Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lactobacillus rhamnosus BST-L.601 and Mashed Sweet Potato Medium
2.3. Cell Culture and Differentiation of Pre-Adipocytes
2.4. Cell Viability Determination
2.5. Oil Red O Staining
2.6. Total RNA Preparation and Reverse Transcription-Polymerase Chain Reaction (RT–PCR)
2.7. Western Blot Analysis
2.8. Animal Care and Diets
2.9. Serum Biochemical Analysis
2.10. Chemical Composition Analysis
2.11. Statistical Analysis
3. Results
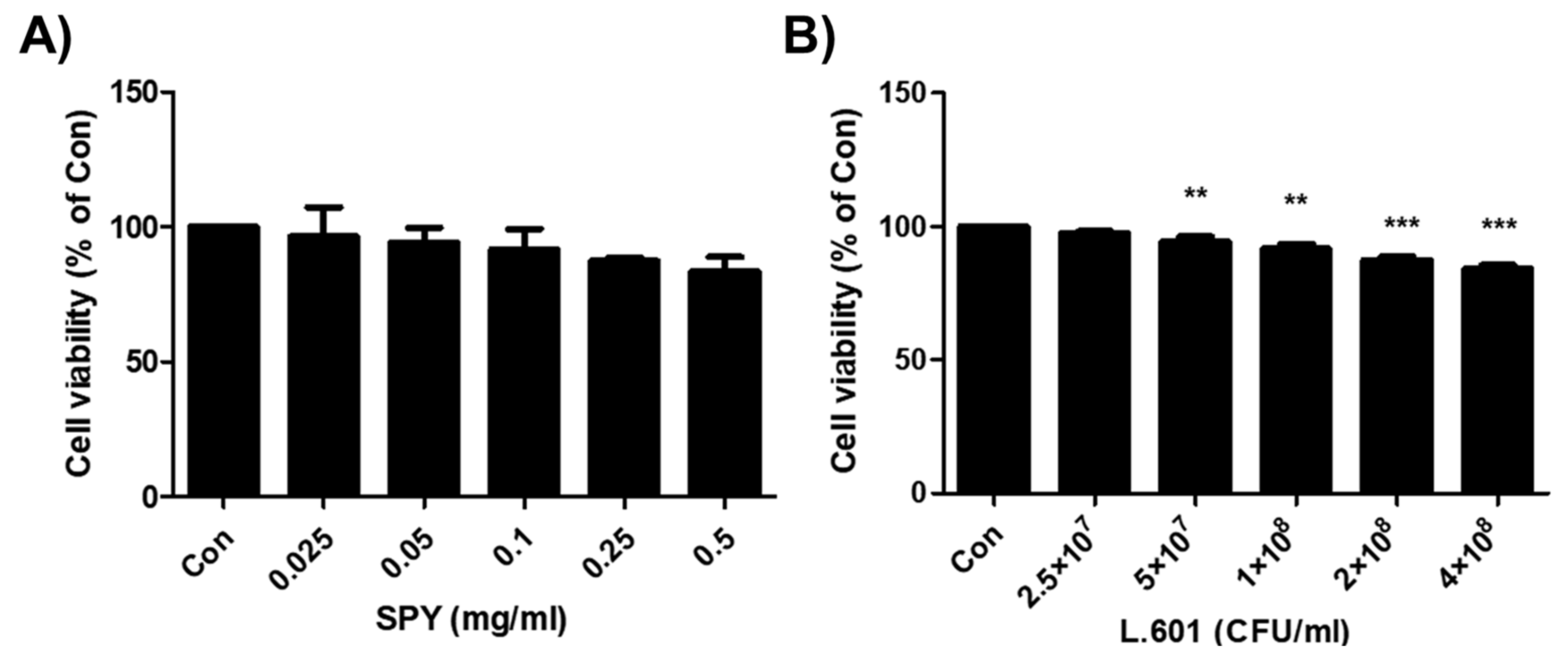
3.1. Effects of SPY and BST-L.601 on the Viability of 3T3-L1 Pre-Adipocytes
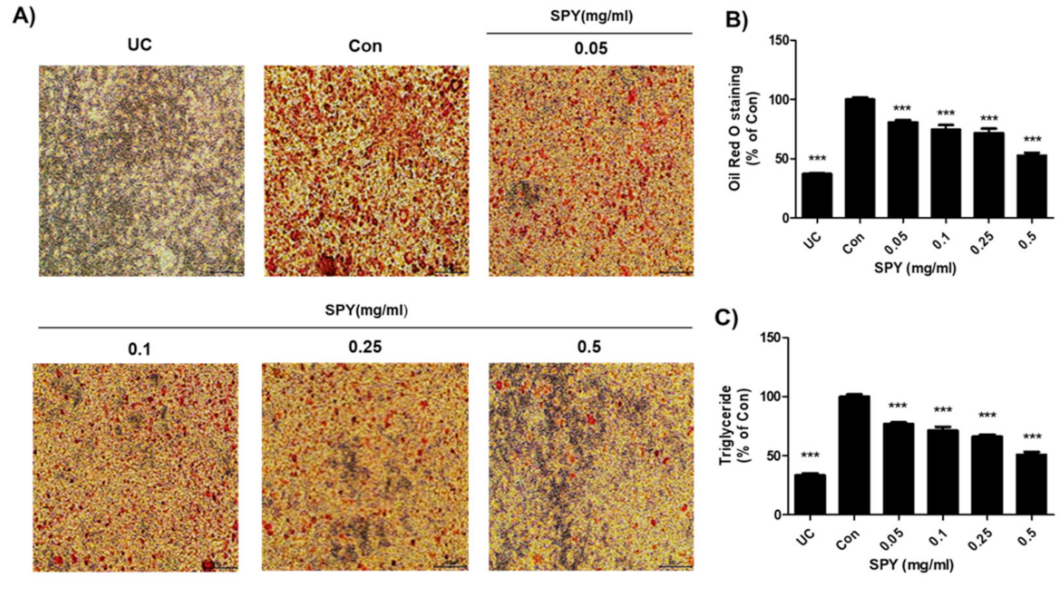
3.2. Effects of SPY on Adipocyte Differentiation
3.3. Inhibitory Effect of SPY on Differentiation and Lipogenesis-Related Protein Expression in 3T3-L1 Cells
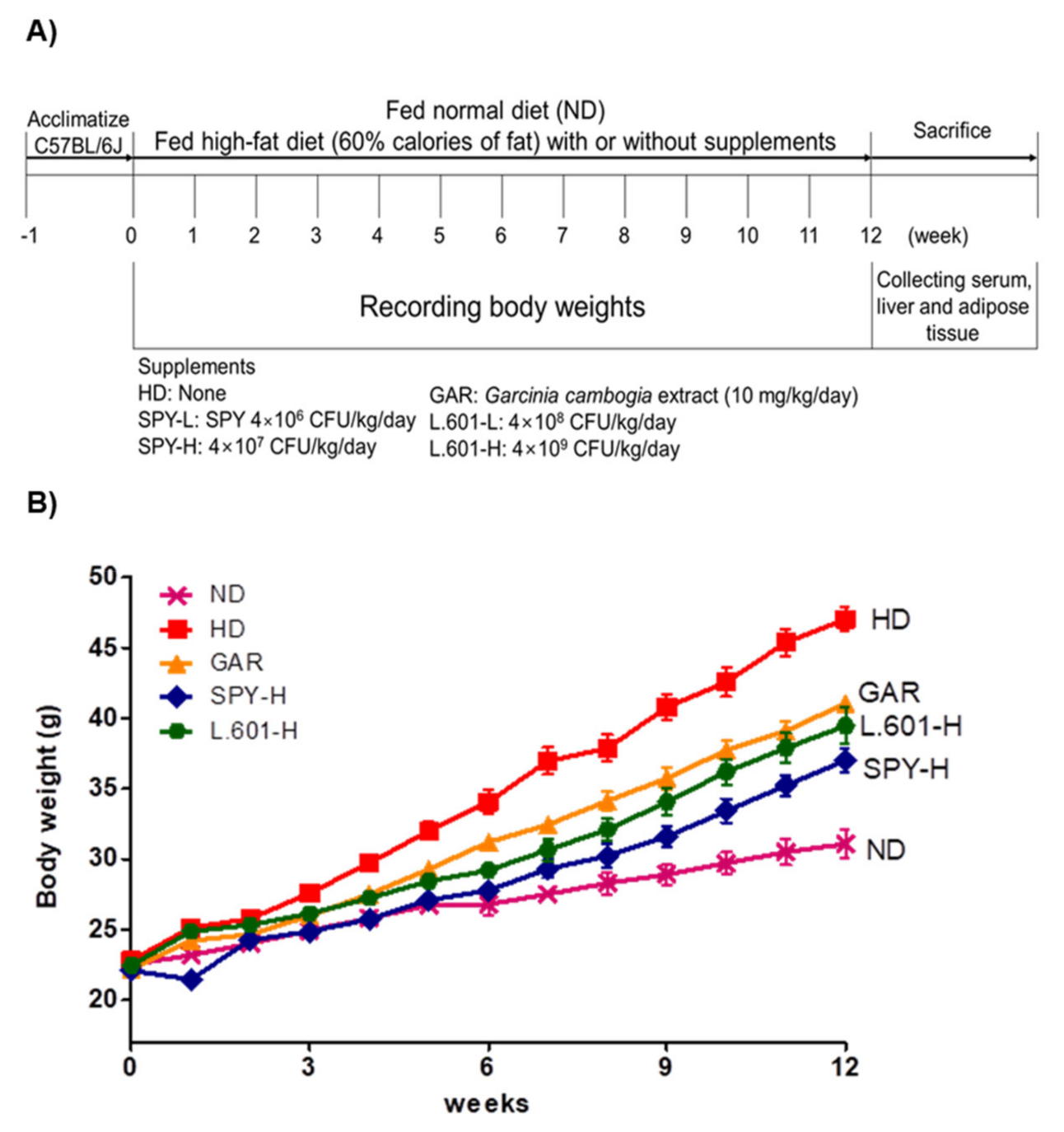
3.4. Body and Organ Weight Changes of Mice Fed the Different Diets
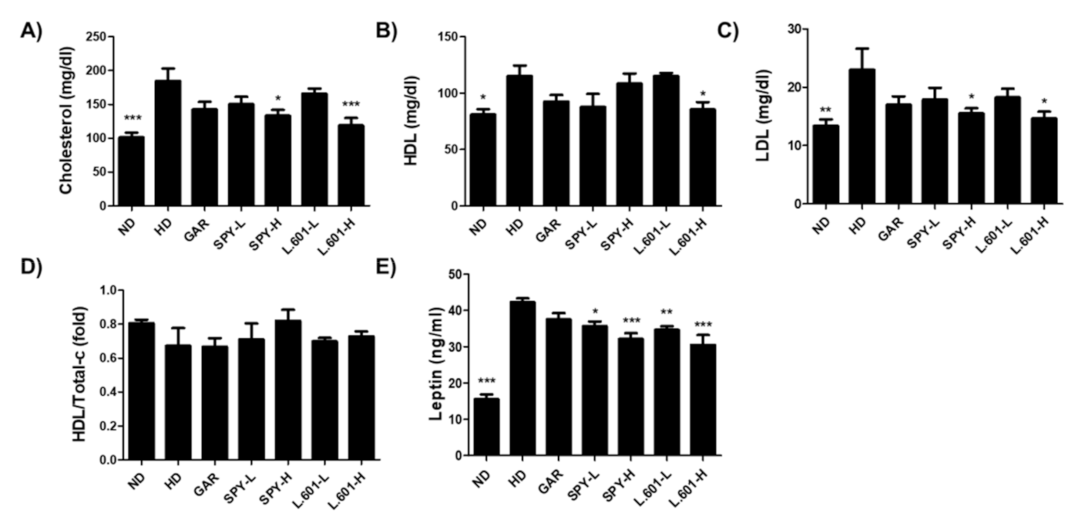
3.5. SPY Prevents Hyperlipidemia in High-Fat Diet Mice
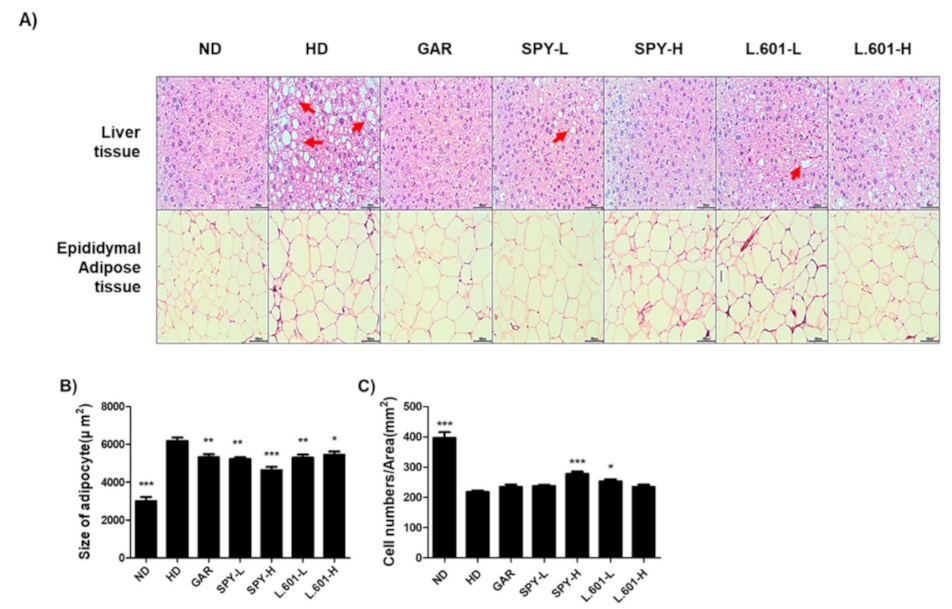
3.6. Effects of SPY and BST-L.601 on the Size and Numbers of Adipocytes in Liver and Epididymal Fat Tissues
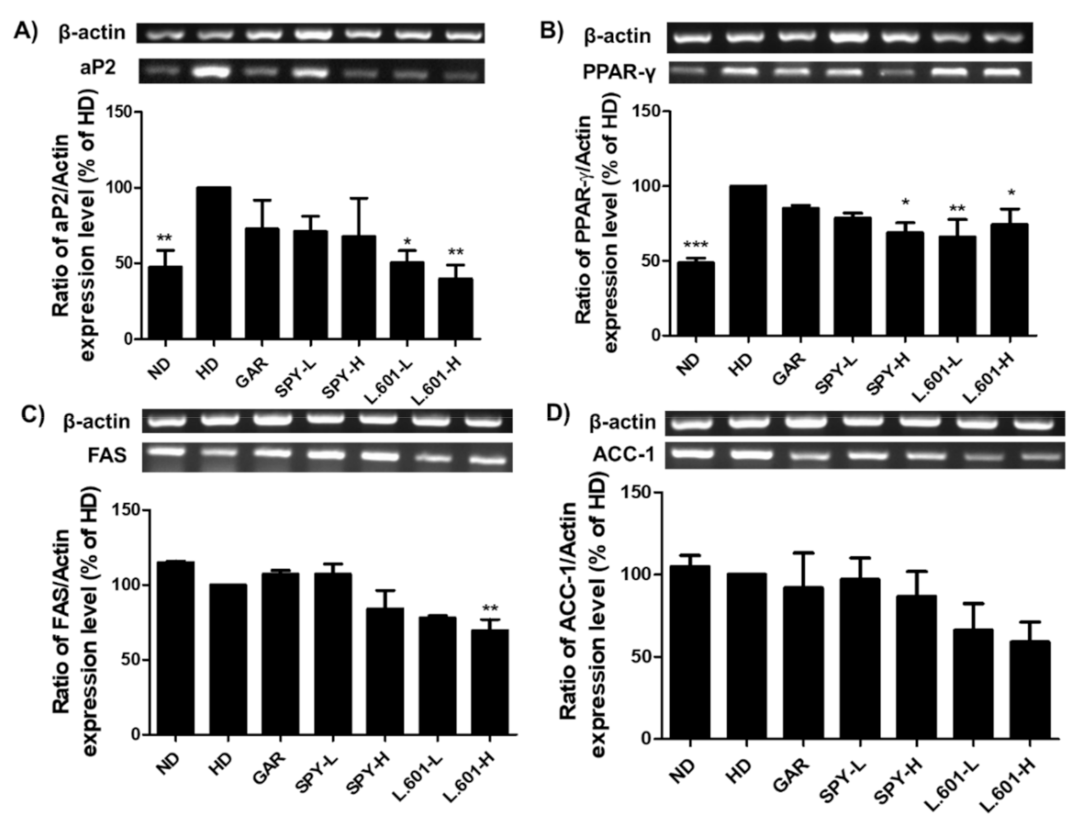
3.7. SPY and BST-L.601 Suppress Adipogenic and Lipogenic Marker Protein mRNA Expression in HD-Induced Obese Mice
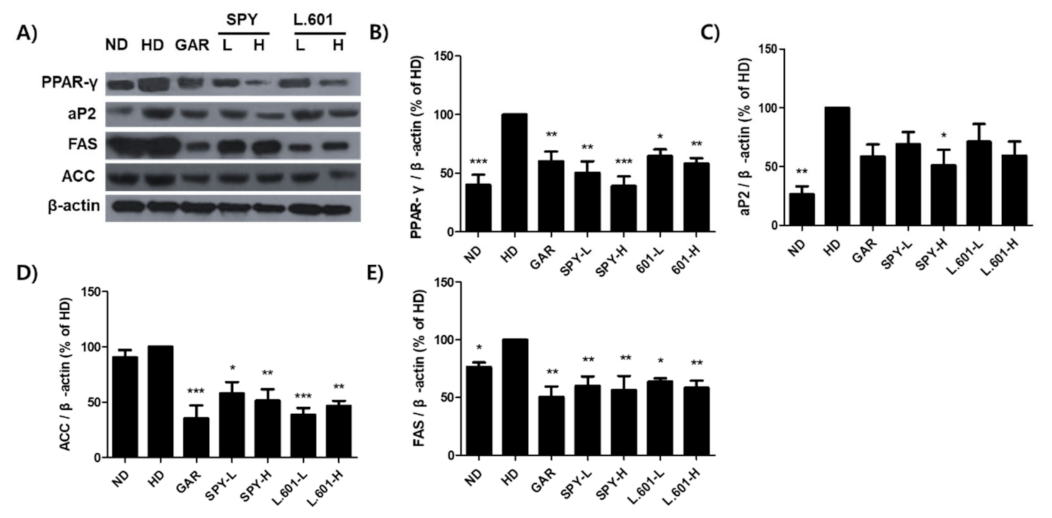
