Abstract
Background: Encapsulation is a valuable method used to protect active substances and enhance their physico-chemical properties. It can also be used as protection from unpleasant scents and flavors or adverse environmental conditions. Methods: In this comprehensive review, we highlight the methods commonly utilized in the food and pharmaceutical industries, along with recent applications of these methods. Results: Through an analysis of numerous articles published in the last decade, we summarize the key methods and physico-chemical properties that are frequently considered with encapsulation techniques. Conclusion: Encapsulation has demonstrated effectiveness and versatility in multiple industries, such as food, nutraceutical, and pharmaceuticals. Moreover, the selection of appropriate encapsulation methods is critical for the effective encapsulation of specific active compounds. Therefore, constant efforts are being made to develop novel encapsulation methods and coating materials for better encapsulation efficiency and to improve properties for specific use.
1. Introduction
Different substances have great health benefits or function as a drug, but they cannot be used directly or stored for a long period. This is due to their low solubility, low bio-availability, tendency to oxidize, or their strong odor or flavor. Encapsulation is a method that has been known for more than 60 years, but it still holds great interest among researchers [1,2]. Typically, a substance is enclosed by a coating material which forms a barrier to protect the substance from the environment and chemical interaction [3,4,5,6]. Encapsulated substances can be also called core, fill, or matrix substances. The coating material is called a shell or wall material, as can be seen in Figure 1. Encapsulation can be used for improving the mentioned properties of different substances as well as protecting the core substance from, for example, light, moisture, and changes in pH [2,7,8,9]. Apart from protecting of core, it can also be used in the prolongation of release. This can be helpful with gradual and controlled release of drugs [10,11].
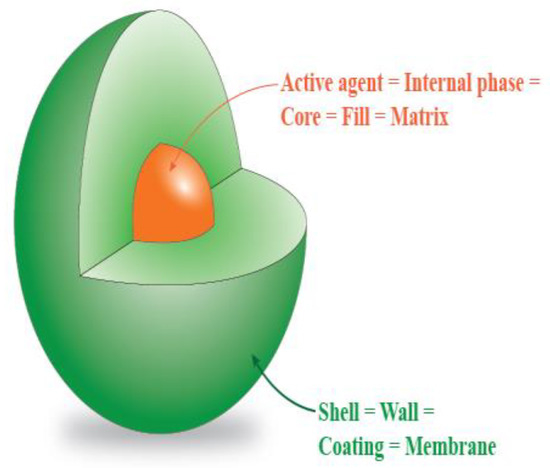
Figure 1.
Substance encapsulated into a coating material.
Encapsulation can be achieved by many methods, some of which are described in this review. These methods can be divided into three main types: chemical, physico-chemical, and physico-mechanical [7,12]. This review does not mention every encapsulation approach since it is a very broad topic, but rather focuses on methods mostly used in pharmaceutical and food industries. The global food encapsulation market has undergone continuous growth and is expected to reach a value of USD 17 billion by 2027 [13].
With regard to encapsulation of pharmacologically active substances, it is important to note that this technology can have broader applications beyond just the pharmaceutical and food industries. Encapsulation can also be utilized in various other industries; for example, it can be used in nutraceuticals or in the construction industry as a self-healing material such as concrete [14,15]. Specifically, in concrete, types of bacteria can be encapsulated for a self-healing effect [16]. The principle of self-healing materials is applied when the treated material is mechanically damaged; the encapsulate is released after a rupture of capsules and the core material is used to repair the damaged part [17,18,19]. In the same industry, the encapsulation is also used for anticorrosive coating. An example of encapsulated food is linseed oil [20]. The anticorrosive coating can have three mechanisms of protection. Firstly, it is a basic barrier on top of the material; secondly, the coating material can carry corrosion inhibitors; lastly, the coating can provide cathodic protection [21]. Self-healing materials and anticorrosion coatings of materials aim to prolong material use and prevent and avoid structural damage that may occur [22]. For example, marine infrastructure such as bridges and tunnels are in hostile environments and are prone to micro-cracks [23].
Quantum dots (QDs) are closely examined within chemistry. QDs are between 2 and 10 nm in diameter and have a semiconductor core with a shell, together with ligands [24]. Quantum dots can be also used together with encapsulation methods to achieve better properties, which are already unique. Quantum dots are widely used in the biomedical industry [24,25], for example, in biomolecular tracking, tumor imaging, and photodynamic therapy [24]. Encapsulated QDs can be used for the detection of heavy metals and other possible harmful substances [26]. Table 1 provides specific examples of the encapsulation methods described in this review, along with several examples from other industries that demonstrate the diversity of encapsulation.

Table 1.
Different methods of encapsulation.
