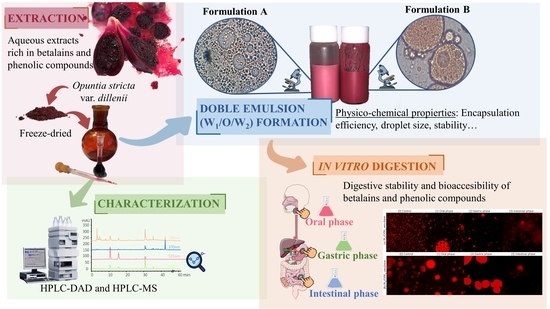
Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccessibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Obtaining OPD’s Betalain- and Phenolic-Rich Green Extracts
2.4. Characterization of Betalains and Phenolic Compounds on OPD Green Extracts
2.5. Formation of Double Emulsion Systems W1/O/W2
2.5.1. Preparation of Formulation A
2.5.2. Preparation of Formulation B
2.6. In Vitro Gastro-Intestinal Digestion
concentration in the fruit) × 100.
2.7. Characteristics of Double Emulsions (W1/O/W2)
2.7.1. Encapsulation Efficiency
2.7.2. Droplet Size and Distribution
2.7.3. Zeta Potential
2.7.4. Physical Stability by Optical Inspection
2.7.5. Optical Microscopy
2.7.6. Confocal Laser Scanning Microscopy
2.7.7. Colour Measurement
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composition of Opuntia stricta var. dillenii (OPD) Green Extract
3.2. Characterization of Double Emulsions (W1/O/W2)
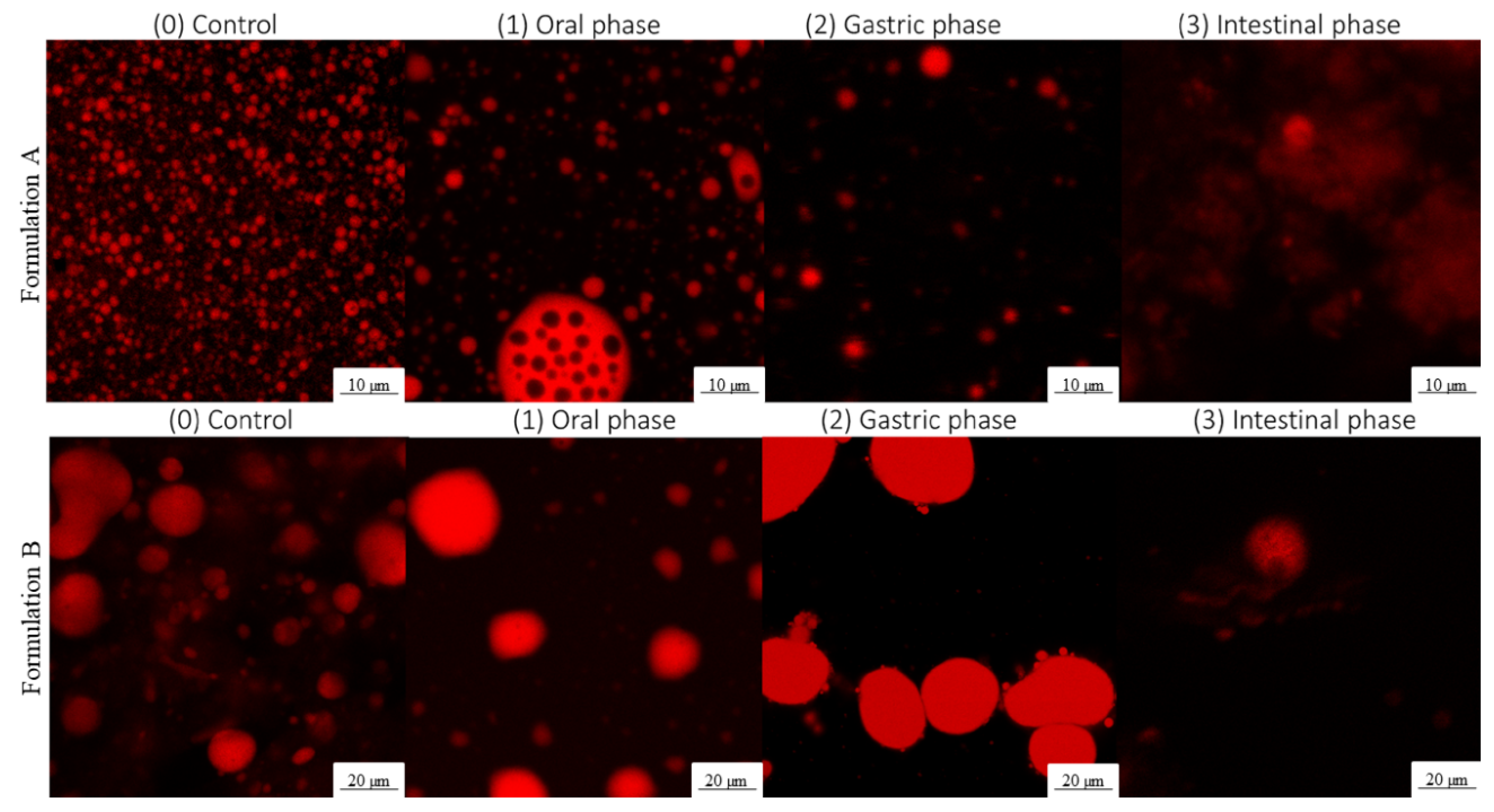
3.2.1. Droplet Morphology
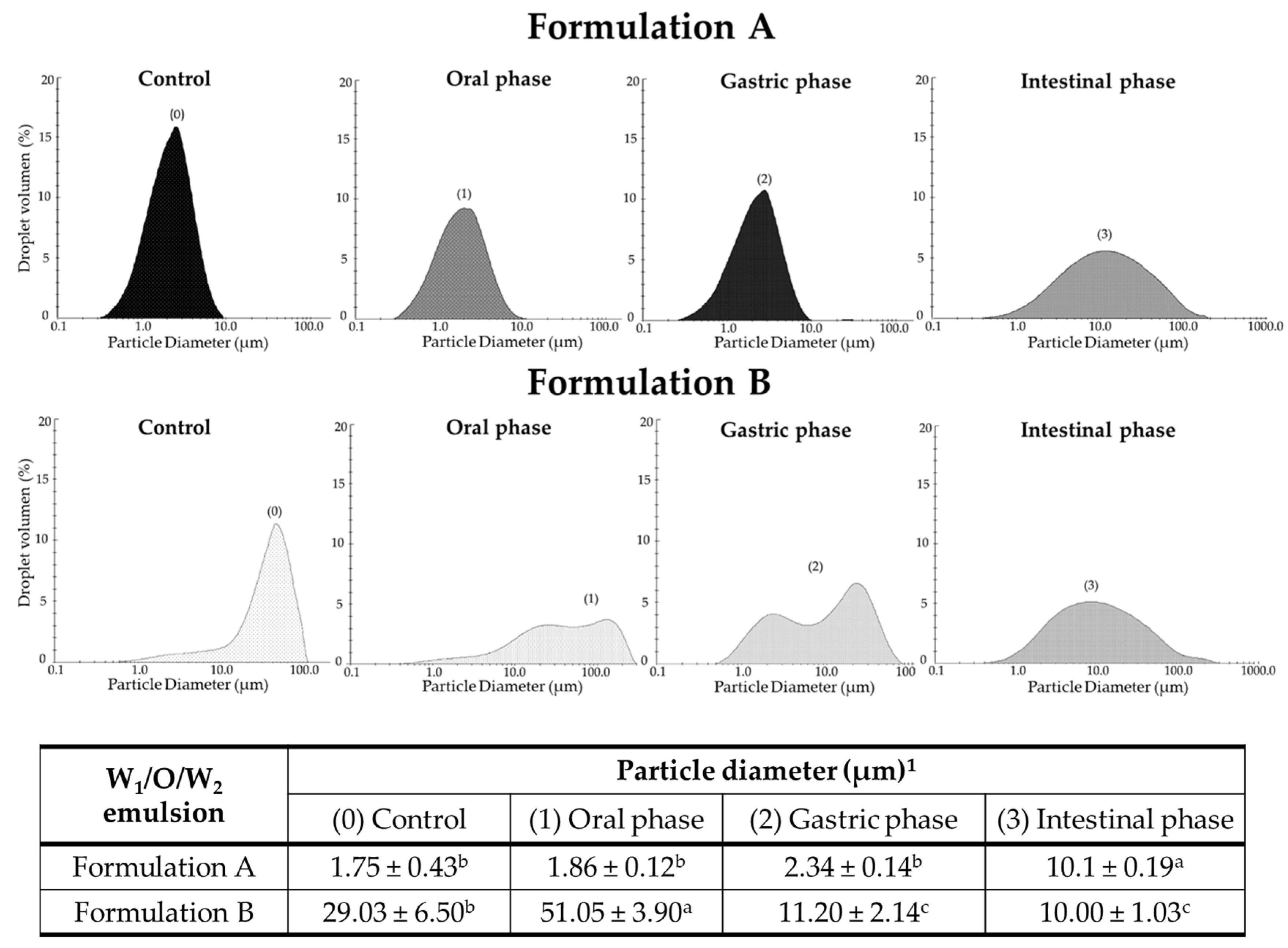
3.2.2. Droplet Size Distribution and Zeta Potential
3.2.3. Encapsulation Efficiency
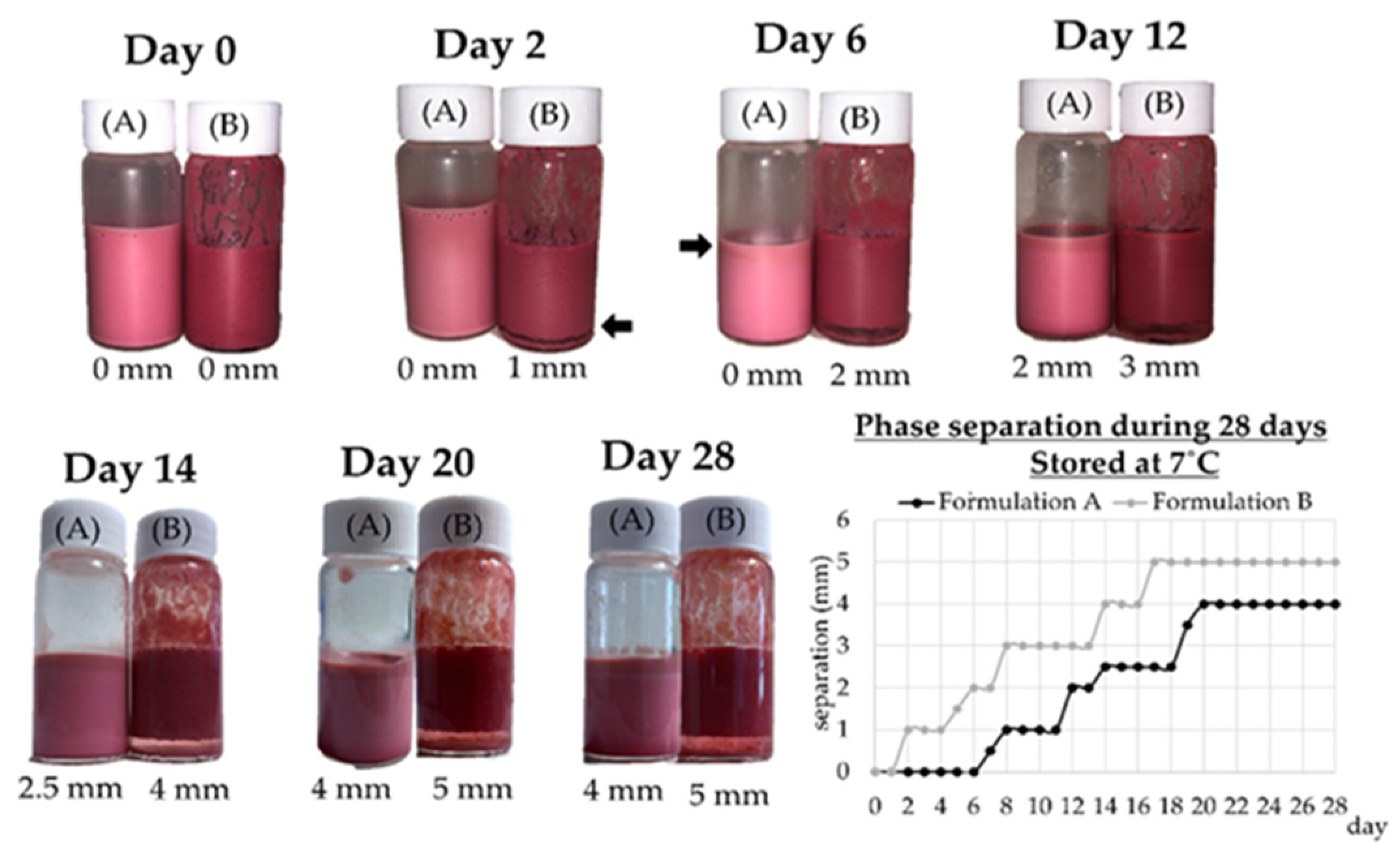
3.2.4. Physical Stability
3.3. In Vitro Gastro-Intestinal Digestion of Double Emulsions (W1/O/W2)
3.3.1. Microstructure of W1/O/W2 Formulations during In Vitro Gastro-Intestinal Digestion
3.3.2. Digestive Stability and Bioaccessibility of Betalains and Phenolic Compounds Encapsulated by Double Emulsions (W1/O/W2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreu-Coll, L.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Rocamora-Montiel, B.; Legua, P.; Hernández, F.; López-Lluch, D. Economic estimation of cactus pear production and its feasibility in Spain. Trends Food Sci. Technol. 2020, 103, 379–385. [Google Scholar] [CrossRef]
