Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Growth Conditions
2.2. Probiotic Drinks Preparation
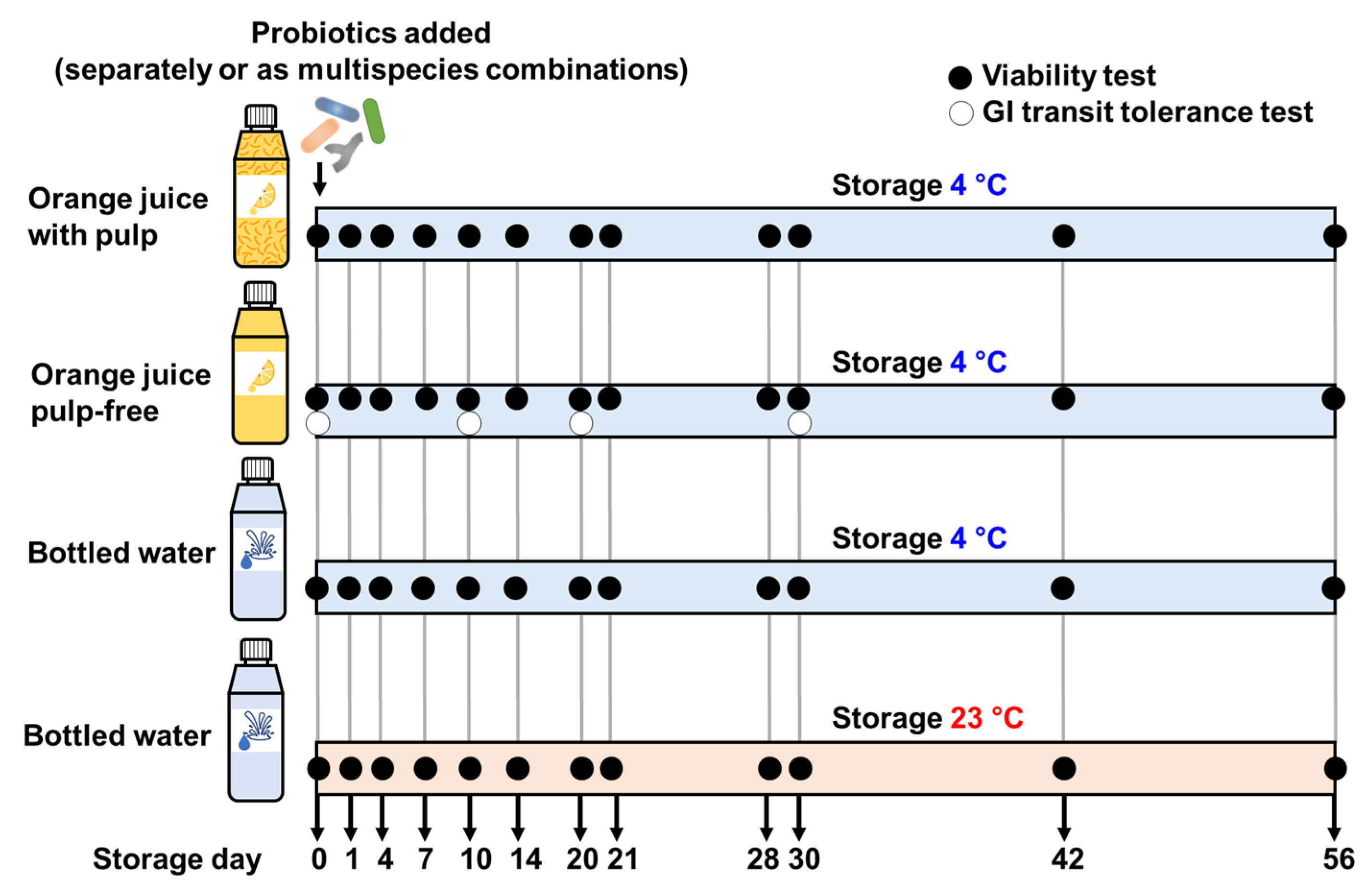
2.3. Viability of Probiotics in the Drinks
2.4. Measurement of pH, Titratable Acidity, and Brix of Drinks
2.5. In Vitro Gastrointestinal Transit Tolerance Assay
2.6. Statistical Analysis
3. Results
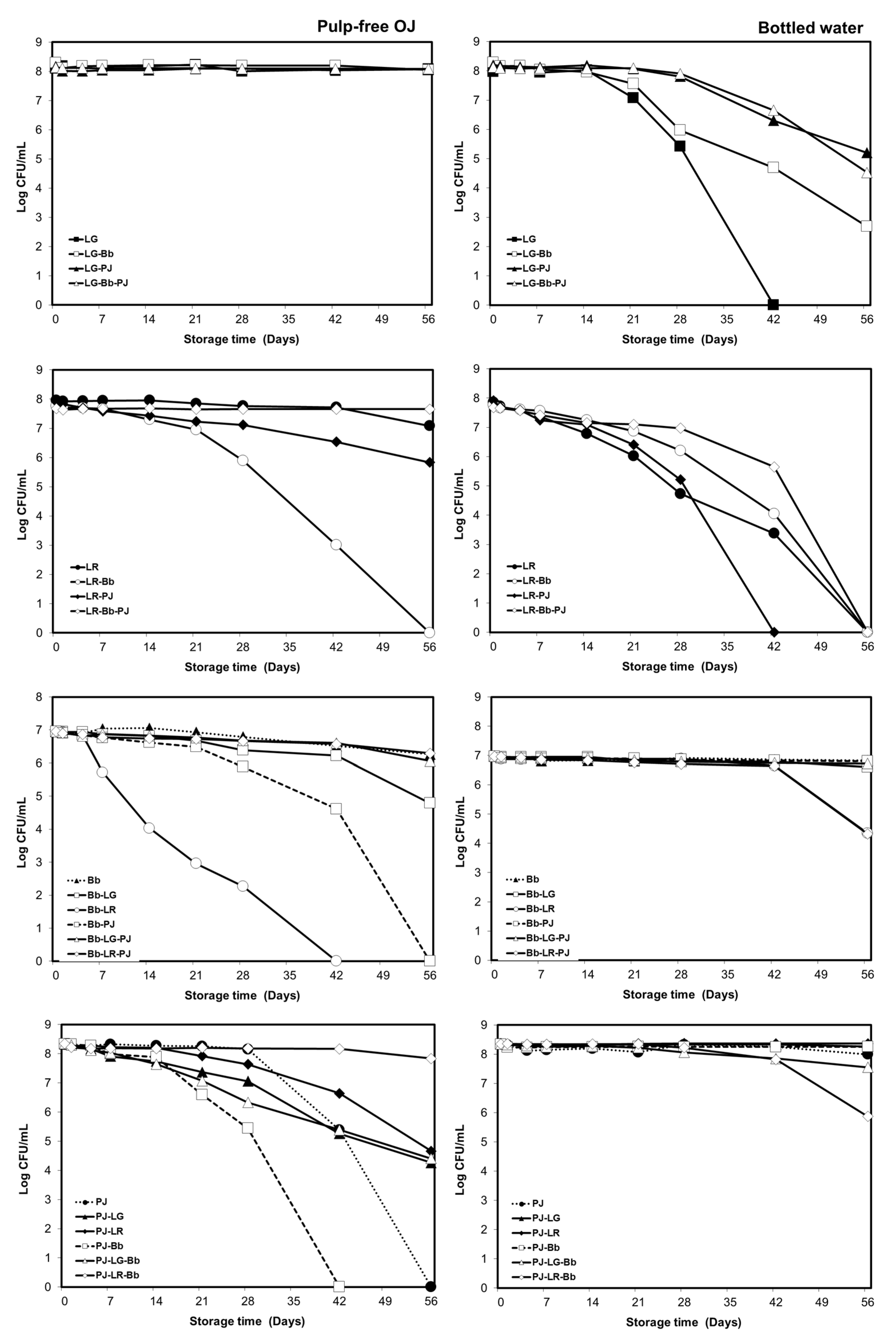
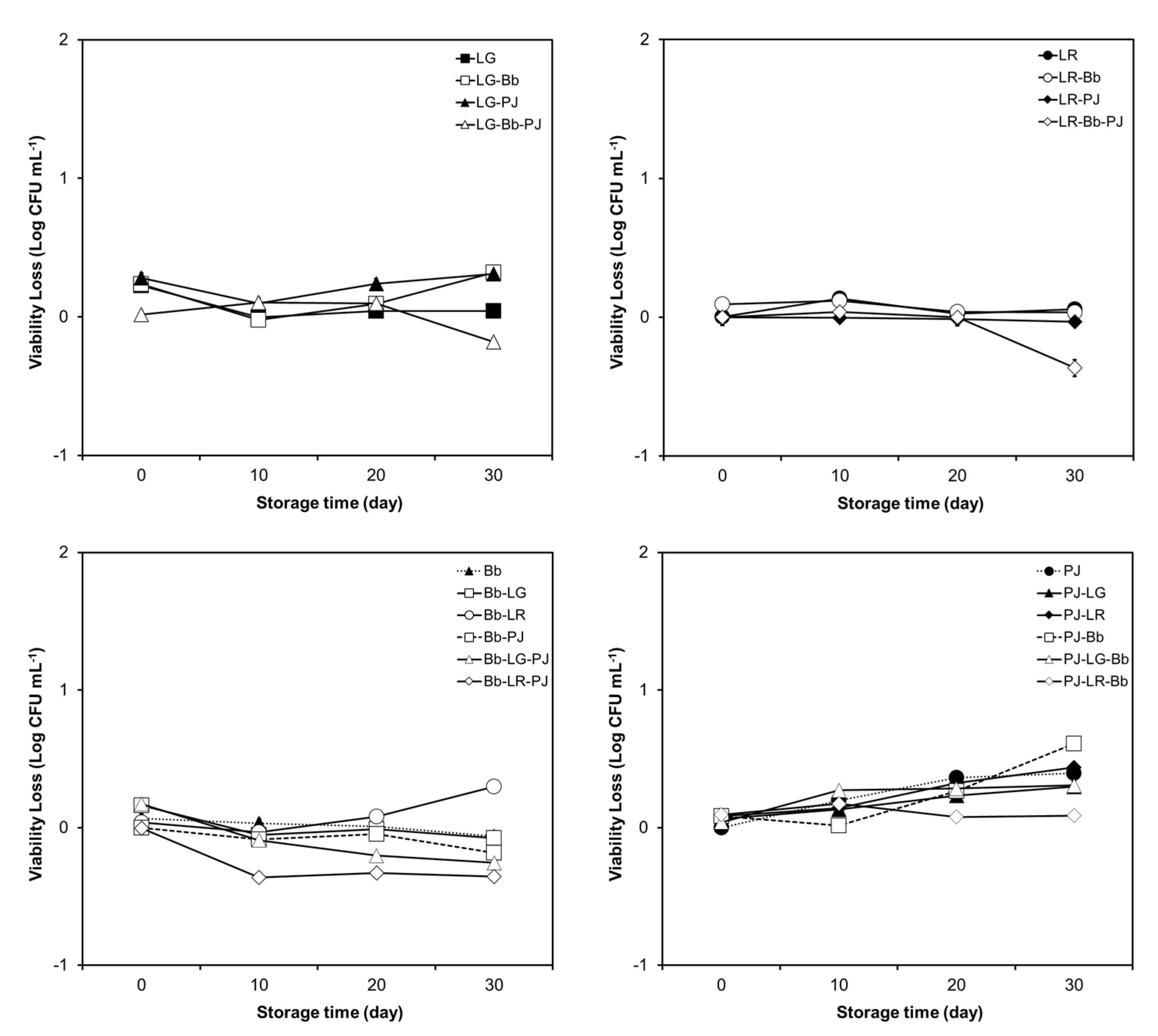
3.1. Viability of Probiotics in the Carrier Drinks during Storage
3.1.1. Orange Pulp and Probiotic Viability
3.1.2. Strain and Carrier Dependent Variation in Probiotic Viability
3.1.3. Storage Temperature and Probiotic Viability in Bottled Drinking Water
3.1.4. Viability of Individual Strains in Probiotic Combinations
3.2. pH and Brix Changes in Orange Juices
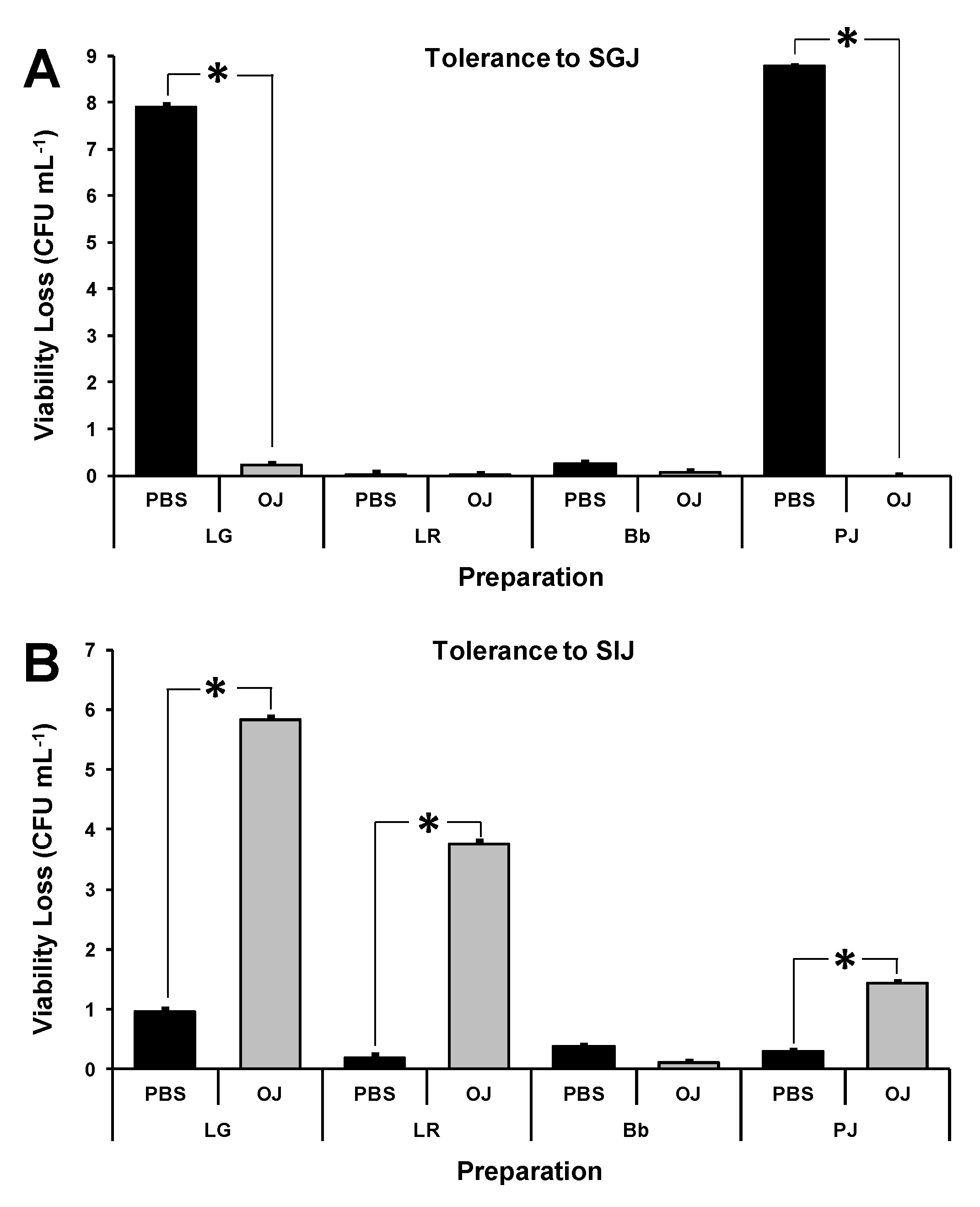
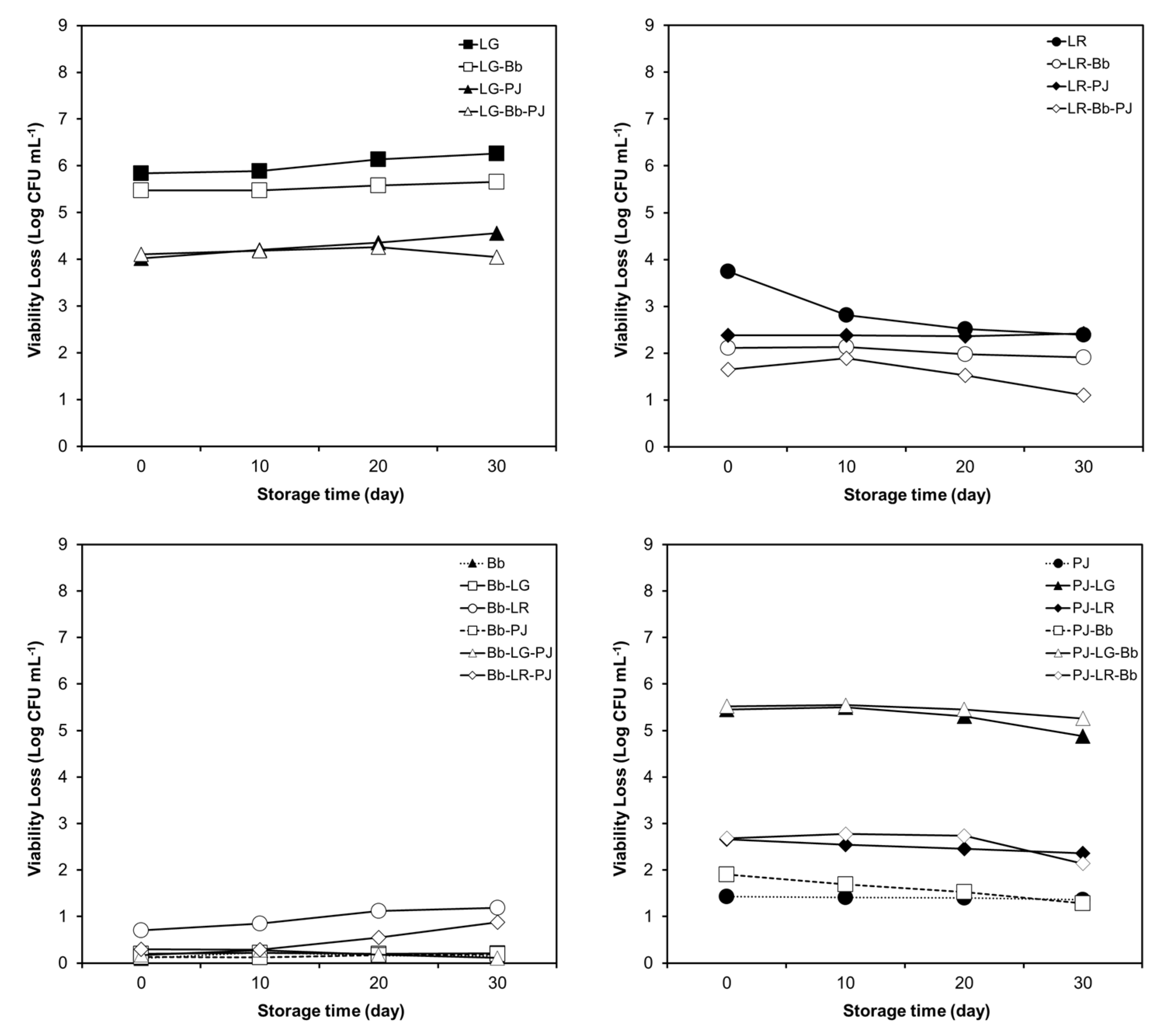
3.3. Effect of Pulp-Free Orange Juice on Simulated GI Tolerance of Probiotics
3.4. Effect of Probiotic Combinations and Storage in Pulp-Free OJ on Simulated GI Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.Q.; Shah, N.P. Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Burns, P.; Reinheimer, J. Probiotics in Nondairy Products. In Vegetarian and Plant-Based Diets in Health and Disease Prevention, 1st ed.; Mariotti, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 809–835. [Google Scholar]