A retrospective analysis was conducted in this review to evaluate advancements in encapsulation techniques used in the food industry over the last decade. The study identified several methods utilized during this period, including liposomal, lipid, simple and double oil/water emulsions, and extrusion. These techniques have shown significant potential in enhancing food quality and safety by protecting and delivering active ingredients while minimizing the degradation of nutritional and sensory properties.
2. Physical-Chemical Approaches in Pharmaceutical Industry
2.1. Encapsulation of Bacteriophages
Encapsulation can be used for the treatment of bacterial infections [46]. Due to the still-greater risk of bacteria obtaining resistance to antibiotics, the encapsulation of bacteriophages can be one of the main options to fight bacterial infection without antibiotics [46]. Bacteriophages are viruses that can infect and kill bacteria with high specificity and do not threaten healthy human microflora [46,47,48,49]. Without encapsulation, the bacteriophages have lower survivability in gastro-intestinal conditions; specifically, they can be affected by gastric enzymes, bile, and the acidic environment when used orally [46,48,50].
2.1.1. Emulsification
This method is based on dispersing one liquid with the active compound into a second liquid that is not miscible, and creating small droplets [51,52,53,54,55]. Emulsions tend to be thermodynamically unstable and surfactants as emulsifiers need to be added to decrease surface tension [56,57,58]. Solid particles, such as nanoparticles, can also function as a stabilizer and emulsions stabilized in such a way are called Pickering emulsions [56,58,59].
The method mentioned by Anna Choinska-Pulit et al. includes a mixture of microorganism cells and polymer, which is added to vegetable oil (canola, sunflower, and corn oil, for example) [46]. Mixtures must be homogenous until a water-in-oil emulsion is formed, and stirring of the emulsion is a key step to obtain droplets having the right size and shape. Emulsification creates droplets called capsules, whereas extrusion droplets are called beads. The capsule core is liquid, and the bead core is porous. Capsules are at least 100 times smaller than beads [60].
Emulsifiers, for example, guar gum or lecithin, must be included for stabilization. Settling is used to recover hardened capsules. Researchers in the publication from Dini et al. used this method to encapsulate bacteriophages to reduce enterohemorrhagic E. coli in the bovine gastric environment; methoxylated pectin was used as the material for encapsulation and was emulsified by mixing with Tween 20 (Polyoxyethylene sorbitan monolaurate) [61]. Homogenization was performed by mixing and oleic acid was added to reach a final concentration of 10 vol%. Additionally, coating was carried out with 0.2 wt% of high-methoxylated pectin or guar gum.
Double water-in-oil-in-water (W/O/W) emulsion was conducted by Kim, S. et al. [62]. PLGA microspheres were prepared using a bacteriophage solution of pAh-6C in phosphate buffered saline (PBS), mixed with PLGA, and dissolved in dichloromethane to form a W/O emulsion. To form a double emulsion, the primary W/O was homogenized together with polyvinyl alcohol (PVA). A second set of microspheres comprised PLGA/alginate composite. This composite was prepared by preparing W/O/W as before. Calcium chloride solution was added and homogenized to crosslink the alginate. To the emulsion was added deionized water and, after stirring and evaporating, microspheres were obtained [62].
2.1.2. Extrusion
The principle of this method is the forceful flow of material through a slit [8]. Extrusion is a suitable method for the preparation of capsules with hydrocolloids by adding microorganisms. The cell suspension is extruded through a syringe needle in the form of droplets into a bath or a hardening solution, which is mostly calcium chloride [46,63,64,65].
The group of Zhenxing Tang et al. used extrusion as the method to encapsulate Felix O1 bacteriophages into alginate-whey protein microspheres [66]. In this work, researchers focused on the growing problem of bacterial contamination in food poisoning. Worldwide treatment of bacterial infections in food animals is carried out using antibiotics, which causes the overuse of these drugs. Bacteriophage treatment can be a good substitute. Encapsulation is needed because gastric acidity decreases the viability of bacteriophages. Within minutes, the phages were found to be inactive in the simulated gastric fluid. The viability of Felix O1 increased to 2 h of incubation in alginate-whey protein microcapsules [66]. Encapsulation efficiency describes how much of the core material is successfully entrapped into the capsules. The encapsulation efficiency of bacteriophages is calculated as the quantity released from capsules divided by the initial quantity in the capsules multiplied by 100% [67]:
where is the initial mass and is the mass of the compound left in solution. Here the encapsulation efficiency reached 99% for mixtures of alginate-whey proteins from 93% of pure alginate microspheres. The extrusion method is simple and cheap but has a big disadvantage for use at a mass-production scale [46].