- Snyman, H.A. Growth Rate and Water-Use Efficiency of Cactus Pears Opuntia ficus-indica and O. robusta. Arid Land Res. Manag. 2013, 27, 337–348. [Google Scholar] [CrossRef]
- Habtemariam, S. Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 435–472. [Google Scholar]
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia dillenii: A Forgotten Plant with Promising Pharmacological Properties. JoP 2019, 22, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. dillenii Fruits and Products of Their Industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, I.; Mendiola, J.A.; Portillo, M.P.; Cano, M.P. Pressurized green liquid extraction of betalains and phenolic compounds from Opuntia stricta var. dillenii whole fruit: Process optimization and biological activities of green extracts. IFSET 2022, 80, 103066. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Soccio, M.; Cano, M.P. In Vitro Antioxidant Capacity of Opuntia spp. Fruits Measured by the LOX-FL Method and its High Sensitivity Towards Betalains. Plant Foods Hum Nutr. 2021, 76, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- González-Arceo, M.; Gómez-López, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int. J. Mol. Sci. 2022, 24, 299. [Google Scholar] [CrossRef]
- Del Socorro Santos-Díaz, M.; De La Rosa, A.P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Fernández-Repetto, A.; Gómez-Maqueo, A.; García-Cayuela, T.; Guajardo-Flores, D.; Cano, M.P. Analysis of hydrocolloid excipients for controlled delivery of high-value microencapsulated prickly pear extracts. FHFH 2023, 3, 100115. [Google Scholar] [CrossRef]
