Stability and Digestive Properties of a Dual-Protein Emulsion System Based on Soy Protein Isolate and Whey Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dual-Protein Emulsions
2.3. Particle Size and Zeta Potential Determination
2.4. Viscosity Determination
2.5. Determination of Emulsion Activity and Stability
2.6. Interfacial Adsorption Protein Determination
2.7. In-Vitro-Simulated Digestion
2.8. Determination of Free Fatty Acid Release
2.9. Determination of Oxidative Stability
2.10. Data Statistics and Analysis
3. Results and Discussion
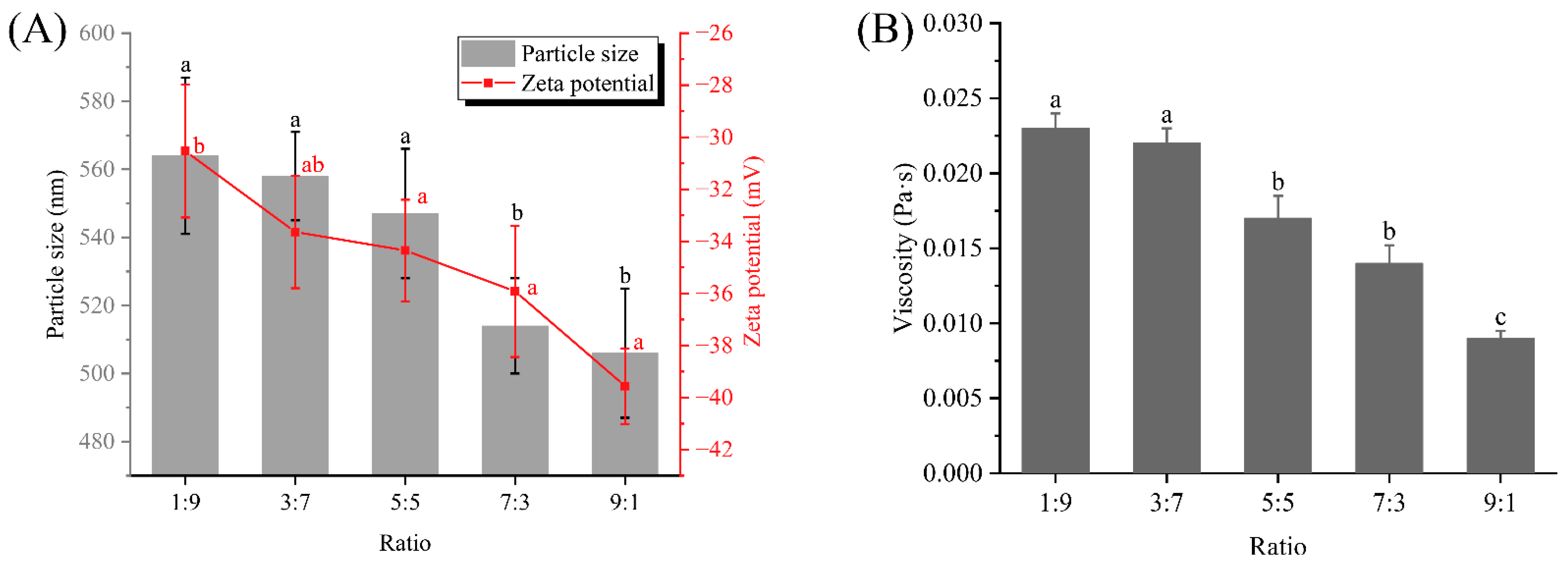
3.1. Particle Size, Zeta Potential and Viscosity Analysis
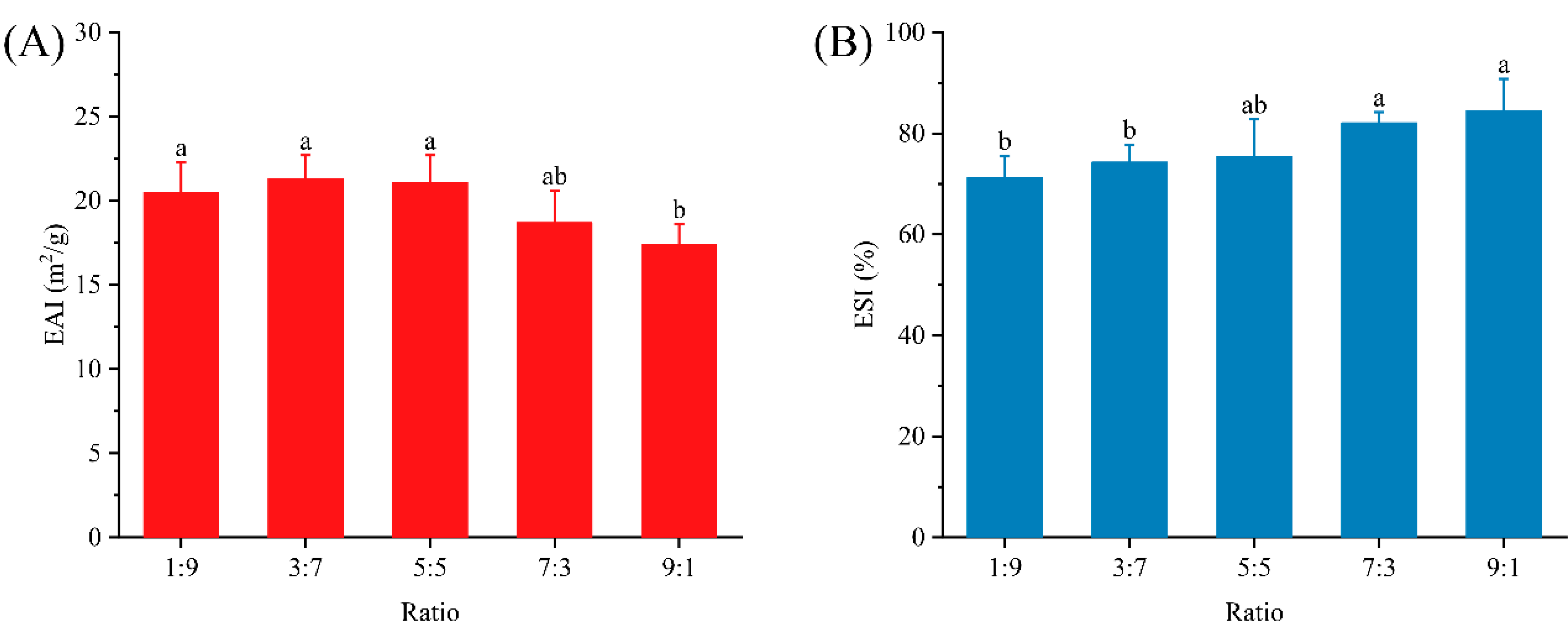
3.2. Emulsification Activity and Stability Analysis
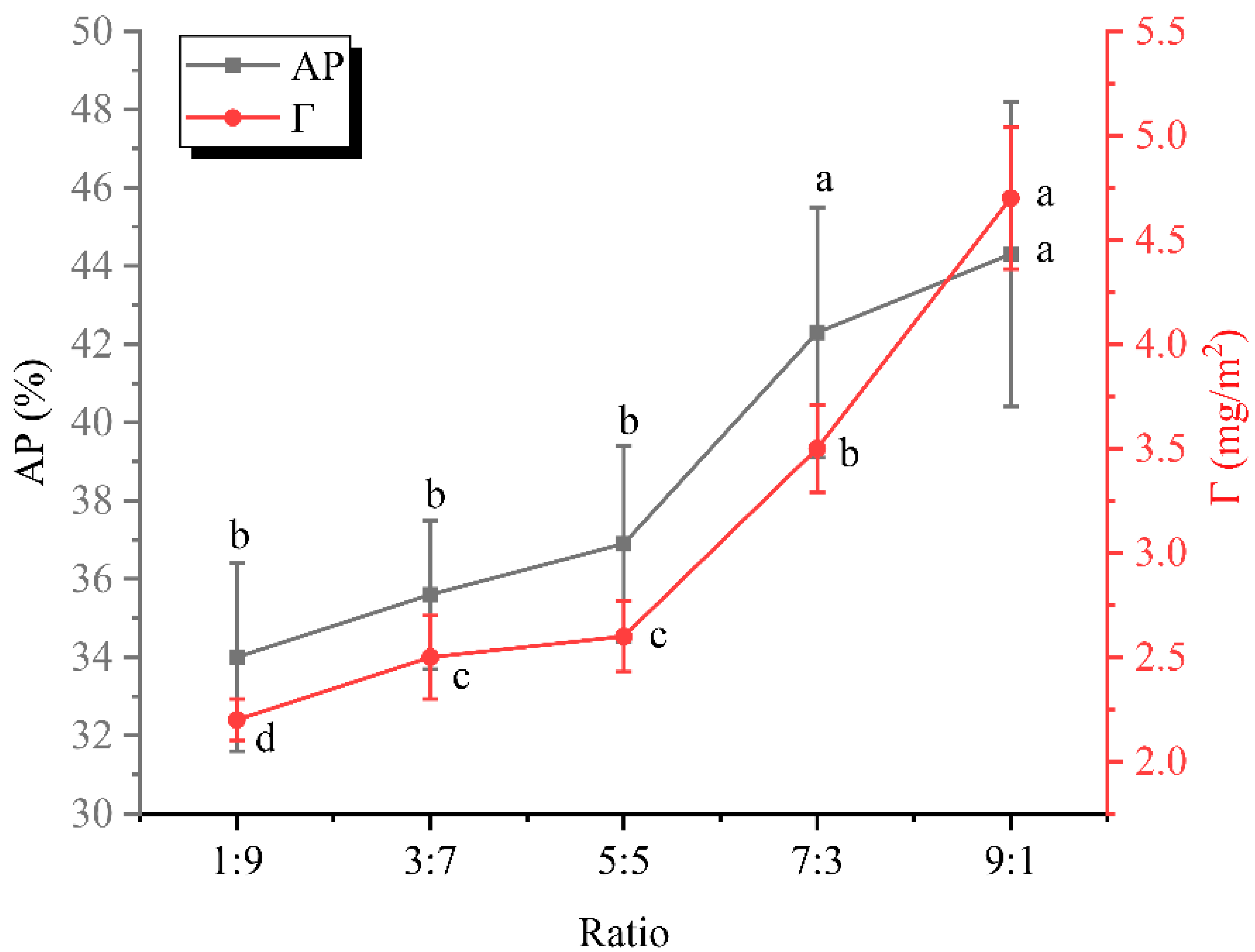
3.3. Interfacial Protein Adsorption Rate and Loading Analysis
3.4. Particle Size and Zeta Potential Analysis after In-Vitro-Simulated Digestion
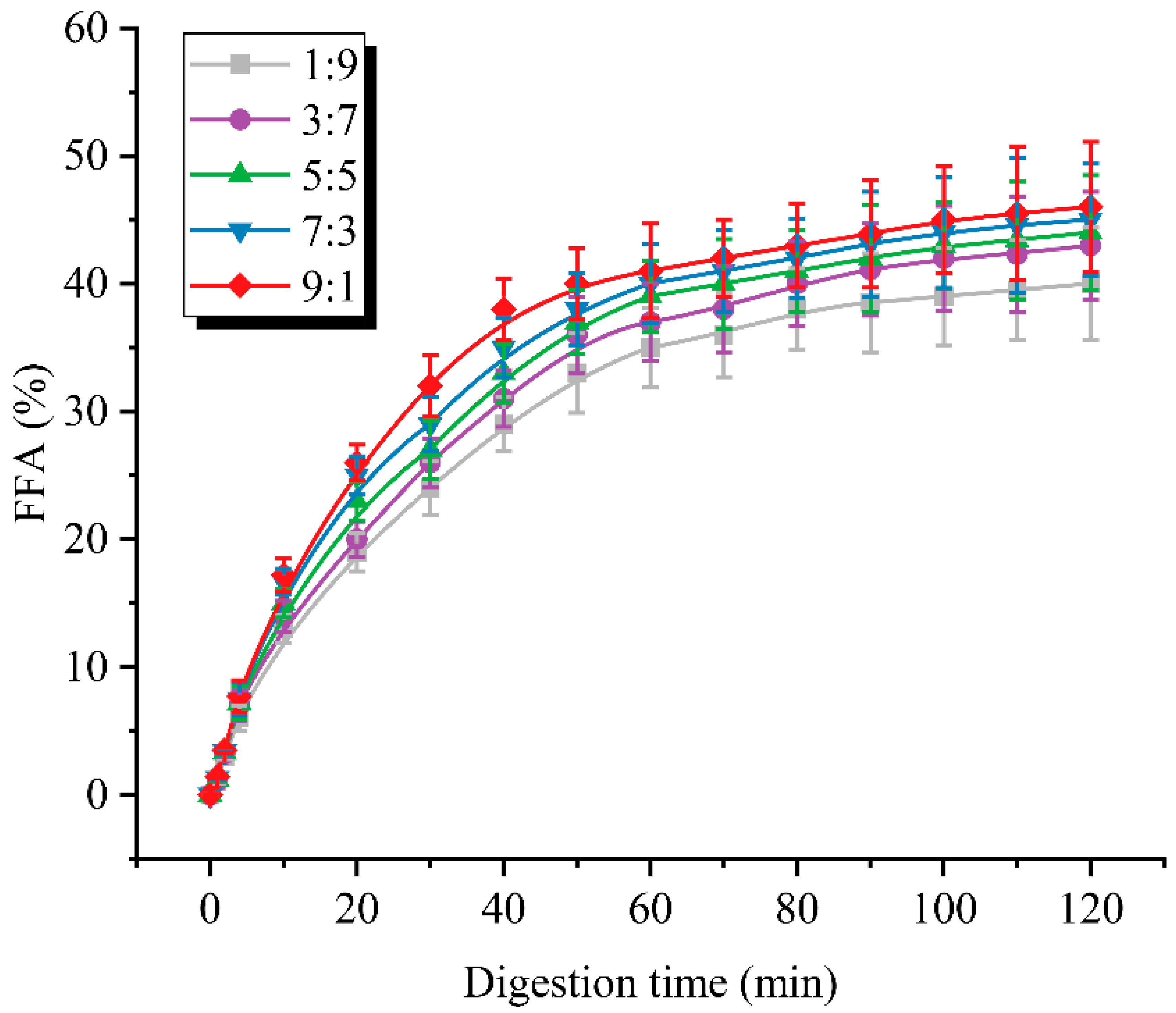
3.5. Free Fatty Acid Release Curve Analysis
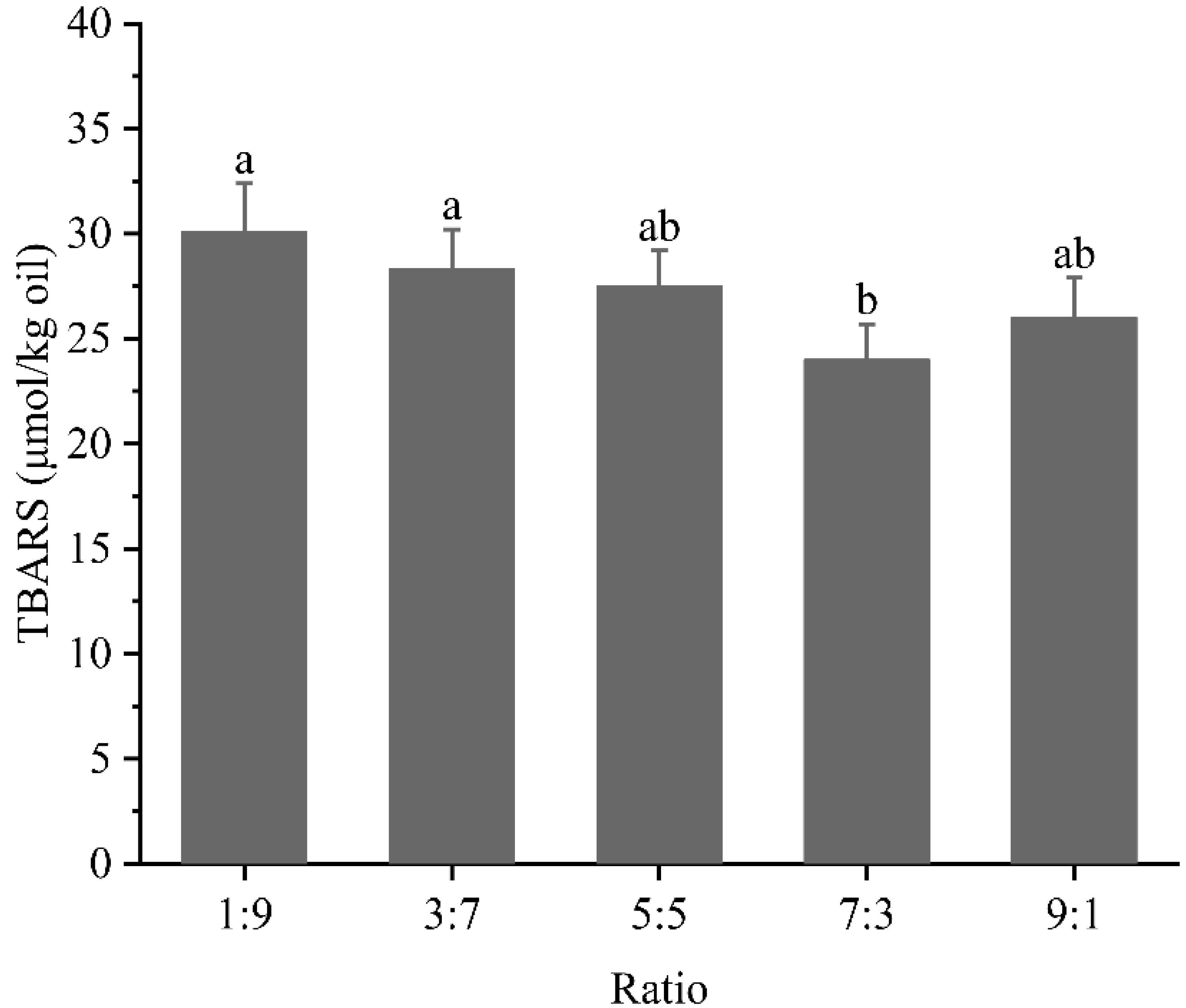
3.6. Oxidation Stability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarahi, M.; Ahmed, J. Recent advances in legume protein-based colloidal systems. Legume Sci. 2023, e185. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhao, L.; Zhou, X.; Cao, W.; Zhou, C. Effect of pre-emulsion of pea-grass carp co-precipitation dual protein on the gel quality of fish sausage. Foods 2022, 11, 3192. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F. Progress in the application of food-grade emulsions. Foods 2022, 11, 2883. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, F.; Han, L.; Li, L.; Jiang, L.; Qi, B.; Li, Y. Soy/whey protein isolates: Interfacial properties and effects on the stability of oil-in-water emulsions. J. Sci. Food Agric. 2021, 101, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Martha, L.; Casanova, F.; Tavares, G. Structural and foaming properties of whey and soy protein isolates in mixed systems before and after heat treatment. Food Sci. Technol. Int. 2022, 28, 545–553. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Tan, Y.; Muriel Mundo, J.L.; McClements, D.J. Comparison of plant-based emulsifier performance in water-in-oil-in-water emulsions: Soy protein isolate, pectin and gum Arabic. J. Food Eng. 2021, 307, 110625. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Shi, Y.; Akhtar, M.; Chen, J.; Ettelaie, R. Emulsifying and emulsion stabilizing properties of soy protein hydrolysates, covalently bonded to polysaccharides: The impact of enzyme choice and the degree of hydrolysis. Food Hydrocoll. 2021, 113, 106519. [Google Scholar] [CrossRef]
