Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Freeze-Dried Enzymatic Extract Preparation
2.2. Characterisation of Thistle Extracts
2.2.1. Total Protein Content
2.2.2. Mineral Content
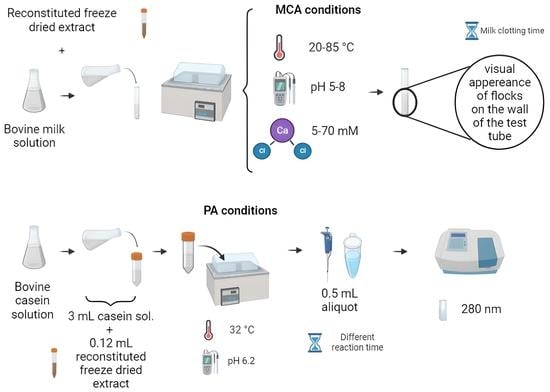
2.2.3. Milk Clotting Activity Assay
2.2.4. Proteolytic Activity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Total Protein Content
3.2. Mineral Content
3.3. Milk Clotting Activity Assay
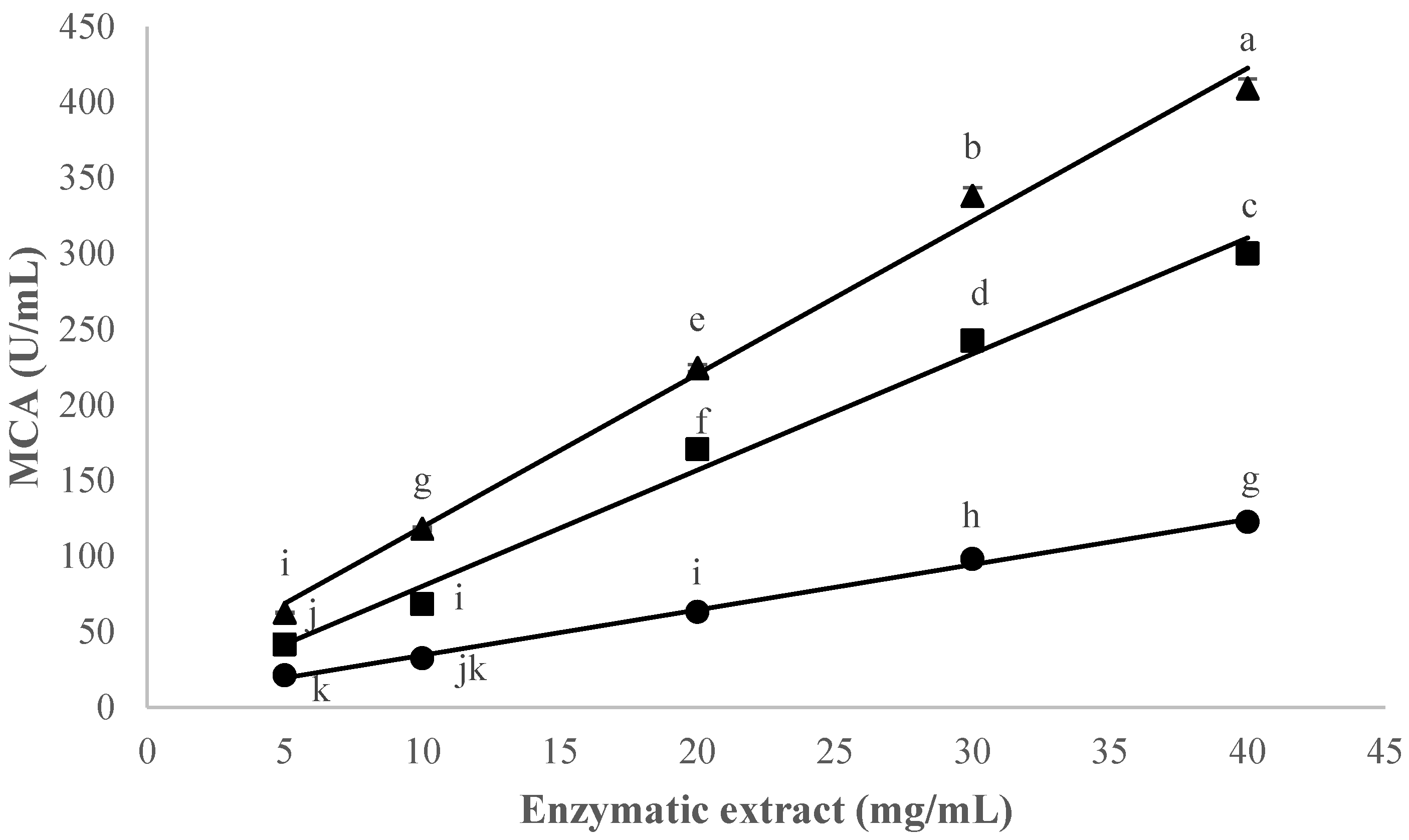
3.3.1. Effect of Thistle Species and Extract Concentration
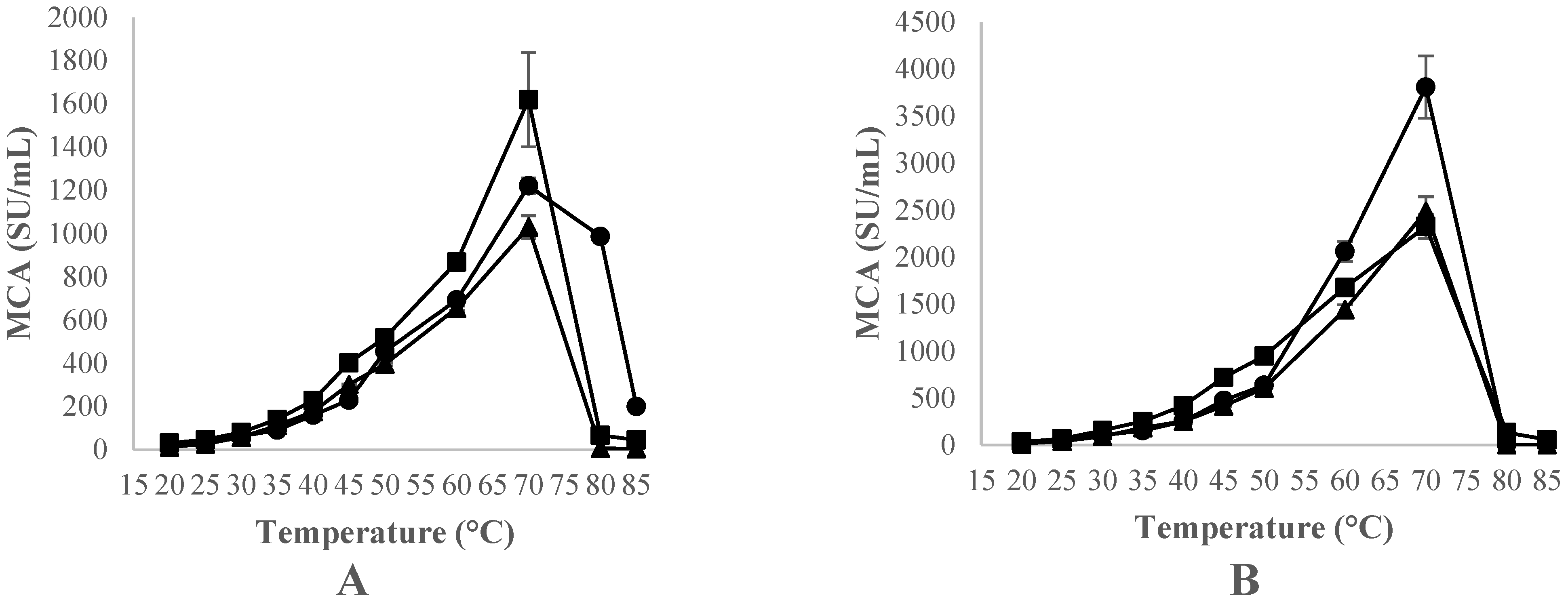
3.3.2. Effect of Thistle Species and Temperature
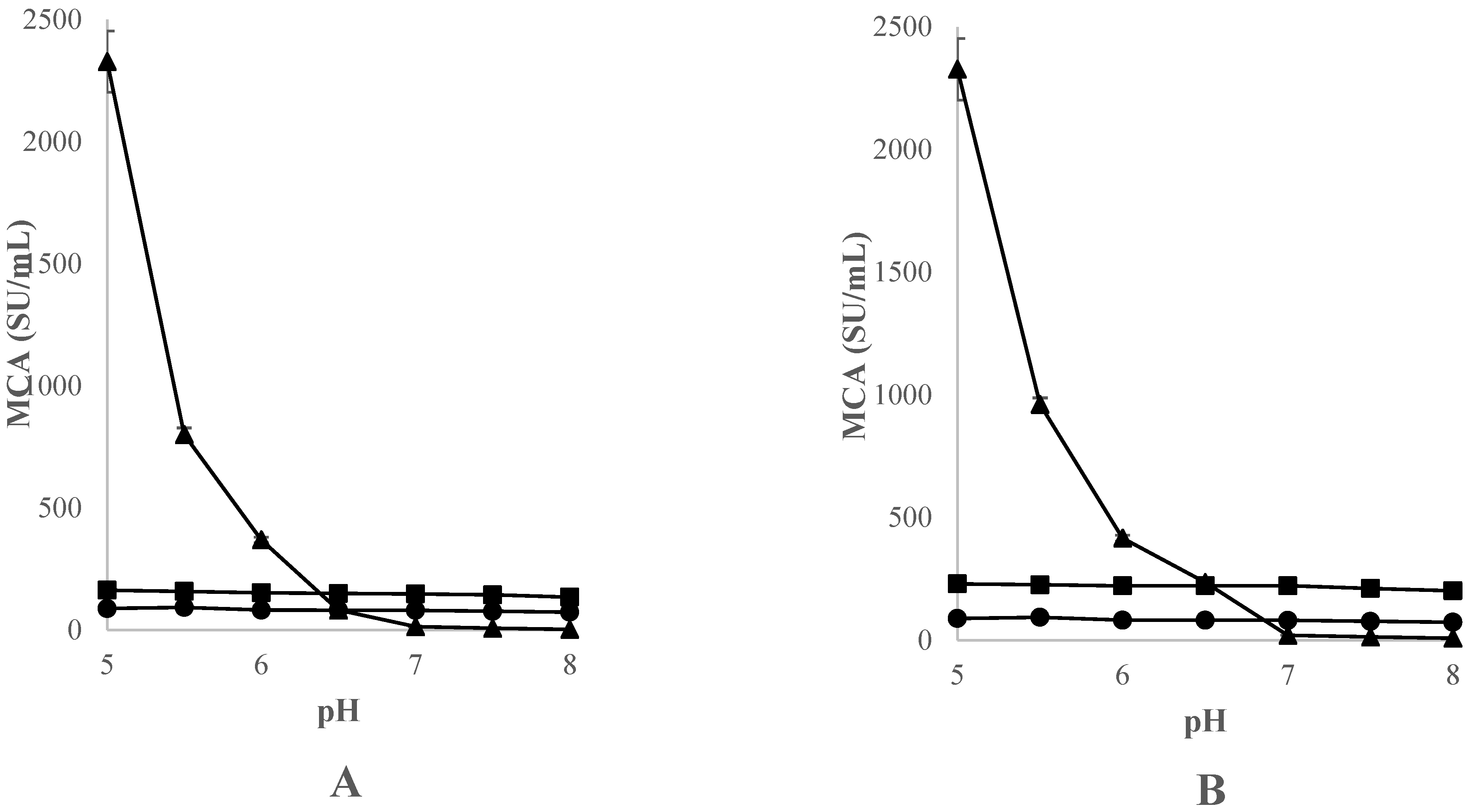
3.3.3. Effect of Thistle Species and pH
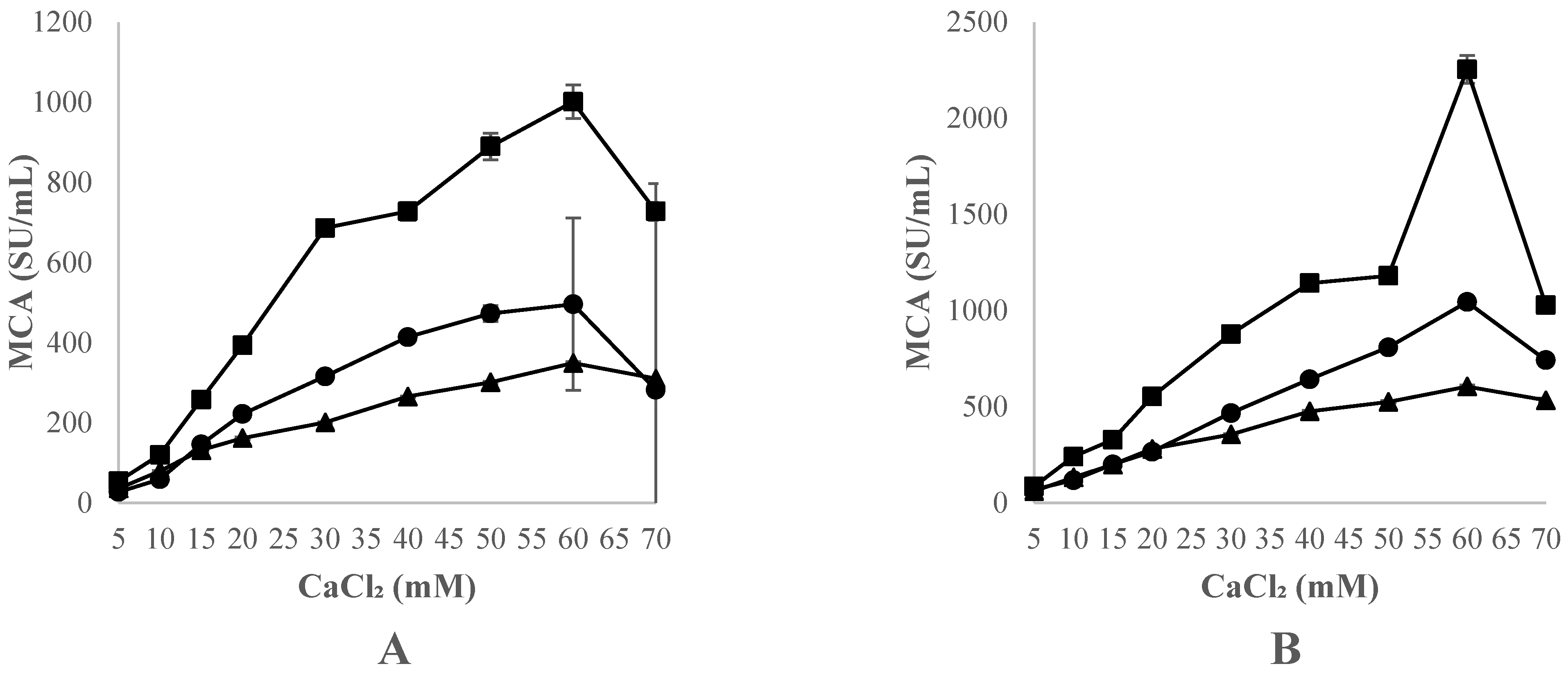
3.3.4. Effect of Thistle Species and CaCl2
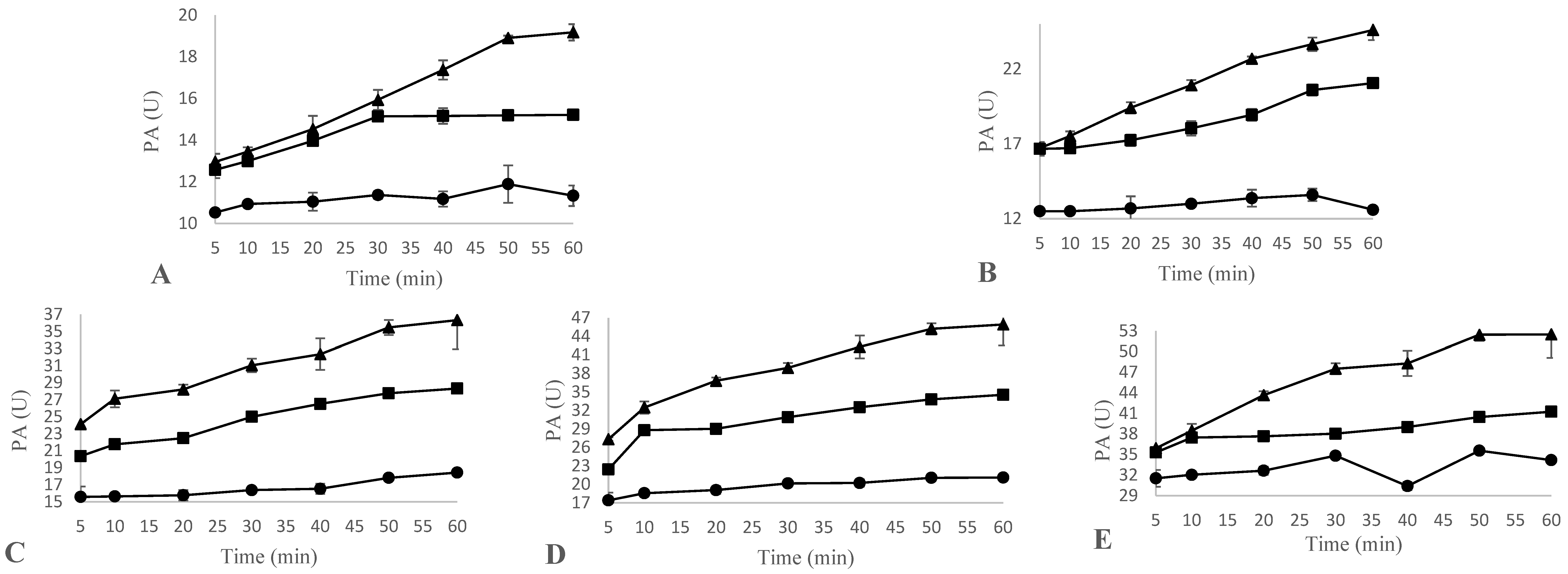
3.4. Proteolytic Activity and MCA/PA Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mazorra-Manzano, M.A.; Perea-Gutiérrez, T.C.; Lugo-Sánchez, M.E.; Ramirez-Suarez, J.C.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Cordoba, B. Comparison of the milk-clotting properties of three plant extracts. Food Chem. 2013, 141, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Jaros, D.; Rohm, H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Shah, M.A.; Mir, S.A.; Paray, M.A. Plant proteases as milk-clotting enzymes in cheesemaking: A review. Dairy Sci. Technol. 2014, 94, 5–16. [Google Scholar] [CrossRef]
- Galán, E.; Cabezas, L.; Fernández-Salguero, J. Proteolysis, microbiology and sensory properties of ewes’ milk cheese produced with plant coagulant from cardoon Cynara cardunculus, calf rennet or a mixture thereof. Int. Dairy J. 2012, 25, 92–96. [Google Scholar] [CrossRef]