- Vinderola, G.; Reinheimer, J.; Salminen, S. The enumeration of probiotic issues: From unavailable standardised culture media to a recommended procedure? Int. Dairy J. 2019, 96, 58–65. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Leonard, W.; Liang, A.; Ranadheera, C.S.; Fang, Z.; Zhang, P. Fruit juices as a carrier of probiotics to modulate gut phenolics and microbiota. Food Funct. 2022, 13, 10333–10346. [Google Scholar] [CrossRef]
- Charnchai, P.; Jantama, S.S.; Prasitpuriprecha, C.; Kanchanatawee, S.; Jantama, K. Effects of the Food Manufacturing Chain on the Viability and Functionality of Bifidobacterium Animalis through Simulated Gastrointestinal Conditions. PLoS ONE 2016, 11, e0157958. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gardner, N.J. Effect of storage in a fruit drink on subsequent survival of probiotic lactobacilli to gastro-intestinal stresses. Food Res. Int. 2008, 41, 539–543. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Gagnon, R. Viability of Lactobacillus rhamnosus R0011 in an apple-based fruit juice under simulated storage conditions at the consumer level. J. Food Sci. 2008, 73, M221–M226. [Google Scholar] [CrossRef]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. Lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef]
- Sagheddu, V.; Elli, M.; Biolchi, C.; Lucido, J.; Morelli, L. Impact of mode of assumption and food matrix on probiotic viability. J. Food Microbiol. 2018, 2, 1–6. [Google Scholar]