- Carreón-Hidalgo, J.P.; Franco-Vásquez, D.C.; Gómez-Linton, D.R.; Pérez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2021, 151, 110821. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, W.; Yong, C.; Lan, Y.; Huang, Q.; Xiao, J. Effects of the Distribution Site of Crystallizable Emulsifiers on the Gastrointestinal Digestion Behavior of Double Emulsions. J. Agric. Food Chem. 2022, 70, 5115–5125. [Google Scholar] [CrossRef]
- Robert, P.; Vergara, C.; Silva-Weiss, A.; Osorio, F.A.; Santander, R.; Sáenz, C.; Giménez, B. Influence of gelation on the retention of purple cactus pear extract in microencapsulated double emulsions. PLoS ONE 2020, 15, e0227866. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Ultrasound-Assisted “Green” Extraction (UAE) of Antioxidant Compounds (Betalains and Phenolics) from Opuntia stricta var. dillenii’s Fruits: Optimization and Biological Activities. Antioxidants 2021, 10, 1786. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. JFF 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT-Food Sci. Technol. 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Casanova, F.Y.H.; Cardona, C.T.S. Emulsiones o/w estabilizadas con caseinato de sodio: Efecto de los iones calcio, concentración de proteína y temperatura. Vitae-Rev. Fac. Quim. Farm. 2009, 11, 13–19. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive stability and bioaccessibility of antioxidants in prickly pear fruits from the Canary Islands: Healthy foods and ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT-Food Sci. Technol. 2014, 59, 621–628. [Google Scholar] [CrossRef]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of xylitol by double emulsion followed by complex coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. JCR 2019, 295, 31–49. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Estefes-Duarte, J.A.; Afanador-Barajas, L.N.; Fernández-Luqueño, F.; Zepeda-Velázquez, A.P.; Franco-Fernández, M.J.; Peláez-Acero, A.; Campos-Montiel, R.G. Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions. Molecules 2020, 25, 5736. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; Taylor & Francis: Oxfordshire, UK, 2002; pp. 355–391. [Google Scholar]
- Stang, M.; Karbstein, H.; Schubert, H. Adsorption kinetics of emulsifiers at oil—Water interfaces and their effect on mechanical emulsification. Chemical. Eng. Process. 1994, 33, 307–311. [Google Scholar] [CrossRef]
- Kamble, S.B.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- De Campo, C.; Dick, M.; Santos, P.R.D.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; De Oliveira Rios, A.; Flôres, S.H. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. 2013, 12, 265–273. [Google Scholar]
- Ribeiro, R.C.D.A.; Barreto, S.M.D.A.G.; Ostrosky, E.A.; Rocha-Filho, P.A.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, E. Characterisation and storage stability of aronia anthocyanins encapsulated with combinations of maltodextrin with carboxymethyl cellulose, gum Arabic, and xanthan gum. Food Chem. 2023, 405, 135002. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Silva, H.D.; Soliva-Fortuny, R.; Martín-Belloso, O.; Vicente, A.A. Formation, stability and antioxidant activity of food-grade multilayer emulsions containing resveratrol. Food Hydrocoll. 2017, 71, 207–215. [Google Scholar] [CrossRef]
- Espert, M.; Borreani, J.; Hernando, I.; Quiles, A.; Salvador, A.; Sanz, T. Relationship between cellulose chemical substitution, structure and fat digestion in o/w emulsions. Food Hydrocoll. 2017, 69, 76–85. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Pena, M.P. Digestibility of (poly) phenols and antioxidant activity in raw and cooked cactus cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef]