- Khaldi, S.; Sonnante, G.; el Gazzah, M. Analysis of molecular genetic diversity of cardoon (Cynara cardunculus L.) in Tunisia. C. R. Biol. 2012, 335, 389–397. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Barbosa, M.; Ames, J.M.; Wilbey, R.A. Cheesemaking with vegetable coagulants—The use of Cynara L. for the production of ovine milk cheeses. Int. J. Dairy Technol. 2003, 56, 76–85. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Prados, F.; Cayuela, J.M. Compositional characteristics of Murcia al Vino goat’s cheese made with calf rennet and plant coagulant. Int. J. Dairy Technol. 2008, 61, 119–125. [Google Scholar] [CrossRef]
- Getu Derso, A.; Gashaw Dagnew, G. Isolate and extract for milk clotting enzymes from the leaves of Moringa oleifera, Carica papaya and Mangifera indica and use in cheese making: The case of Western Hararage Region, Ethiopia. J. Food Nutr. Res. 2019, 7, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Espinoza, J.A.; Domínguez-Lujan, B.; Guitierrez-Méndez, N.; Chávez-Garay, D.R.; Nájera-Domínguez, C.; Leal-Ramos, M.Y. The impact of chymosin and plant-derived proteases on the acid-induced gelation of milk. Int. J. Dairy Technol. 2021, 74, 297–306. [Google Scholar] [CrossRef]
- Feijoo-Siota, L.; Villa, T.G. Native and biotechnologically engineered plant proteases with industrial applications. Food Bioproc Technol. 2011, 4, 1066–1088. [Google Scholar] [CrossRef]
- Gomes, S.; Belo, A.T.; Alvarenga, N.; Dias, J.; Lage, P.; Pinheiro, C.; Pinto-Cruz, C.; Brás, T.; Duarte, M.F.; Martins, A.P.L. Characterization of Cynara cardunculus L. flower from Alentejo as a coagulant agent for cheesemaking. Int. Dairy J. 2019, 91, 178–184. [Google Scholar] [CrossRef]
- Sarmento, A.C.; Lopes, H.; Oliveira, C.S.; Vitorino, R.; Samyn, B.; Sergeant, K.; Debyser, G.; Van Beeumen, J.; Domingues, P.; Amado, F.; et al. Multiplicity of aspartic proteinases from Cynara cardunculus L. Planta 2009, 230, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.M.; Lourenço, P.L.; Domingos, A.; Clemente, A.F.; Pais, M.S.; Malcata, F.X. Applicability of extracts from Centaurea calcitrapa in ripening of bovine cheese. Int. Dairy J. 2000, 10, 775–780. [Google Scholar] [CrossRef]