3.8. SPY and BST-L.601 Suppress Adipocyte Differentiation and Lipogenic Marker Protein Expression in Liver Tissue of HD-Induced Obese Mice
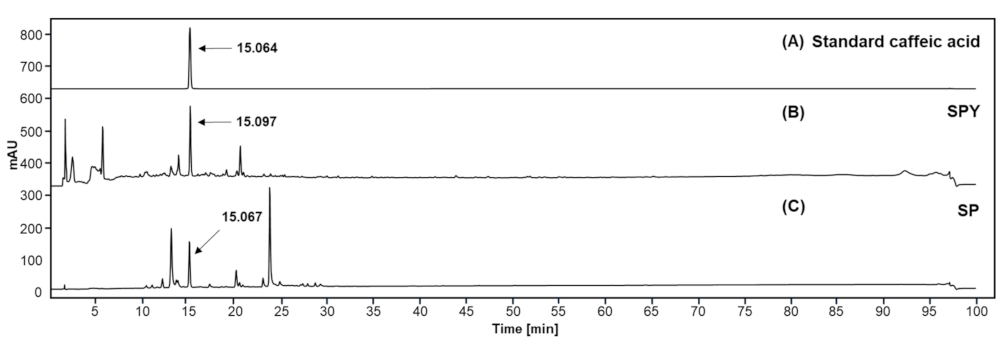
3.9. Chemical Composition Profile of SPY and MSPP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popkin, B.M.; Kim, S.; Rusev, E.R.; Du, S.; Zizza, C. Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes. Rev. 2006, 7, 271–293. [Google Scholar] [CrossRef] [PubMed]
- OECD. Obesity Update 2017. Available online: https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf (accessed on 22 December 2022).
- Kim, J.H.; Kim, O.K.; Yoon, H.G.; Park, J.; You, Y.; Kim, K.; Lee, Y.H.; Choi, K.C.; Lee, J.; Jun, W. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr Res. 2016, 60, 30428. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Sorisky, A.; Pardasani, D.; Gagnon, A.; Smith, T.J. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J. Clin. Endocrinol. Metab. 1996, 81, 3428–3431. [Google Scholar]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Bazin, R.; Ferré, P. Assays of lipogenic enzymes. Methods Mol. Biol. 2001, 155, 121–127. [Google Scholar]
- DiBaise, J.K.; Frank, D.N.; Mathur, R. Impact of the gut microbiota on the development of obesity: Current concepts. Am. J. Gastroenterol. Suppl. 2012, 1, 22. [Google Scholar] [CrossRef]