- Cheng, Y.; Ofori Donkor, P.; Yeboah, G.B.; Ayim, I.; Wu, J.; Ma, H. Modulating the in vitro digestion of heat-set whey protein emulsion gels via gelling properties modification with sequential ultrasound pretreatment. LWT 2021, 149, 111856. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Tomé, A.S.; Pires, C.; Batista, I.; Sousa, I.; Raymundo, A. Protein gels and emulsions from mixtures of Cape hake and pea proteins. J. Sci. Food Agric. 2015, 95, 289–298. [Google Scholar] [CrossRef]
- Santiago, L.; Carrara, C.; González, R. Interaction of Soy Protein Isolate and Meat Protein in a Model Emulsion System. Effect of Emulsification Order and Characteristics of Soy Isolate Used. Food Sci. Technol. Int. 2005, 11, 79–88. [Google Scholar] [CrossRef]
- Wang, T.; Li, F.; Zhang, H.; Feng, W.; Wang, R. Plant-based high internal phase emulsions stabilized by dual protein nanostructures with heat and freeze–thaw tolerance. Food Chem. 2022, 373, 131458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, M.; Xie, F.; Sun, Y.; Zhang, S.; Qi, B. The effect of pH on the stabilization and digestive characteristics of soybean lipophilic protein oil-in-water emulsions with hypromellose. Food Chem. 2020, 309, 125579. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Tang, C. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. LWT 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Liu, N.; Li, N.; Faiza, M.; Li, D.; Yao, X.; Zhao, M. Stability and in vitro digestion of high purity diacylglycerol oil-in-water emulsions. LWT 2021, 148, 111744. [Google Scholar] [CrossRef]
- Keramat, M.; Ehsandoost, E.; Golmakani, M.-T. Recent trends in improving the oxidative stability of oil-based food products by inhibiting oxidation at the interfacial region. Foods 2023, 12, 1191. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Hu, T.; Xu, X.; Pan, S.; Hu, H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason. Sonochem. 2019, 58, 104627. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.; Xie, F.; Hu, M.; Sun, Y.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 113, 106391. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Schroën, K.; San Martín-González, M.F.; Berton-Carabin, C.C. Synergistic and antagonistic effects of plant and dairy protein blends on the physicochemical stability of lycopene-loaded emulsions. Food Hydrocoll. 2018, 81, 180–190. [Google Scholar] [CrossRef]
- Ahn, N.; Imm, J.-Y. Effect of phospholipid matrix on emulsion stability, microstructure, proteolysis, and in vitro digestibility in model infant formula emulsion. Food Res. Int. 2023, 163, 112218. [Google Scholar] [CrossRef] [PubMed]
- Euston, S.; Al-Bakkush, A.; Campbell, L. Comparing the heat stability of soya protein and milk whey protein emulsions. Food Hydrocoll. 2009, 23, 2485–2492. [Google Scholar] [CrossRef]
- Zhai, J.; Wooster, T.J.; Hoffmann, S.V.; Lee, T.-H.; Augustin, M.A.; Aguilar, M.-I. Structural Rearrangement of β-Lactoglobulin at Different Oil–Water Interfaces and Its Effect on Emulsion Stability. Langmuir 2011, 27, 9227–9236. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Li, Q.; He, S.; Xu, W.; Peng, F.; Gu, C.; Wang, R.; Ma, Y. Formation, Stability and In Vitro Digestion of β-carotene in Oil-in-Water Milk Fat Globule Membrane Protein Emulsions. Food Biophys. 2018, 13, 198–207. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Feng, J.; Schroën, K.; Fogliano, V.; Berton-Carabin, C. Antioxidant potential of non-modified and glycated soy proteins in the continuous phase of oil-in-water emulsions. Food Hydrocoll. 2021, 114, 106564. [Google Scholar] [CrossRef]
- Faraji, H.; McClements, D.J.; Decker, E.A. Role of Continuous Phase Protein on the Oxidative Stability of Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2004, 52, 4558–4564. [Google Scholar] [CrossRef]