- Silva, S.V.; Barros, R.M.; Malcata, F.X. Hydrolysis of caseins by extracts of Cynara cardunculus precipitated by ammonium sulfate. J. Food Sci. 2002, 67, 1746–1751. [Google Scholar] [CrossRef]
- Fernández-Salguero, J.; Sánchez, E.; Gómez, R.; Mata, C.; Vioque, M.; Tejada, L. A preliminary study of microbiological quality of cardoons of genus Cynara L. used in manufacture of traditional cheese. Milchwissenschaft 1999, 54, 688–690. [Google Scholar]
- Vioque, M.; Gómez, R.; Sánchez, E.; Mata, C.; Tejada, L.; Fernández-Salguero, J. Chemical and microbiological characteristics of ewes’ milk cheese manufactured with extracts from flowers of Cynara cardunculus and Cynara humilis as coagulants. J. Agric. Food Chem. 2000, 48, 451–456. [Google Scholar] [CrossRef]
- Fernández-Salguero, J.; Tejada, L.; Gómez, R. Use of powdered vegetable coagulant in the manufacture of ewe’s milk cheeses. J. Sci. Food Agric. 2002, 82, 464–468. [Google Scholar] [CrossRef]
- Tejada, L.; Gómez, R.; Fernández-Salguero, J. Sensory characteristics of ewe milk cheese made with three types of coagulant: Calf rennet, powdered vegetable coagulant and crude aqueous extract from Cynara cardunculus. J. Food Qual. 2007, 30, 91–103. [Google Scholar] [CrossRef]
- Tejada, L.; Vioque, M.; Gómez, R.; Fernández-Salguero, J. Effect of lyophilisation, refrigerated storage and frozen storage on the coagulant activity and microbiological quality of Cynara cardunculus L. extracts. J. Sci. Food Agric. 2008, 88, 1301–1306. [Google Scholar] [CrossRef]
- Abellán, A.; Cayuela, J.M.; Pino, A.; Martínez-Cachá, A.; Salazar, E.; Tejada, L. Free amino acid content of goat’s milk cheese made with animal rennet and plant coagulant. J. Sci. Food Agric. 2012, 92, 1657–1664. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cachá, A.; Fernández-Salguero, J. Proteolysis in goats’ milk cheese made with calf rennet and plant coagulant. Int. Dairy J. 2008, 18, 139–146. [Google Scholar] [CrossRef]