In the publication by Savic et al., researchers encapsulated extracted antioxidants from orange peel into alginate-chitosan microparticles [68]. The extrusion method with coaxial airflow was used for encapsulation. To the solution of alginate 1.5% (v/v) was added ethanol extract of orange peels. From the homogeneous solution, which was in a plastic syringe, drops were torn using coaxial airflow. Droplets fell into the crosslinking solution while stirring and solidifying. The crosslinking solution was prepared using calcium chloride 2% (w/v) and chitosan 0.5% (w/v). The chitosan was prepared using 0.5% (v/v) acetic acid. The encapsulation efficiency was 89.2% [68].
2.2. Probiotic Encapsulation by Chitosan-Gel Particles
Probiotics are helpful bacteria for maintaining a healthy bowel environment. A problem in administering probiotics is that they are prone to degradation because of humidity and low pH in the human intestines. The group of Albadran H. et al. devised a novel method to encapsulate probiotics in chitosan-coated agar-gelatin particles for releasing probiotics into the large intestine [69]. This method should be scalable and thus more suitable than commonly used extrusion methods. Firstly, they prepared agar-gelatin particles loaded with bacteria. This was undertaken by separately dissolving agar and gelatin in deionized water for 2 h at 70–80 °C. Both substances were mixed together at a 1:1 ratio and autoclaved. A small volume of cell suspension was mixed with agar-gelatin and poured into a petri dish, left at room temperature to solidify, and cut into particles having a size of approximately 6 mm. Secondly, particles were coated with chitosan. Agar-gelatin particles were added into the chitosan solution and stirred. Particles were then collected by filtration and washed with phosphate buffered saline (PBS). As a result, particles prepared by the method described by Albadran H. et al. showed great potential for delivery of probiotics into the large intestine [69]. Coated particles showed the ability to withstand the environment of simulated gastric fluid (SGF) for 2 h of incubation and 3 h in simulated intestinal fluid (SIF). X-ray diffraction analysis showed a change in the physico-chemical properties of agar. This change caused by thermal treatment resulted in a strong and tight polymer network.
2.3. Nanoemulsion
The definition of a nanoemulsion system can vary as some sources describe that the droplet size is smaller than 500 nm whereas others claim the droplet size is up to 1000 nm [70,71,72,73,74]. The system is made of two immiscible liquids, as stated previously, which are stabilized using surfactant [75,76,77,78]. Nanoemulsions, as opposed to macro and microemulsions, have improved physico-chemical stability [79]. The immiscible liquids used most are water and oil [74,80]. There are two main types of nanoemulsion. The first type can be formed as oil-in-water (O/W); the second type is water-in-oil (W/O), depending on the dispersed liquid. Apart from these, they are also water-in-oil-in-water (W/O/W), and vice versa, and bi-continuous types [55,72,81,82]. Two main approaches are used for preparation, the so-called high and low-energy methods [83,84].
High-energy methods include high-pressure homogenization, microfluidization, and ultrasonication [74,85,86]. With these methods, mechanical energy is used to break large droplets. The main disadvantage of high-energy methods is cost, due to energy demand. Conversely, the advantages are good control of droplet size and possibility of choosing the formulation composition [85,87].
Low-energy methods include the phase inversion temperature and the emulsion inversion point [74,85]. Their principle uses internal chemical energy and formation of droplets with a change in, for example, temperature or chemical composition [86,87,88].
Particles are spheres with amorphous, lipophilic, and negatively charged surfaces [73,89]. A significant number of newly investigated drugs have problems with water solubility and thus their bioavailability is very low [78]. In the study by Dey et al. [90]., it was found that nanoemulsion enhances the absorption of lipids in the small intestine of rats more than conventional emulsion.
The group of Oh et al. [91]. developed a lecithin nano-liposol system loaded with astaxanthin (ASTA). Like other antioxidants, ASTA is susceptible to degradation and thus needs to be encapsulated. The method used for preparation was emulsion evaporation. Chloroform with different concentrations of ASTA was added to lecithin and mixed for 2 h. The final mixture was then added to deionized water and homogenized. Chloroform was removed by drying. The last steps were carried out using ultrasonication and purification by centrifuge. The best results were achieved with a loading of 15 wt% as it had a similar hydrodynamic diameter to that prior of loading. The diameter was around 140 nm ± 4 nm. The encapsulation efficiency for 15 wt% was 98.8% [91].
The publication by Tayeb and Sainsbury describes different uses of nanoemulsion in the pharmaceutical industry [78]. For the delivery of the drug, different ways of application can be used, i.e., nasal, ocular, oral, and parenteral [78,92]. The application of drugs through the skin can be challenging because of the protectiveness of skin layers. Nanoemulsion encapsulation can improve both bioavailability and penetration chance because of the nano-dimensions and low surface tension [78]. Quercetin as a well-known antioxidant with various health benefits, which can be encapsulated. It is necessary to do so because of its poor water solubility, skin absorption, and penetration ability, which are improved by nanoemulsion encapsulation [78,93,94]. Oral drug intake is specific because of the acidic environment and enzymes [95,96]. Encapsulation can decrease the impact of the environment and help with absorption of active substances [97].