- Conterno, L.; Fava, F.; Viola, R.; Tuohy, K.M. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011, 6, 241–260. [Google Scholar] [CrossRef]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The human gut microbiome and body metabolism: Implications for obesity and diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Demchenko, O.A.; Nechypurenko, O.V.; Spivak, M.Y. Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice. Epma. J. 2017, 8, 357–376. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: Current evidence, controversies, and perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- Choque Delgado, G.T.; Tamashiro, W. Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res. Int. 2018, 113, 183–188. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Li, B.; Lin, L.; Tundis, R.; Loizzo, M.R.; Zheng, B.; Xiao, J. Characterization and prebiotic effect of the resistant starch from purple sweet potato. Molecules 2016, 21, 932. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1–10. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Zhou, S.; Wang, T.T.Y.; Zhou, S.; Yang, K.; Li, Y.; Tian, J.; Wang, J. Dietary fiber isolated from sweet potato residues promotes a healthy gut microbiome profile. Food Funct. 2020, 11, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Ouwens, D.M.; Hörbelt, T.; Van de Velde, F.; Fahlbusch, P.; Herzfeld de Wiza, D.; Van Nieuwenhove, Y.; Calders, P.; Praet, M.; Hoorens, A.; et al. Reduced expression of chemerin in visceral adipose tissue associates with hepatic steatosis in patients with obesity. Obesity 2016, 24, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Frece, J.; Kos, B.; Svetec, I.K.; Zgaga, Z.; Beganović, J.; Lebos, A.; Susković, J. Synbiotic effect of Lactobacillus helveticus M92 and prebiotics on the intestinal microflora and immune system of mice. J. Dairy Res. 2009, 76, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.; Ostovar-Ravari, A.; Shokri-Afra, H. Characterization and improvement of phenol-sulfuric acid microassay for glucose-based glycogen. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2020–2024. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hong, J.-I.; Kim, H.-J.; Kim, J.-Y. Factors affecting reactivity of various phenolic compounds with the Folin-Ciocalteu reagent. J. Korean Soc. Food Sci. Nutr. 2011, 40, 205–213. [Google Scholar] [CrossRef]
- Xu, J.; Ge, J.; He, X.; Sheng, Y.; Zheng, S.; Zhang, C.; Xu, W.; Huang, K. Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice. J. Funct. Foods 2020, 74, 104061. [Google Scholar] [CrossRef]
- Meng, X.J.; Tan, C.; Feng, Y. Solvent extraction and in vitro simulated gastrointestinal digestion of phenolic compounds from purple sweet potato. Int. J. Food Sci. Technol. 2019, 54, 2887–2896. [Google Scholar] [CrossRef]