- Tejada, L.; Fernández-Salguero, J. Chemical and microbiological characteristics of ewe milk cheese (Los Pedroches) made with a powdered vegetable coagulant or calf rennet. Ital. J. Food Sci. 2003, 15, 125–131. [Google Scholar]
- Bouazzi, S.; El Mokni, R.; Nakbi, H.; Dhaouadi, H.; Joshi, R.K.; Hammami, S. Chemical composition and antioxidant activity of essential oils and hexane extract of Onopordum arenarium from Tunisia. J. Chromatogr. Sci. 2020, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Zenobi, S.; Fiorentini, M.; Aquilanti, L.; Foligni, R.; Mannozzi, C.; Mozzon, M.; Zitti, S.; Casavecchia, S.; Al Mohandes Dridi, B.; Orsini, R. Effect of planting density in two thistle species used for vegetable rennet production in marginal Mediterranean areas. Agronomy 2021, 11, 135. [Google Scholar] [CrossRef]
- Mozzon, M.; Foligni, R.; Mannozzi, C.; Zamporlini, F.; Raffaelli, N.; Aquilanti, L. Clotting properties of Onopordum tauricum (Willd.) aqueous extract in milk of different species. Foods 2020, 9, 692. [Google Scholar] [CrossRef]
- Foligni, R.; Mannozzi, C.; Gasparrini, M.; Raffaelli, N.; Zamporlini, F.; Tejada, L.; Bande-De León, C.; Orsini, R.; Manzi, P.; Di Costanzo, M.G.; et al. Potentialities of aqueous extract from cultivated Onopordum tauricum (Willd.) as milk clotting agent for cheesemaking. Food Res. Int. 2022, 158, 111592. [Google Scholar] [CrossRef] [PubMed]
- Heimgartner, U.; Pietrzak, M.; Geertsen, R.; Brodelius, P.; da Silva Figueiredo, A.C.; Pais, M.S.S. Purification and partial characterization of milk clotting proteases from flowers of Cynara cardunculus. Phytochemistry 1990, 29, 1405–1410. [Google Scholar] [CrossRef]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and characterization of a milk-clotting aspartic proteinase from globe artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef]
- Llorente, B.E.; Obregón, W.D.; Avilés, F.X.; Caffini, N.O.; Vairo-Cavalli, S. Use of artichoke (Cynara scolymus) flower extract as a substitute for bovine rennet in the manufacture of Gouda-type cheese: Characterization of aspartic proteases. Food Chem. 2014, 159, 55–63. [Google Scholar] [CrossRef]