- Kiokias, S.; Gordon, M.; Oreopoulou, V. Effects of composition and processing variables on the oxidative stability of protein-based and oil-in-water food emulsions. Crit. Rev. Food Sci. Nutr. 2017, 57, 549–558. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.-T.; Liu, J.-X.; Gao, X.; Zhang, G.-Q.; Tang, X.-Z. Stability and Digestive Properties of a Dual-Protein Emulsion System Based on Soy Protein Isolate and Whey Protein Isolate. Foods 2023, 12, 2247. https://doi.org/10.3390/foods12112247
Gao T-T, Liu J-X, Gao X, Zhang G-Q, Tang X-Z. Stability and Digestive Properties of a Dual-Protein Emulsion System Based on Soy Protein Isolate and Whey Protein Isolate. Foods. 2023; 12(11):2247. https://doi.org/10.3390/foods12112247
Chicago/Turabian StyleGao, Ting-Ting, Jing-Xue Liu, Xin Gao, Guo-Qi Zhang, and Xiao-Zhi Tang. 2023. "Stability and Digestive Properties of a Dual-Protein Emulsion System Based on Soy Protein Isolate and Whey Protein Isolate" Foods 12, no. 11: 2247. https://doi.org/10.3390/foods12112247
APA StyleGao, T.-T., Liu, J.-X., Gao, X., Zhang, G.-Q., & Tang, X.-Z. (2023). Stability and Digestive Properties of a Dual-Protein Emulsion System Based on Soy Protein Isolate and Whey Protein Isolate. Foods, 12(11), 2247. https://doi.org/10.3390/foods12112247