- Philpott, M.; Ferguson, L.R.; Gould, K.S.; Harris, P.J. Anthocyanidin-containing compounds occur in the periderm cell walls of the storage roots of sweet potato (Ipomoea batatas). J. Plant Physiol. 2009, 166, 1112–1117. [Google Scholar] [CrossRef]
- Zhao, J.G.; Yan, Q.Q.; Xue, R.Y.; Zhang, J.; Zhang, Y.Q. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD-ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E.; Watt, M.J. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E958–E964. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122s–3126s. [Google Scholar] [CrossRef]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Li, B.; Davis, M.E.; Suh, Y.; Lee, K. Comparative analysis of fatty acid-binding protein 4 promoters: Conservation of peroxisome proliferator-activated receptor binding sites. J. Anim. Sci. 2009, 87, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Aja, S.; Wei, Z.; Wong, G.W. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J. Biol. Chem. 2012, 287, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.D.; Wolfgang, M.; Cha, S.H.; Dai, Y. Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. Int. J. Obes. 2008, 32, S49–S54. [Google Scholar] [CrossRef]
- Jones, S.F.; Infante, J.R. Molecular pathways: Fatty acid synthase. Clin. Cancer Res. 2015, 21, 5434–5438. [Google Scholar] [CrossRef]
- Saggerson, E.D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem. J. 1972, 128, 1057–1067. [Google Scholar] [CrossRef]