- Martínez, E.; Esteban, M.A. Clotting activity of an extract from the flowers of the cardon Cynara humilis. Arch. Zootec. 1980, 29, 107–116. [Google Scholar]
- Silva, S.V.; Malcata, F.X. Influence of the coagulant level on early proteolysis in ovine cheese-like systems made with sterilized milk and Cynara cardunculus. J. Food Sci. 2004, 69, C579–C584. [Google Scholar] [CrossRef]
- Serrano, E.; Marcos, A. Proteolytic activity of the extract from the flowers of Cynara humilis L. Arch. Zootec. 1980, 29, 11. [Google Scholar]
- Bueno-Gavilá, E.; Abellán, A.; Bermejo, M.S.; Salazar, E.; Cayuela, J.M.; Prieto-merino, D.; Tejada, L. Characterization of proteolytic activity of artichoke (Cynara scolymus L.) flower extracts on bovine casein to obtain bioactive peptides. Animals 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Esteves, C.L.C.; Lucey, J.A.; Hyslop, D.B.; Pires, E.M.V. Effect of gelation temperature on the properties of skim milk gels made from plant coagulants and chymosin. Int. Dairy J. 2003, 13, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Esteves, C.L.C.; Lucey, J.A.; Wang, T.; Pires, E.M.V. Effect of pH on the gelation properties of skim milk gels made from plant coagulants and chymosin. J. Dairy Sci. 2003, 86, 2558–2567. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Official Method 991.25. Calcium, Magnesium, and Phosphorus in Cheese, Atomic Absorption Spectrophotometric and Colorimetric Method. In Official Methods of Analysis, 17th ed.; AOAC International: Arlington, VA, USA, 2002. [Google Scholar]
- IDF Standard 110A; Determination of the Clotting Time of Milk to Which a Milk Clotting Enzyme Solution has Been Added. International Dairy Federation: Brussels, Belgium, 1987.