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of micronutrients, bioaccessibility and antioxidant activity of prickly pear cladodes as functional ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef]


| Type of Emulsion | Reagent and Solutions 1 | ||
|---|---|---|---|
| Formulation A W1/O/W2 | Primary emulsion (W1/O) 25% | Aqueous phase (W1) 25% | NaCl (0.1 M) |
| Glycerol | |||
| OPD extract | |||
| Oil phase (O) 75% | MCT oil | ||
| PGPR | |||
| Aqueous phase (W2) 75% | NaCl (0.1 M) | ||
| Tween 20 (2%) | |||
| Formulation B W1/O/W2 | Primary emulsion (W1/O) 30% | Aqueous phase (W1) 30% | Gelatine 6% in TRIS buffer (pH 9) |
| OPD extract | |||
| Oil phase (O) 70% | MCT oil | ||
| PC | |||
| PGPR | |||
| Aqueous phase (W2) 70% | Caseinate at 3% | ||
| NaCl 10% in glycerol | |||
| Guar gum | |||
| Acacia gum (Arabic) | |||
| Peak 1 | Compound | Content (mg/g Dry Weight) 2 | ||
|---|---|---|---|---|
| Control 3 | Formulation A 4 | Formulation B 4 | ||
| 1 | Piscidic acid | 7.29 ± 0.05 | 2.00 ± 0.04 a | 1.98 ± 0.16 a |
| 2 | Betanin | 5.41 ± 0.9 | 2.91 ± 0.22 a | 2.83 ± 0.08 a |
| 3 | Isobetanin | 3.71 ± 0.02 | 1.79 ± 0.02 a | 1.95 ± 0.15 a |
| 4 | Neobetanin | 0.66 ± 0.01 | 0.36 ± 0.06 b | 0.47 ± 0.06 a |
| 5 | Quercetin-3-O-rhamnosyl-rutinoside (QG3) | 0.13 ± 0.01 | 0.09 ± 0.02 a | 0.04 ± 0.02 b |
| 6 | Quercetin hexosyl pentosyl rhamnoside (QG1) | 0.13 ± 0.0 | 0.05 ± 0.02 a | 0.03 ± 0.0 a |
| 7 | Quercetin hexose pentoside (QG2) | 0.17 ± 0.0 | 0.13 ± 0.02 a | 0.05 ± 0.02 b |
| 8 | Isorhamnetin glucoxyl-rhamnosyl-rhamnoside (IG1) | 0.05 ± 0.0 | 0.01 ± 0.0 a | 0.01 ± 0.0 a |