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How probiotics face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr. 2020, 60, 1552–1580. [Google Scholar] [CrossRef] [PubMed]
- Post, G. Probiotic fruit juice beverages. Fruit Process. 2002, 12, 212–217. [Google Scholar]
- Saarela, M.; Virkajarvi, I.; Alakomi, H.L.; Sigvart-Mattila, P.; Matto, J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int. Dairy J. 2006, 16, 1477–1482. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multi-strain Versus Single-strain Probiotics, Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- Mirandaa, R.F.; de Paulab, M.M.; da Costab, G.M.; Barãob, C.E.; da Silvac, A.C.R.; Raicesc, R.S.L.; Gomesa, R.G.; Pimentel, T.C. Orange juice added with L. casei: Is there an impact of the probiotic addition methodology on the quality parameters? LWT 2019, 106, 186–193. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Which juice is ‘healthier’? A consumer study of probiotic non-dairy juice drinks. Food Qual. Prefer. 2004, 15, 751–759. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Consumer acceptance of orange juice containing functional ingredients. Food Res. Int. 2004, 37, 805–814. [Google Scholar] [CrossRef]
- Kardooni, Z.; Alizadeh Behbahani, B.; Jooyandeh, H.; Noshad, M. Probiotic viability, physicochemical, and sensory properties of probiotic orange juice. Food Meas. 2023, 17, 1817–1822. [Google Scholar] [CrossRef]