- Silva, S.V.; Malcata, F.X. Studies pertaining to coagulant and proteolytic activities of plant proteases from Cynara cardunculus. Food Chem. 2005, 89, 19–26. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Lucini, L.; Rea, E.; Colla, G. Nutrient solution concentration affects growth, mineral composition, phenolic acids, and flavonoids in leaves of artichoke and cardoon. HortScience 2012, 47, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive Compounds from Cardoon as Health Promoters in Metabolic Disorders. Foods 2022, 11, 336. [Google Scholar] [CrossRef]
- Hajji Nabih, M.; El Hajam, M.; Boulika, H.; Hassan, M.M.; Idrissi Kandri, N.; Hedfi, A.; Zerouale, A.; Boufahja, F. Physicochemical Characterization of Cardoon “Cynara cardunculus” Wastes (Leaves and Stems): A Comparative Study. Sustainability 2021, 13, 13905. [Google Scholar] [CrossRef]
- Vieira de Sá, F.; Barbosa, M. Cheese-making with a vegetable rennet from cardo (Cynara cardunculus). J. Dairy Res. 1972, 39, 335–343. [Google Scholar] [CrossRef]
- Galán, E.; Prados, F.; Pino, A.; Tejada, L.; Fernández-Salguero, J. Influence of different amounts of vegetable coagulant from cardoon Cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. Int. Dairy J. 2008, 18, 93–98. [Google Scholar] [CrossRef]
- Chazarra, S.; Sidrach, L.; López-Molina, D.; Rodríguez-López, J.N. Characterization of the milk-clotting properties of extracts from artichoke (Cynara scolymus, L.) flowers. Int. Dairy J. 2007, 17, 1393–1400. [Google Scholar] [CrossRef]
- Brutti, C.B.; Pardo, M.F.; Caffini, N.O.; Natalucci, C.L. Onopordum acanthium L. (Asteraceae) flowers as coagulating agent for cheesemaking. LWT-Food Sci. Technol. 2012, 45, 172–179. [Google Scholar] [CrossRef]
- Campos, R.; Guerra, R.; Aguilar, M.; Ventura, O.; Camacho, L. Chemical characterization of proteases extracted from wild thistle (Cynara cardunculus). Food Chem. 1990, 35, 89–97. [Google Scholar] [CrossRef]
- Christen, C.; Virasoro, E. Présures végétales. Extraction et propriétés. Le Lait 1935, 15, 354–363. [Google Scholar] [CrossRef]
- Marcos, A.; Esteban, M.A.; Martínez, E.; Alcala, M.; Fernández-Salguero, J. Thermal inactivation of proteinases from the flowers of the cardoon Cynara humilis L.: Kinetic and thermodynamic parameters. Arch. Zootec. 1980, 29, 283–294. [Google Scholar]