3. Physical-Chemical Approaches in Food Industry
3.1. Spray Drying
The spray-drying process is a common encapsulation method used for the food and pharmaceutical industries due to its cost and ability for use in industrial conditions, for which it has been used since the 1950s [46,98,99,100,101]. The principle of the spray-drying method is the induction of particle agglomeration due to elevated temperatures. Partial melting of the particles and increasing their kinetic energy results in multiple particle collisions [102]. One of the examples of spray-drying encapsulation in the food industry is the encapsulation of oils. This is carried out to protect the oil from oxidization, light, evaporation, and many more effects. One of the side benefits of encapsulating oils or vitamins and antioxidants for supplementation is covering their possible undesirable smell and taste, especially with fish oil [98,103,104,105].
This method is based on three steps. The first is to make small droplets from an emulsion of the core substance with a coating material. This step is called atomization. By producing small droplets, the surface area increases, resulting in faster and easier solvent evaporation [99,106,107,108].
Secondly, this emulsion is then applied and mixed in hot air (100–300 °C) for evaporation of water or another solvent. Liquid is sprayed using various types of nozzles, such as a centrifugal or rotary wheel atomizer, a pressure nozzle, or a two-fluid or pneumatic nozzle [99,100,109].
The last step in spray drying is the separation of dried powder [98,99]. This step is mostly executed by cyclones, which use centrifugal forces to collect particles [100].
Spray drying was used, for example, in encapsulation of flaxseed oil with up to 84% encapsulation efficiency in a study of Fioramonti et al. [110]. In this work, double-layer O/W emulsion was prepared using whey protein concentrate and sodium alginate. The study of Santana Aguiar et al. describes the production of microcapsules using a spray-drying process [111]. Microcapsules loaded with orange essential oil using gelatin and lignin as biopolymers were prepared under optimized conditions. The most important parameters for the atomization efficiency were the inlet air temperature (150 °C), low flow transfer rate (0.15 L/h) of the colloidal suspension, and a higher drying air flow rate (536 L/h). The average particle size was less than 4 μm.
One of the main limitations of spray drying is reduced yield. The reasons for this are associated with the accumulation of powder on the walls during drying. Losing powder through the exhaust air is another reason [7]. Another limitation is due to the high temperature used, and therefore decreased viability can occur for some active substances [46,103,112,113,114]. The encapsulation efficiency, among other things, is affected by the wall material and the material’s physico-chemical properties, such as solubility, viscosity, and thermal stability [104,115].
3.2. Freeze Drying
Freeze drying, or lyophilization, is a technique used to retrieve moisture from frozen samples by using sublimation. Sublimation is the phase change when a solid (ice) directly converts to a vapor phase. The process requires heat energy and low pressures for the frozen product to occur. It consists of three main steps: freezing, primary drying, and secondary drying [7,116,117,118]. The first step involves freezing moisture, i.e., crystallization [117]. The product in a vial or a flask is frozen at atmospheric pressure. The result of this phase is the formation of ice crystals. In the second step, the frozen product is placed under a vacuum and the sublimation process takes the main part in primary drying. Lastly, secondary drying is conducted using desorption to remove moisture that was not frozen. Freeze drying is mostly used for food samples and samples with bioactive compounds that are prone to degrading in higher temperatures [7,119,120,121].
The downside of freeze drying is its high operating cost together with a longer process. The freeze-drying process can also cause cell injury, but this can be prevented by using cryoprotectants [7,119,122]. Generally, disaccharides, polyalcohols, amino acids, or proteins function as cryoprotectants [122,123]. To ensure the cryoprotection of the liposomes’ structure, sugar macromolecules (sucrose, lactose, trehalose) are typically included in the liposome systems. Due to the rehydration process, molecules of water are able to replace sugars and reconstitute the liposomes without remarkable changes in their sizes. Sugars such as trehalose are capable of imitating the presence of water and protecting the integrity of dry liposomes and membranes [124].
In a study performed by Li and Deng, a phospholipid t-butyl alcohol water-sucrose solution was initially frozen for 8 h at the temperature T = −40 °C; then, the sample was dried for 48 h at the same temperature, and finally the product was dried at T = 25 °C for 10 h [125]. As a result, the size of the liposomes and polydispersity decreased with the increased concentration of sucrose [124].