- Khairani, A.F.; Islami, U.; Syamsunarno, M.; Lantika, U. Synbiotic purple sweet potato yogurt ameliorate lipid metabolism in high fat diet mice model. Biomed. Pharmacol. J. 2020, 13, 175–184. [Google Scholar] [CrossRef]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Jung, H.H.; Kim, Y.H.; Kim, T.H. Antiobesity effects of Lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of diet-induced obese mice. J. Med. Food 2019, 22, 560–566. [Google Scholar] [CrossRef]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on antiobesity effect of Garcinia origin (-)-HCA. Evid. Based Complem. Alternat. Med. 2013, 2013, 751658. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K.; Ishii, Y.; Kaneko, I.; Shen, M.; Okuhara, Y.; Shigematsu, N.; Tomi, H.; Furuse, M.; Yoshino, G.; Shimasaki, H. Effects of Garcinia cambogia (hydroxycitric acid) on visceral fat accumulation: A double-blind, randomized, placebo-controlled trial. Curr. Ther. Re.s Clin. Exp. 2003, 64, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Seo, J.Y.; Lee, J.; Choi, J.W.; Cho, S.; Bae, J.Y.; Sohng, J.K.; Kim, S.O.; Kim, J.; Park, Y.I. 3-O-Glucosylation of quercetin enhances inhibitory effects on the adipocyte differentiation and lipogenesis. Biomed. Pharmacother. 2017, 95, 589–598. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Yang, D.K.; Jo, D.-G. Mulberry fruit extract ameliorates nonalcoholic fatty liver disease (NAFLD) through inhibition of mitochondrial oxidative stress in rats. Evid. Based Complem. Alternat. Med. 2018, 2018, 8165716. [Google Scholar] [CrossRef]
