 3.57 |
| Without SO2 Advanced maturity 2018 | 219 | 2.65 | 3.61 |
| With SO2 Advanced maturity 2018 | 219 | 2.65 | 3.61 |
| No. | Name | Molecular Formula [M]+ | m/z | Retention Time (min) |
|---|---|---|---|---|
| P1 | F-Mv-(3-O-glc) | [C38H37O18]+ | 781.1974 | 4.720 |
| P2 | 5.404 | |||
| P3 | 6.679 | |||
| P4 | 7.172 | |||
| P5 | Mv-(3-O-glc)-F(A type) | [C38H37O18]+ | 783.2131 | 6.690 |
| P6 | 7.514 | |||
| P11 | F-(Mv-(3-O-glc)-F(Atype))-phloroglucinol | [C44H41O18]+ | 907.2291 | 5.490 |
| P12 | 5.648 | |||
| P13 | Mv-(3-O-glc)-ethylidene bridge-F | [C40H41O18]+ | 809.2287 | 7.839 |
| P14 | 7.980 | |||
| P15 | 8.263 | |||
| P16 | 8.396 | |||
| P17 | Mv-(3-O-glc)-ethylidene bridge-F-phloroglucinol | [C46H45O21]+ | 933.2448 | 7.562 |
| P18 | 7.845 | |||
| P19 | 8.053 | |||
| P21 | Mv-(3-O-glc)-ethylidene bridge- Mv-(3-O-glc) | [C48H51O24]+ | 1011.2765 | 9.536 |
| P22 | 11.209 | |||
| P25 | Mv-(3-O-glc-acetylated)-ethylidene bridge- Mv-(3-O-glc-acetylated) | [C52H55O26]+ | 1095.2976 | 11.028 |
| P26 | 12.886 | |||
| P42 | PyranoMv-(3-O-glc)-F | [C40H37O18]+ | 805.1974 | 9.336 |
| P43 | 9.886 | |||
| P44 | PyranoMv-(3-O-glc-acetylated)-F | [C42H39O19]+ | 847.2080 | 9.611 |
| P45 | 9.819 | |||
| P46 | PyranoMv-(3-O-glc-p-coumaroylated)-F | [C49H43O20]+ | 951.2342 | 9.769 |
| P47 | PyranoMv-(3-O-glc-)-F-F | [C45H49O24]+ | 1093.2610 | 7.353 |
| P48 | 7.436 | |||
| P49 | PyranoMv-(3-O-glc-acetylated)-F-F | [C57H51O25]+ | 1135.2714 | 7.561 |
| P50 | 7.644 | |||
| P51 | PyranoMv-(3-O-glc-p-coumaroylated)-F-F | [C64H55O26]+ | 1239.2976 | 8.468 |
| P52 | 8.468 | |||
| P53 | 8.468 | |||
| P54 | 8.468 |
| Vintage | Wine | L* | a* | b* | C* | h* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 2015 | With added SO2 | 21.38 | 4.66 | 50.88 | 2.99 | 34.71 | 6.26 | 61.69 | 5.84 | 0.59 | 0.06 |
| Without added SO2 | 15.91 | 4.80 | 46.72 | 4.56 | 26.56 | 7.25 | 53.87 | 7.53 | 0.50 | 0.03 | |
| 2016 | With added SO2 | 20.13 | 3.91 | 50.82 | 2.17 | 32.33 | 4.21 | 60.26 | 3.92 | 0.56 | 0.07 |
| Without added SO2 | 12.16 | 2.52 | 43.37 | 3.11 | 20.71 | 3.9 | 48.12 | 4.61 | 0.44 | 0.05 | |
References
- Dubernet, M. Les “polyphenoloxydases” du raisin sain et du raisin parasite par Botrytis cinerea. CR Acad. Sci. 1973, 277, 975–978. [Google Scholar]
- Carr, J.G.; Davies, P.A.; Sparks, A.H. The Toxicity of Sulphur Dioxide towards Certain Lactic Acid Bacteria from Fermented Apple Juice. J. Appl. Bacteriol. 1976, 40, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Albertin, W.; Miot-Sertier, C.; Bely, M.; Marullo, P.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Masneuf-Pomarede, I. Oenological prefermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int. J. Food Microbiol. 2014, 178, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Usseglio-Tomasset, L. Properties and use of sulphur dioxide. Food Addit. Contam. 1992, 9, 399–404. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Traité D’oenologie, Tome 1—Microbiologie du Vin—Vinification, 6th ed.; Dunot: Paris, France, 2012; Volume 1. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Amato, M.; Ballco, P.; López-Galán, B.; De Magistris, T.; Verneau, F. Exploring consumers’ perception and willingness to pay for “Non-Added Sulphite” wines through experimental auctions: A case study in Italy and Spain. Wine Econ. Policy 2017, 6, 146–154. [Google Scholar] [CrossRef]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- García-Estévez, I.; Cruz, L.; Oliveira, J.; Mateus, N.; de Freitas, V.; Soares, S. First evidences of interaction between pyranoanthocyanins and salivary proline-rich proteins. Food Chem. 2017, 228, 574–581. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Winemaking-Stabilization, Aging Chemistry and Biochemistry; IntechOpen: London, UK, 2020. [Google Scholar]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Péros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, T. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Kennedy, J.A. Mass spectrometric evidence for the formation of pigmented polymers in red wine. Aust. J. Grape Wine Res. 2003, 9, 210–220. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Competition between (+)-catechin and (−)-epicatechin in acetaldehyde-induced polymerization of flavanols. J. Agric. Food Chem. 1999, 47, 2088–2095. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Blouin, J. Le SO2 en Œnologie; Dunod: Paris, France, 2014; ISBN 9782100710492. [Google Scholar]
- Gambuti, A.; Strollo, D.; Erbaggio, A.; Lecce, L.; Moio, L. Effect of winemaking practices on color indexes and selected bioactive phenolics of Aglianico wine. J. Food Sci. 2007, 72, S623–S628. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Bellworthy, S.J.; Garcia-Viguera, C.; Reader, H.P.; Watkins, S.J. Effect of sulphur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J. Sci. Food Agric. 1998, 78, 297–307. [Google Scholar] [CrossRef]
- Pelonnier-Magimel, E.; Windholtz, S.; Pomarède, I.M.; Barbe, J.-C. Sensory characterisation of wines without added sulfites via specific and adapted sensory profile. OENO One 2020, 54, 671–685. [Google Scholar] [CrossRef]
- Pelonnier-Magimel, E.; Mangiorou, P.; Darriet, P.; de Revel, G.; Jourdes, M.; Marchal, A.; Marchand, S.; Pons, A.; Riquier, L.; Teissedre, P.-L.; et al. Sensory characterisation of Bordeaux red wines produced without added sulfites. OENO One 2020, 54, 733–743. [Google Scholar] [CrossRef]
- Zeng, L.; Teissèdre, P.; Jourdes, M. Structures of polymeric pigments in red wine and their derived quantification markers revealed by high-resolution quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Paul, F. Die alkalimetrische Bestimmung der freien, gebundenen und gesamten schwefligen Säure mittels des Apparates von Lieb und Zacherl. Mitt. Klosterneuburg 1958, 8, 21–27. [Google Scholar]
- Commission Internationale de l’Eclairage. Colorimetry, 2nd ed.; Publication CIE no. 15.2 (TC-1.3); Bureau Central de la CIE: Paris, France, 1986.