- Claverie-Martín, F.; Vega-Hernández, M.C. Aspartic proteases used in cheese making. In Industrial Enzymes; Polaina, J., MacCabe, A.P., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 207–219. [Google Scholar] [CrossRef]
- Sousa-Gallagher, M.J.; Malcata, F. Effects of processing conditions on the caseinolytic activity of crude extracts of Cynara cardunculus L. Food Sci. Technol. Int. 1996, 2, 255–263. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhao, J.; Agboola, S. Isolation and partial characterization of rennet-like proteases from Australian cardoon (Cynara cardunculus L.). J. Agric. Food Chem. 2003, 51, 3127–3134. [Google Scholar] [CrossRef]
- Zikiou, A.; Zidoune, M.N. Enzymatic extract from flowers of Algerian spontaneous Cynara cardunculus: Milk-clotting properties and use in the manufacture of a Camembert-type cheese. Int. J. Dairy Technol. 2019, 72, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Bencini, R. Factors affecting the clotting properties of sheep milk. J. Sci. Food Agric. 2002, 82, 705–719. [Google Scholar] [CrossRef]
- Lagaude, A.; Fernandez, L.; Cuq, J.L.; Marchesseau, S. Characterization of curd formation during the rennet coagulation of milk by an optical microscopic method. Int. Dairy J. 2004, 14, 1033–1039. [Google Scholar] [CrossRef]
- Nájera, A.I.; de Renobales, M.; Barron, L.J.R. Effects of pH, temperature, CaCl2 and enzyme concentrations on the rennet-clotting properties of milk: A multifactorial study. Food Chem. 2003, 80, 345–352. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Guan, R.; Jia, G.; Ma, Y.; Zhang, Y. Advances in Research on Calf Rennet Substitutes and Their Effects on Cheese Quality. Food Res. Int. 2021, 149, 110704. [Google Scholar] [CrossRef]