The study conducted by Ghasemi et al. focused on encapsulation of orange peel oil [126]. Orange peel oil (OPO) is commonly used as a flavoring in the food industry. Encapsulation is needed because of high volatility and low stability. Pectin solution, whey protein concentrate solution, and maltodextrin solution were mixed to create a biopolymer solution. Tween 80 was added to the solution and mixed until it completely dissolved. OPO was added and homogenized. The resulting solution was created using freeze drying converted into powder. The encapsulation efficiency was in the range from 70% to 88% based on the altered pH of samples. The mean size of particles ranged between 20 and 110 nm.
3.3. Encapsulation Using Liposomes
Liposomes are particles made of at least one lipid bilayer shaped into spheres. The bilayer is mostly made of phospholipids and contains hydrophobic and hydrophilic parts [127,128,129,130]. Liposomes, due to their character, can encapsulate substances in both hydrophobic and hydrophilic parts [131,132]. Apart from these two substances, liposomes can be used to encapsulate substances with an amphiphilic character [133,134]. Liposomes are great in drug delivery. High biocompatibility, variability in structural properties, and simple preparation are only a few of the advantages mentioned for the use of liposomes in the biomedical and nutraceutical fields [124]. The group of Hudiyanti, Fawrin, and Siahaan encapsulated vitamin C and beta carotene simultaneously in sesame liposomes [128]. A mixture of beta-carotene and phospholipid was prepared with a ratio of 20% (w/w). Cholesterol was added to the mixture with the same ratio. For preparation of a thin layer, chloroform was added. Lastly, the vitamin C in the phosphate buffer was added and the mixture was centrifuged. The encapsulation efficiency was different based on the cholesterol used. The highest EE for vitamin C was 89% with 20% (w/w) of cholesterol used. The highest EE for beta-carotene was 77% without the use of any cholesterol [128].
The researchers Tripathy and Srivastav prepared an extract of Centella asiatica leaf encapsulated in liposome [135]. Soy lecithin and stigmasterol were mixed together with ethanol. The solvent was evaporated using a rotary evaporator. To hydrate the newly formed thin lipid film, phosphate buffer solution with the extract was used. Particle sizes ranged from 512.67 to 787.78 nm. The encapsulation efficiency ranged between 40.36 and 67.80% depending on the ratio of used chemicals.
In the work from Mohammadi et al., Spirulina protein hydrolysates were encapsulated [136]. For preparation, lecithin and γ-oryzanol were dissolved in ethanol. With the use of a rotary evaporator, the solvent was removed and a thin phospholipid bilayer was created as the coating of the container. After adding hydrolyzed Spirulina, the liposomes were spontaneously formed. The final size was achieved using a probe sonicator. The encapsulation efficiency reached 90% and the size of particles was approximately 100 nm.
3.4. Lipid Encapsulation
Apart from using liposomes as a shell material for encapsulation, lipid nanoparticles can be also used. The main difference can be seen in Figure 2. Liposomes encapsulate active substances in an aqueous environment. By comparison, the lipid nanoparticles do not have a continuous bilayer and the active substance is not encapsulated in an aqueous environment.
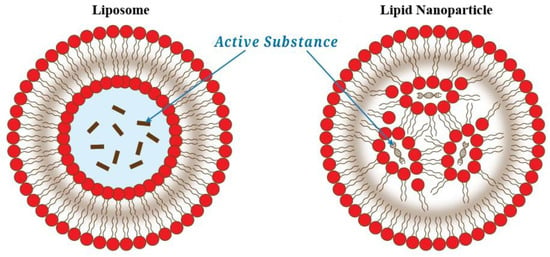
Figure 2.
The difference between liposomes and lipid nanoparticles for encapsulation. Redrawn from [137].
Lipids are suitable for encapsulation of drugs and can be used as a drug delivery system [138]. Based on the matrix of lipids they can be separated into different categories. Firstly, if they are composed of only liquid lipids, they are called nanoemulsions. Secondly, those that are made only by solid lipids are called solid lipid nanoparticles (SLNs). Lastly, there can be a crossover of the above. Nanostructured lipid carriers have liquid as well as solid lipids, which are stabilized by the use of surfactants [102,138,139,140].
SLN has some disadvantages, for example, it is prone to gelation and polymorphic transition, and has a low encapsulation efficiency [139]. Based on these disadvantages, the NCL was developed. Physical, chemical, and colloidal stability, as well as the release of the drug, are based on the ratio of solid and liquid lipids in NCL. The ratio can vary from 70:30 in favor of liquid lipids up to 99.9:0.1 [97]. For preparation of SLNs and NCLs, nanoemulsion preparation can be utilized. It is essential to ensure that these procedures are carried out above the melting temperature of the lipid used [141].