- Tran, N.K.S.; Kim, G.T.; Park, S.H.; Lee, D.; Shim, S.M.; Park, T.S. Fermented Cordyceps militaris extract prevents hepatosteatosis and adipocyte hypertrophy in high fat diet-fed mice. Nutrients 2019, 11, 1015. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef]
- Havel, P.J. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000, 59, 359–371. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]

| Group | ND | HD | GAR | ||||
|---|---|---|---|---|---|---|---|
| Liver weight (g) | 1.59 ± 0.17 | 1.93 ± 0.44 | 1.61 ± 0.16 | 1.71 ± 0.47 | 1.49 ± 0.19 * | 1.52 ± 0.19 * | 1.49 ± 0.17 * |
| Kidney weight (g) | 0.45 ± 0.06 | 0.45 ± 0.04 | 0.48 ± 0.04 | 0.47 ± 0.08 | 0.49 ± 0.06 | 0.43 ± 0.06 | 0.48 ± 0.04 |
| Spleen weight (g) | 0.081 ± 0.016 | 0.083 ± 0.007 | 0.079 ± 0.012 | 0.078 ± 0.009 | 0.079 ± 0.007 | 0.072 ± 0.01 | 0.074 ± 0.012 |
| Epididymis fat tissue (g) | 1.029 ± 0.26 *** | 3.246 ± 0.55 | 3.151 ± 0.21 | 2.920 ± 0.24 | 2.725 ± 0.41 | 3.446 ± 0.14 | 2.533 ± 0.48 ** |
| Visceral fat tissue (g) | 0.449 ± 0.11 *** | 1.752 ± 0.17 | 1.394 ± 0.19 ** | 1.480 ± 0.17 * | 1.321 ± 0.12 *** | 1.578 ± 0.33 | 1.240 ± 0.11 *** |