- Esteves, C.L.C.; Lucey, J.A.; Pires, E.M.V. Rheological properties of milk gels made with coagulants of plant origin and chymosin. Int. Dairy J. 2002, 12, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.; Faro, C.; Macedo, I.; Esteves, C.; Morgado, J.; Veríssimo, P.; Pereira, C.; Gomes, D. Flor do cardo versus quimosina no fabrico de queijos artesanais. Bol. Soc. Port. Química 1994, 54, 66–68. [Google Scholar] [CrossRef]
| Minerals | Species | |
|---|---|---|
| CH | OP | |
| Ca | 346.1 ± 2.3 a | 330.7 ± 4.1 b |
| P | 638.9 ± 7.6 b | 778.1 ± 13.3 a |
| Na | 77.3 ± 3.4 a | 73.6 ± 1.5 a |
| K | 7577.7± 156.2 a | 5918.6 ± 86.4 b |
| Mg | 393.3 ± 0.4 a | 311.1 ± 1.4 b |
| Zn | 3.2 ± 0.1 a | 2.5 ± 0.1 b |
| Mn | 2.0 ± 0.0 b | 2.2 ± 0.1 a |
| [Extract] (mg/mL) | MCA/PA 1 | ||
|---|---|---|---|
| CC | CH | OP | |
| 5 | 3.2740 | 2.7359 | 1.8765 |
| 10 | 3.3861 | 3.2477 | 2.5854 |
| 20 | 4.3226 | 6.0250 | 3.4263 |
| 30 | 7.3651 | 7.0204 | 4.6564 |
| 40 | 7.7977 | 7.2768 | 3.5884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bande-De León, C.; Buendía-Moreno, L.; Abellán, A.; Manzi, P.; Al Mohandes Dridi, B.; Essaidi, I.; Aquilanti, L.; Tejada, L. Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb. Foods 2023, 12, 2325. https://doi.org/10.3390/foods12122325
Bande-De León C, Buendía-Moreno L, Abellán A, Manzi P, Al Mohandes Dridi B, Essaidi I, Aquilanti L, Tejada L. Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb. Foods. 2023; 12(12):2325. https://doi.org/10.3390/foods12122325
Chicago/Turabian StyleBande-De León, Cindy, Laura Buendía-Moreno, Adela Abellán, Pamela Manzi, Bouthaina Al Mohandes Dridi, Ismahen Essaidi, Lucia Aquilanti, and Luis Tejada. 2023. "Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb." Foods 12, no. 12: 2325. https://doi.org/10.3390/foods12122325
APA StyleBande-De León, C., Buendía-Moreno, L., Abellán, A., Manzi, P., Al Mohandes Dridi, B., Essaidi, I., Aquilanti, L., & Tejada, L. (2023). Clotting and Proteolytic Activity of Freeze-Dried Crude Extracts Obtained from Wild Thistles Cynara humilis L. and Onopordum platylepis Murb. Foods, 12(12), 2325. https://doi.org/10.3390/foods12122325