Researchers from the group of Lin et al. prepared lipid nanoparticles with entrapped krill oil [142]. Solid lipids were melted with krill oil to form the lipid phase. To form the water phase, distilled water was mixed with lecithin. Both phases were homogenized together and dispersed using an ultrasonic cell crusher. The particle size varied based on the type of solid lipid used. In their work, the scientists achieved a particle size from 112.4 to 989.4 nm. EE was then from 72.86% to 99.37%. The lipid used with the smallest particle size and highest EE was glycerol 1,3-distearate.
3.5. Microoemulsion
In the study performed by Ziani et al., the scientists focused on encapsulation of vitamin E, vitamin D, and lemon oil [143]. They used colloidal systems based on surfactants to protect the substances. Emulsion was prepared using 1% (w/w) of the chosen surfactant and by dissolving it in an acidic buffer solution consisting of 0.8% citric acid and 0.08% sodium benzoate with a final pH of 2.6. A quantity of 10% (w/w) oil-in-water emulsion was prepared by blending 10% w/w oil phase with 90% w/w aqueous phase using a high-speed blender and passing the emulsion through a high-pressure homogenizer [143].
The emulsion titration method was used for preparation of the microemulsion. Emulsion droplets were titrated into aqueous micelle solution. First, the oil solubilized into the surfactants; after a critical concentration of the oil, its droplets remained in solution. In this study it was found that lemon oil was successfully encapsulated into the microemulsion. Both vitamins were not able to form microemulsions [143].
3.6. Electrospinning and Electrospraying
Electrospinning and electrospraying are relatively new non-thermal methods producing micro- and nanofibers or particles [144]. These techniques rely on a strong electric field between a polymeric or biopolymeric solution and a grounded collector [145]. One of the primary advantages of these methods is their non-thermal encapsulation, which makes them useful in food and nutraceutical applications [146].
3.6.1. Electrospraying
This method is capable of producing micro/nano thin films, particles, or capsules using a high-voltage electric field [144]. The process is based on atomization while applying an electric force. The solution is pumped into a nozzle where it is further forced by an electric force to create droplets [146]. The surface of the solution is subjected to shear stress because of the high electric potential. As soon as the electrostatic force in the solution is greater than the surface tension, droplets move to the collecting plate [147]. The solvent then evaporates. Electrospraying is used to encapsulate unstable and poorly water-soluble bioactive substances or drugs [148]. Using this method, homogenous nano-sized particles can be formed. It is possible to control particle size by changing the operating parameters; for example, the carrier material has a significant impact on the process [148]. Such a material can be protein-based, which provides aggregation stability, the possibility of different structure forms, and better water dispersibility [148]. Other parameters that have an impact on particle size are electric potential, flow rate, collector distance, viscosity, electrical conductivity, and surface tension [149].
In the study by Mahalakshmi et al., electrospraying was used for encapsulation of curcumin into whey protein [148]. First, they prepared an oil-in-water emulsion. The water phase was achieving by dissolving whey protein in distilled water. For preparation of the oil phase, different quantities of curcumin were dissolved in coconut oil. As an emulsifier in the water phase, Tween 80 was used. Both phases were mixed together to form the final O/W emulsion. The emulsion was sprayed through a needle with the use of electrospraying to obtain nanoparticles. The particles were formed with the use of a 14 kV voltage source at a flow rate of 0.2 mL/h. The particles had a size of less than 500 nm and the electrospraying method achieved 5 to 7% better encapsulation efficiency than the same experiment conducted with conventional spray drying. The encapsulated particles produced using spray drying had a size ranging from 2 to 10 μm [148].
3.6.2. Electrospinning
The electrospinning method produces continuous nanofibers by applying an electrical charge to a polymeric solution. The solution forms a cone on the tip of nozzle because of the electrification. A fluid jet is ejected from the cone toward the grounded collector [150]. Based on different parameters, the size and morphology of fibers can be changed. Apart from this advantage, the electrospinning method manufactures nanofibers that are light, with high porosity and excellent mechanical properties [151]. This method has found application in, for example, food packaging, drug delivery, water treatment, antibacterial and antioxidant materials, catalysis, and wound healing [151,152].
In the study by Pires et al., electrospinning was used for the encapsulation of curcumin into potato starch [153]. The starch solution was mixed with formic acid and shaken for 24 h. Curcumin was added to the starch solution at different dry base concentrations. After different periods of time, the polymer solution was used in electrospraying. The flow of the solution was 0.60 mL/h with an applied +23 kV voltage. The fibers had a mean diameter ranging from 94 nm to 464 nm. The achieved loading capacity was 79.01% to 97.09%.
The advantages and disadvantages of encapsulation methods are summarized in Table 2.

Table 2.
Advantages and disadvantages of encapsulation methods.