- Malik, A.C.; Reinbold, G.W.; Vedamuthu, E.R. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 1968, 14, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Rogosa, M.; Mitchell, J.A.; Wiseman, R.F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J. Bacteriol. 1952, 62, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraj, N.; Shah, N.P. Selective enumeration of Lactobacillus delbrueckii ssp. Bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J. Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Solids (Soluble) in Citrus Fruit Juices: Refractometer Method.Section 983.17. In Official Method of Analysis, 15th ed.; Association of Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Huang, Y.; Adams, M.C. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 2004, 91, 253–260. [Google Scholar] [CrossRef]
- Basit, A.W.; Newton, J.M.; Short, M.D.; Waddington, W.A.; Ell, P.J.; Lacey, L.F. The effect of polyethylene glycol 400 on gastrointestinal transit: Implications for the formulation of poorly-water soluble drugs. Pharm. Res. 2001, 18, 1146–1150. [Google Scholar] [CrossRef]
- de Palencia, P.F.; Lopez, P.; Corbi, A.L.; Pelaez, C.; Requena, T. Probiotic strains: Survival under simulated gastrointestinal conditions, in vitro adhesion to Caco-2 cells and effect on cytokine secretion. Eur. Food Res. Technol. 2008, 227, 1475–1484. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 869–884. [Google Scholar]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Boylston, T.D.; Vinderola, C.G.; Ghoddusi, H.B.; Reinheimer, J.A. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int. Dairy J. 2004, 14, 375–387. [Google Scholar] [CrossRef]