| Abdominal fat tissue (g) | 0.889 ± 0.19 *** | 4.797 ± 0.37 | 3.512 ± 0.56 *** | 3.855 ± 0.39 ** | 3.035 ± 0.56 *** | 3.849 ± 0.34 ** | 3.373 ± 0.85 *** |
| Total fat (g) | 2.48 ± 0.66 *** | 10.14 ± 1.06 | 7.92 ± 0.67 ** | 8.35 ± 0.62 * | 7.12 ± 1.11 *** | 8.50 ± 0.46 | 7.57 ± 1.93 *** |
| Total Carbohydrate A (mg GE/g) | Total Protein B (mg BE/g) | Total Polyphenols C (mg GAE/g) | Caffeic Acid (mg/mL) | |
|---|---|---|---|---|
| MSPP D | 922.4 (±7.69) | 30.7 (±1.69) | 1.65 (±0.01) | 0.26 |
| SPY | 810.5 (±17.40) | 16.3 (±1.00) | 1.99 (±0.01) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.; Ree, J.; Park, J.-W.; Choe, H.; Park, Y.I. Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice. Foods 2023, 12, 2202. https://doi.org/10.3390/foods12112202
Kang T, Ree J, Park J-W, Choe H, Park YI. Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice. Foods. 2023; 12(11):2202. https://doi.org/10.3390/foods12112202
Chicago/Turabian StyleKang, Taewook, Jin Ree, Joo-Woong Park, Hyewon Choe, and Yong Il Park. 2023. "Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice" Foods 12, no. 11: 2202. https://doi.org/10.3390/foods12112202
APA StyleKang, T., Ree, J., Park, J.-W., Choe, H., & Park, Y. I. (2023). Anti-Obesity Effects of SPY Fermented with Lactobacillus rhamnosus BST-L.601 via Suppression of Adipogenesis and Lipogenesis in High-Fat Diet-Induced Obese Mice. Foods, 12(11), 2202. https://doi.org/10.3390/foods12112202