| 9 | Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | 0.47 ± 0.05 | 0.19 ± 0.02 b | 0.31 ± 0.04 a |
| Encapsulation Efficiency (%) 1 | ||
|---|---|---|
| Compound | Formulation A 2 | Formulation B 2 |
| BETALAINS | ||
| Betanin | 96.9 ± 3.3 a | 94.2 ± 2.5 a |
| Isobetanin | 92.2 ± 0.1 a | 98 ± 3.9 a |
| Neobetanin | 73.7 ± 6.7 b | 95.2 ± 3.2 a |
| PHENOLIC ACIDS | ||
| Piscidic acid | 71 ± 1.3 a | 70.2 ± 5.7 a |
| FLAVONOIDS | ||
| Quercetin-3-O-rhamnosyl-rutinoside (QG3) | 87.7 ± 3.1 a | 88.5 ± 1.5 a |
| Quercetin hexosyl pentosyl rhamnoside (QG1) | 89.7 ± 3.2 a | 81.4 ± 1.5 b |
| Isorhamnetin glucoxyl–rhamnosyl–rhamnoside (QG2) | 84.4 ± 6.2 a | 68.2 ± 5.9 b |
| Isorhamnetin glucoxyl–rhamnosyl–rhamnoside (IG1) | 89.9 ± 1.4 a | 91.7 ± 3.4 a |
| Isorhamnetin glucoxyl–rhamnosyl–pentoside (IG2) | 74.2 ± 3.6 b | 95.9 ± 7.7 a |
| Total Betalains | 93.2 ± 2.2 a | 96.6 ± 1.8 a |
| Total Phenolic Acids | 71 ± 1.3 a | 70.2 ± 5.7 a |
| Total Flavonoids | 85.8 ± 2.7 a | 91.5 ± 7.9 a |
| Stability (mg Comp./g of Dry Extract) 1 | Bioaccessibility (%) 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | In Vitro Phase | Extract 1 | Formulation A 2 | Formulation B 3 | Control 1 | Formulation A 2 | Formulation B 3 | ||||
| w/o Lipase | w/ Lipase | w/o Lipase | w/ Lipase | w/o Lipase | w/ Lipase | w/o Lipase | w/ Lipase | ||||
| BETALAINS | |||||||||||
| Betanin | Control | 5.41 ± 0.09 | 1.39 ± 0.23 Ab | 1.41 ± 0.3 Ab | 2.88 ± 0.08 Aa | 2.79 ± 0.2 Aa | - | - | - | - | - |
| Oral | 4.91 ± 0.02 | 1.73 ± 0.28 Ab | 1.46 ± 0.05 Ab | 3.20 ± 0.14 Aa | 2.55 ± 0.22 Ba | - | - | - | - | - | |
| Gastric | 4.67 ± 0.12 | 1.54 ± 0.06 Ab | 1.15 ± 0.08 Bb | 2.01 ± 0.04 Aa | 2.10 ± 0.58 Aa | - | - | - | - | - | |