4. Future Trends
As previously mentioned, the encapsulation approach holds a promising future in pharmaceutical, food, cosmetics, biomedical, construction, biophysical, and biochemical industries. The most significant advantage would come from developing systems that deliver ingredients precisely where and when they are needed. A controlled and prompt release of the ingredients would also be advantageous. Vitamins, minerals, and other bioactives, which are highly volatile, can be encapsulated to help extend the shelf life and quality of food. In addition, the clever improvement in the sensor-empowered exemplification of medications and prescriptions for constant observation and input of the embodiment frameworks would be a significant advancement, thus enhancing the preparation of the well-being ventures and improving their applicability.
5. Conclusions
The process of encapsulation is a highly effective method for preserving and enhancing the physico-chemical properties of active compounds in a range of different applications. These can be, for example, in food, pharmaceutical, nutraceutical, and other industries. In food and pharmaceutical applications, encapsulation has gained more attention in recent years, as evidenced by the increasing number of publications in the Web of Science and the growing market demand. In this review, we focused mainly on food and pharmaceutical industries, where we looked at the methods most commonly used in the past decade. Future research aims are to develop methods with higher encapsulation efficiency that can be easily scaled up for different industries to reduce costs. The selection of an appropriate encapsulation method for a specific group of active compounds is crucial. Therefore, ongoing research is likely to be focused on developing new encapsulation methods and coating materials.
Author Contributions
Conceptualization, L.L. and D.Ř.; methodology, D.Ř. and Y.M.; resources, L.L.; data curation, D.Ř.; writing—original draft preparation, D.Ř.; writing—review and editing, A.K.; visualization, Y.M.; supervision, L.L.; project administration, L.L.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project number IGA_PrF_2023_024 of Internal Student Grant Agency of the Palacký University in Olomouc, Czech Republic.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials—A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.-S.; Pushpamalar, J.; Choo, W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process. Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; Fernandes, R.V.D.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable by-products for food application—A review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Food Encapsulation Market Size, Share, Global Trends, Forecasts to 2027. Available online: https://www.marketsandmarkets.com/Market-Reports/food-encapsulation-advanced-technologies-and-global-market-68.html (accessed on 10 May 2023).
- Gao, J.; Jin, P.; Zhang, Y.; Dong, H.; Wang, R. Fast-responsive capsule based on two soluble components for self-healing concrete. Cem. Concr. Compos. 2022, 133, 104711. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Mahmood, F.; Rehman, S.K.U.; Jameel, M.; Riaz, N.; Javed, M.F.; Salmi, A.; Awad, Y.A. Self-Healing Bio-Concrete Using Bacillus subtilis Encapsulated in Iron Oxide Nanoparticles. Materials 2022, 15, 7731. [Google Scholar] [CrossRef]
- Li, H.; Wang, X. Preparation of microcapsules with IPDI monomer and isocyanate prepolymer as self-healing agent and their application in self-healing materials. Polymer 2022, 262, 125478. [Google Scholar] [CrossRef]
- Papaioannou, S.; Amenta, M.; Kilikoglou, V.; Gournis, D.; Karatasios, I. Critical Aspects in the Development and Integration of Encapsulated Healing Agents in Cement and Concrete. J. Adv. Concr. Technol. 2021, 19, 301–320. [Google Scholar] [CrossRef]
- Reda, M.A.; Chidiac, S.E. Performance of Capsules in Self-Healing Cementitious Material. Materials 2022, 15, 7302. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Evaluation and failure analysis of linseed oil encapsulated self-healing anticorrosive coating. Prog. Org. Coat. 2018, 118, 108–115. [Google Scholar] [CrossRef]
- He, S.; Gao, Y.; Gong, X.; Wu, C.; Cen, H. Advance of design and application in self-healing anticorrosive coating: A review. J. Coat. Technol. Res. 2023, 20, 819–841. [Google Scholar] [CrossRef]