- Moussavi, M.; Adams, M.C. An in vitro study on bacterial growth interactions and intestinal epithelial cell adhesion characteristics of probiotic combinations. Curr. Microbiol. 2010, 60, 327–335. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Ding, W.K.; Fallourd, M.J.; Leyer, G. Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. J. Food Sci. 2010, 75, M278–M282. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Schöpping, M.; Zeidan, A.A.; Franzén, C.J. Stress Response in Bifidobacteria. Microbiol. Mol. Biol. Rev. 2022, 86, e00170-21. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addition of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Development of new probiotics by strain combinations: Is it possible to improve the adhesion to intestinal mucus? J. Dairy Sci. 2007, 90, 2710–2716. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Isolauri, E.; Kirjavainen, P.V.; Tolkko, S.; Salminen, S.J. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. Delbrueckii subsp bulgaricus. Lett. Appl. Microbiol. 2000, 30, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Falentin, H.; Deutsch, S.M.; Jan, G.; Loux, V.; Thierry, A.; Parayre, S.; Maillard, M.B.; Dherbecourt, J.; Cousin, F.J.; Jardin, J.; et al. The complete genome of Propionibacterium freudenreichii CIRM-BIA1, a hardy actinobacterium with food and probiotic applications. PLoS ONE 2010, 5, e11748. [Google Scholar] [CrossRef]