- Ayala, F.; Echávarri, J.; Negueruela, A. A new simplified method for measuring the color of wines. I. Red and rose wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar] [CrossRef]
- Sudraud, P. Interpretation des courbes d’absorption des vins rouges. Ann. Technol. Agric 1958, 7, 203–208. [Google Scholar]
- Glories, Y. La couleur des vins rouges: 2e. Partie: Mesure, origine et interprétation. Connaiss. Vigne Vin 1984, 18. [Google Scholar] [CrossRef]
- Organisation Internationale de la Vigne et du Vin. Recueil des Méthodes Internationales D’analyse des Vins et des Moûts; OIV: Dijon, France, 1990. [Google Scholar]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.-L.; Saucier, C. Ethylidene-Bridged Flavan-3-ols in Red Wine and Correlation with Wine Age. J. Agric. Food Chem. 2007, 55, 6292–6299. [Google Scholar] [CrossRef]
- Martínez, J.; Melgosa, M.; Pérez, M.; Hita, E.; Negueruela, A. Note. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Picinelli, A.; Bakker, J.; Bridle, P. Model wine solutions: Effect of sulphur dioxide on colour and composition during ageing. Vitis 1994, 33, 31–35. [Google Scholar]
- Santos-Buelga, C.; Bravo-Haro, S.; Rivas-Gonzalo, J.C. Interactions between catechin and malvidin-3-monoglucoside in model solutions. Z. Lebensm.-Unters. Forsch. 1995, 201, 269–274. [Google Scholar] [CrossRef]
- Lee, D.F.; Swinny, E.E.; Jones, G.P. NMR identification of ethyl-linked anthocyanin–flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004, 45, 1671–1674. [Google Scholar] [CrossRef]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.-L.; Saucier, C. Analysis of ethylidene-bridged flavan-3-ols in wine. J. Agric. Food Chem. 2007, 55, 1109–1116. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.; Gómez-Cordovés, M.; Suárez, J. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Avizcuri, J.-M.; Sáenz-Navajas, M.-P.; Echávarri, J.-F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the impact of initial red wine composition on changes in color and anthocyanin content during bottle storage. Food Chem. 2016, 213, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Products formed in model wine solutions involving anthocyanins, procyanidin B2, and acetaldehyde. J. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Structure of a new dimeric acetaldehyde malvidin 3-glucoside condensation product. Tetrahedron Lett. 2002, 43, 6151–6153. [Google Scholar] [CrossRef]
- Cheynier, V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Sims, C.A.; Morris, J.R. Effects of pH, Sulfur Dioxide, Storage Time, and Temperature on the Color and Stability of Red Muscadine Grape Wine. Am. J. Enol Vitic. 1984, 35, 35–39. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [Green Version]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (−)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef]
- Weber, F.; Winterhalter, P. Synthesis and structure elucidation of ethyliden-linked anthocyanin—Flavan-3-ol oligomers. Food Res. Int. 2014, 65, 69–76. [Google Scholar] [CrossRef]
- Zeng, L. Étude de la Composition Macromoléculaire du Raisin et des Vins: Impact sur la Qualité Sensorielle. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2015. [Google Scholar]

| L* | a* | b* | CI | CD | ΔE*ab | |||
|---|---|---|---|---|---|---|---|---|
| 2017 | Technological maturity | With SO2 | 16.75 | 46.45 | 27.88 | 0.643 | 0.729 | 17.62 |
| Without SO2 | 8.64 | 38.05 | 14.68 | 0.937 | 1.087 | |||
| Advanced maturity | With SO2 | 8.66 | 38.35 | 14.73 | 0.999 | 1.085 | 4.22 | |
| Without SO2 | 7.06 | 35.60 | 11.95 | 1.071 | 1.238 | |||
| 2018 | Technological maturity | With SO2 | 17.51 | 48.39 | 28.88 | 0.715 | 0.817 | 4.81 |
| Without SO2 | 15.50 | 45.65 | 25.48 | 0.713 | 0.823 | |||
| Advanced maturity | With SO2 | 12.46 | 43.30 | 21.22 | 0.887 | 1.023 | 2.44 | |
| Without SO2 | 13.71 | 44.49 | 22.99 | 0.676 | 0.805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelonnier-Magimel, E.; Chira, K.; Teissèdre, P.-L.; Jourdes, M.; Barbe, J.-C. Color Characterization of Bordeaux Red Wines Produced without Added Sulfites. Foods 2023, 12, 2358. https://doi.org/10.3390/foods12122358
Pelonnier-Magimel E, Chira K, Teissèdre P-L, Jourdes M, Barbe J-C. Color Characterization of Bordeaux Red Wines Produced without Added Sulfites. Foods. 2023; 12(12):2358. https://doi.org/10.3390/foods12122358
Chicago/Turabian StylePelonnier-Magimel, Edouard, Kléopatra Chira, Pierre-Louis Teissèdre, Michaël Jourdes, and Jean-Christophe Barbe. 2023. "Color Characterization of Bordeaux Red Wines Produced without Added Sulfites" Foods 12, no. 12: 2358. https://doi.org/10.3390/foods12122358
APA StylePelonnier-Magimel, E., Chira, K., Teissèdre, P.-L., Jourdes, M., & Barbe, J.-C. (2023). Color Characterization of Bordeaux Red Wines Produced without Added Sulfites. Foods, 12(12), 2358. https://doi.org/10.3390/foods12122358