| Intestinal | 1.89 ± 0.01 | 1.31 ± 0.19 Ab | 0.99 ± 0.2 Bb | 1.89 ± 0.05 Aa | 1.59 ± 0.05 Aa | 35.3 ± 0.2 | 93.4 ± 0.9 Aa | 70.8 ± 1.8 Ba | 67.1 ± 0.1 Ab | 56.3 ± 0.2 Bb | |
| Isobetanin | Control | 3.71 ± 0.02 | 1.1 ± 0.17 Ab | 1.08 ± 0.1 Ab | 1.96 ± 0.08 Aa | 1.95 ± 0.1 Aa | - | - | - | - | - |
| Oral | 2.97 ± 0.01 | 1.39 ± 0.05 Ab | 0.96 ± 0.18 Bb | 2.03 ± 0.03 Aa | 1.72 ± 0.22 Ba | - | - | - | - | - | |
| Gastric | 2.56 ± 0.0 | 1.04 ± 0.06 Ab | 0.79 ± 0.0 Bb | 1.34 ± 0.08 Aa | 1.31 ± 0.27 Aa | - | - | - | - | - | |
| Intestinal | 1.12 ± 0.0 | 0.88 ± 0.15 Ab | 0.66 ± 0.09 Bb | 1.32 ± 0.07 Aa | 1.08 ± 0.01 Ba | 30.1 ± 0.1 | 90.9 ± 0.9 Aa | 68.1 ± 0.6 Ba | 67.7 ± 1 Ab | 55.8 ± 3.6 Bb | |
| Neobetanin | Control | 0.66 ± 0.01 | 0.25 ± 0.01 Ab | 0.23 ± 0.01 Ab | 0.5 ± 0.1 Aa | 0.47 ± 0.06 Aa | - | - | - | - | - |
| Oral | 0.35 ± 0.0 | 0.17 ± 0.02 Ab | 0.19 ± 0.01 Aa | 0.28 ± 0.01 Aa | 0.18 ± 0.02 Ba | - | - | - | - | - | |
| Gastric | 0.32 ± 0.03 | 0.14 ± 0.04 Ab | n.d. | 0.25 ± 0.01 Aa | 0.09 ± 0.02 B | - | - | - | - | - | |
| Intestinal | 0.31 ± 0.0 | 0.11 ± 0.03 Ab | 0.08 ± 0.0 Aa | 0.21 ± 0.02 Aa | 0.05 ± 0.01 Bb | 47.2 ± 0.1 | 59.9 ± 9 Aa | 41.7 ± 1 Ba | 44.5 ± 1.9 Ab | 11.5 ± 0.7 Bb | |
| PHENOLIC ACIDS | |||||||||||
| Piscidic acid | Control | 7.29 ± 0.05 | 4.43 ± 0.1 Aa | 4.39 ± 0.02 Aa | 2.27 ± 0.05 Ab | 2.23 ± 0.09 Ab | - | - | - | - | - |
| Oral | 7.01 ± 0.27 | 2.08 ± 0.05 Ab | 2.06 ± 0.29 Aa | 2.64 ± 0.13 Aa | 2.01 ± 0.57 Ba | - | - | - | - | - | |
| Gastric | 7.01 ± 0.24 | 3.44 ± 0.03 Aa | 1.84 ± 0.4 Bb | 2.52 ± 0.02 Ab | 2.06 ± 0.85 Ba | - | - | - | - | - | |
| Intestinal | 4.69 ± 0.21 | 10.99 ± 0.5 Aa | 10.94 ± 0.1 Aa | 5.06 ± 0.17 Bb | 8.48 ± 2.86 Ab | 64.3 ± 3.3 | 253.1 ± 5.8 Aa | 251.9 ± 4.7 Aa | 226.9 ± 1 Bb | 247.3 ± 5.4 Aa | |
| FLAVONOIDS | |||||||||||
| Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | Control | 0.47 ± 0.0 | 0.2 ± 0.01 Ab | 0.19 ± 0.01 Ab | 0.3 ± 0.05 Aa | 0.31 ± 0.03 Aa | - | - | - | - | - |
| Oral | 0.35 ± 0.01 | 0.21 ± 0.02 Ab | 0.17 ± 0.01 Ba | 0.28 ± 0.02 Aa | 0.19 ± 0.0 Ba | - | - | - | - | - | |
| Gastric | 0.33 ± 0.0 | 0.18 ± 0.01 Ab | 0.17 ± 0.0 Aa | 0.22 ± 0.01 Aa | 0.19 ± 0.03 Ba | - | - | - | - | - | |
| Intestinal | 0.11 ± 0.0 | 0.18 ± 0.01 Aa | 0.15 ± 0.01 Ba | 0.19 ± 0.0 Aa | 0.14 ± 0.01 Ba | 23.5 ± 0.3 | 91.1 ± 2.8 Aa | 75.7 ± 3.8 Ba | 58.7 ± 0.5 Ab | 46.2 ± 5.3 Bb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parralejo-Sanz, S.; Gómez-López, I.; González-Álvarez, E.; Montiel-Sánchez, M.; Cano, M.P. Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccessibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts. Foods 2023, 12, 2243. https://doi.org/10.3390/foods12112243
Parralejo-Sanz S, Gómez-López I, González-Álvarez E, Montiel-Sánchez M, Cano MP. Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccessibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts. Foods. 2023; 12(11):2243. https://doi.org/10.3390/foods12112243
Chicago/Turabian StyleParralejo-Sanz, Sara, Iván Gómez-López, Erika González-Álvarez, Mara Montiel-Sánchez, and M. Pilar Cano. 2023. "Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccessibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts" Foods 12, no. 11: 2243. https://doi.org/10.3390/foods12112243
APA StyleParralejo-Sanz, S., Gómez-López, I., González-Álvarez, E., Montiel-Sánchez, M., & Cano, M. P. (2023). Oil-Based Double Emulsion Microcarriers for Enhanced Stability and Bioaccessibility of Betalains and Phenolic Compounds from Opuntia stricta var. dillenii Green Extracts. Foods, 12(11), 2243. https://doi.org/10.3390/foods12112243