- Callaway, T.R.; Carroll, J.A.; Arthington, J.D.; Pratt, C.; Edrington, T.S.; Anderson, R.C.; Galyean, M.L.; Ricke, S.C.; Crandall, P.; Nisbet, D.J. Citrus products decrease growth of E. coli O157:H7 and Salmonella typhimurium in pure culture and in fermentation with mixed ruminal microorganisms in vitro. Foodborne Pathog. Dis. 2008, 5, 621–627. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef] [PubMed]
- van de Casteele, S.; Vanheuverzwijn, T.; Ruyssen, T.; van Assche, P.; Swings, J.; Huys, G. Evaluation of culture media for selective enumeration of probiotic strains of lactobacilli and bifidobacteria in combination with yoghurt or cheese starters. Int. Dairy J. 2006, 16, 1470–1476. [Google Scholar] [CrossRef]
- Saarela, M.; Rantala, M.; Hallamaa, K.; Nohynek, L.; Virkajarvi, I.; Matto, J. Stationary-phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J. Appl. Microbiol. 2004, 96, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Rouault, A.; Maubois, J.L. Acid stress susceptibility and acid adaptation of Propionibacterium freudenreichii subsp shermanii. Dair Sci. Technol. 2000, 80, 325–336. [Google Scholar] [CrossRef]
- Leverrier, P.; Dimova, D.; Pichereau, V.; Auffray, Y.; Boyaval, P.; Jan, G. Susceptibility and adaptive response to bile salts in Propionibacterium freudenreichii: Physiological and proteomic analysis. Appl. Environ. Microbiol. 2003, 69, 3809–3818. [Google Scholar] [CrossRef]


| Probiotic(s) | Shelf-Life (Days) | |||
|---|---|---|---|---|
| Pulp Free OJ | OJ with Pulp | BW 4 °C | BW 23 °C | |
| LG | >56 | >56 | 21–28 | 4–7 |
| LR | >56 | 42–56 | 14–21 | 4–7 |
| Bb | >56 | >56 | >56 | 14–21 |
| PJ | 28–42 | 28–42 | >56 | >56 |
| LG-Bb | 42–56 | 28–42 | 28–42 | 0–4 |
| LG-PJ | 28–42 | 28–42 | 42–56 | 1–4 |
| LR-Bb | 7–14 | 7–14 | 28–42 | 4–7 |
| LR-PJ | 42–56 | 42–56 | 21–28 | 4–7 |
| Bb-PJ | 21–28 | 21–28 | >56 | 7–14 |
| LG-Bb-PJ | 42–56 | 42–56 | 42–56 | 4–7 |
| LR-Bb-PJ | >56 | 42–56 | 42–56 | 4–7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussavi, M.; Barouei, J.; Evans, C.; Adams, M.C.; Baines, S. Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water. Foods 2023, 12, 2249. https://doi.org/10.3390/foods12112249
Moussavi M, Barouei J, Evans C, Adams MC, Baines S. Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water. Foods. 2023; 12(11):2249. https://doi.org/10.3390/foods12112249
Chicago/Turabian StyleMoussavi, Mahta, Javad Barouei, Craig Evans, Michelle C. Adams, and Surinder Baines. 2023. "Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water" Foods 12, no. 11: 2249. https://doi.org/10.3390/foods12112249
APA StyleMoussavi, M., Barouei, J., Evans, C., Adams, M. C., & Baines, S. (2023). Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water. Foods, 12(11), 2249. https://doi.org/10.3390/foods12112249