- Ouarga, A.; Lebaz, N.; Tarhini, M.; Noukrati, H.; Barroug, A.; Elaissari, A.; Ben Youcef, H. Towards smart self-healing coatings: Advances in micro/nano-encapsulation processes as carriers for anti-corrosion coatings development. J. Mol. Liq. 2022, 354, 118862. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Chen, M.; Li, X.; Zhu, Z.; Yan, J. Effects of independently designed and prepared self-healing granules on self-healing efficiency for cement cracks. Constr. Build. Mater. 2022, 347, 128626. [Google Scholar] [CrossRef]
- Reshma, V.; Mohanan, P. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Lisi, F.; Sawayama, J.; Gautam, S.; Rubanov, S.; Duan, X.; Kirkwood, N. Re-Examination of the Polymer Encapsulation of Quantum Dots for Biological Applications. ACS Appl. Nano Mater. 2023, 6, 4046–4055. [Google Scholar] [CrossRef]
- Ahmed, S.; Lahkar, S.; Doley, S.; Mohanta, D.; Dolui, S.K. A hierarchically porous MOF confined CsPbBr3 quantum dots: Fluorescence switching probe for detecting Cu (II) and melamine in food samples. J. Photochem. Photobiol. A Chem. 2023, 443, 114821. [Google Scholar] [CrossRef]
- Prieto, C.; Talón, E.; Lagaron, J. Room temperature encapsulation of algae oil in water insoluble gluten extract. Food Hydrocoll. Health 2021, 1, 100022. [Google Scholar] [CrossRef]
- Noor, A.; Al Murad, M.; Chitra, A.J.; Babu, S.N.; Govindarajan, S. Alginate based encapsulation of polyphenols of Piper betel leaves: Development, stability, bio-accessibility and biological activities. Food Biosci. 2022, 47, 101715. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.O.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis Benth Extract in Alginate Beads Enhances the Stability and Antioxidant Activity of Polyphenols under Simulated Gastrointestinal Digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, D.; Yu, H.; Han, J.; Liu, W.; Qu, D. Encapsulation of Salmonella phage SL01 in alginate/carrageenan micro-capsules as a delivery system and its application in vitro. Front. Microbiol. 2022, 13, 2718. [Google Scholar]
- Gupta, P.; Preet, S.; Ananya; Singh, N. Preparation of Thymus vulgaris (L.) essential oil nanoemulsion and its chitosan encapsulation for controlling mosquito vectors. Sci. Rep. 2022, 12, 4335. [Google Scholar] [CrossRef]
- Chomchoey, S.; Klongdee, S.; Peanparkdee, M.; Klinkesorn, U. Fabrication and characterization of nanoemulsions for encapsulation and delivery of vitexin: Antioxidant activity, storage stability and in vitro digestibility. J. Sci. Food Agric. 2023, 103, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Opustilová, K.; Lapčíková, B.; Lapčík, L.; Gautam, S.; Valenta, T.; Li, P. Physico-Chemical Study of Curcumin and Its Application in O/W/O Multiple Emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Dutta, A.; Kundu, A.; Saha, S. Resin Assisted Purification of Anthocyanins and Their Encapsulation. J. Chem. Educ. 2023, 100, 885–892. [Google Scholar] [CrossRef]
- Savoldi, T.E.; Scheufele, F.B.; Drunkler, D.A.; da Silva, G.J.; de Lima, J.D.; Maestre, K.L.; Triques, C.C.; da Silva, E.A.; Fiorese, M.L. Microencapsulation of Saccharomyces boulardii using vegan and vegetarian wall materials. J. Food Process. Preserv. 2022, 46, e16596. [Google Scholar] [CrossRef]
- Blagojević, B.; Četojević-Simin, D.; Djurić, S.; Lazzara, G.; Milioto, S.; Agić, D.; Vasile, B.S.; Popović, B.M. Anthocyanins and phenolic acids from Prunus spinosa L. encapsulation in halloysite and maltodextrin based carriers. Appl. Clay Sci. 2022, 222, 106489. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Shakoury, N.; Aliyari, M.A.; Salami, M.; Emam-Djomeh, Z.; Vardhanabhuti, B.; Moosavi-Movahedi, A.A. Encapsulation of propolis extract in whey protein nanoparticles. LWT 2022, 158, 113138. [Google Scholar] [CrossRef]
- Villar, M.A.L.; Vidallon, M.L.P.; Rodriguez, E.B. Nanostructured lipid carrier for bioactive rice bran gamma-oryzanol. Food Biosci. 2022, 50, 102064. [Google Scholar] [CrossRef]
- Enayati, M.; Madarshahian, S.; Yan, B.; Ufheil, G.; Abbaspourrad, A. Granulation and encapsulation of N-Acetylcysteine (NAC) by internal phase separation. Food Hydrocoll. 2022, 130, 107699. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; McClements, D.J. Biopolymer nanoparticles as potential delivery systems for anthocyanins: Fabrication and properties. Food Res. Int. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Yin, Z.; Dong, J. Geotrichum candidum arthrospore cell wall particles as a novel carrier for curcumin encapsulation. Food Chem. 2023, 404, 134308. [Google Scholar] [CrossRef] [PubMed]
- Thauer, E.; Shi, X.; Zhang, S.; Chen, X.; Deeg, L.; Klingeler, R.; Wenelska, K.; Mijowska, E. Mn3O4 encapsulated in hollow carbon spheres coated by graphene layer for enhanced magnetization and lithium-ion batteries performance. Energy 2021, 217, 119399. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Doong, R.-A.; Li, T.-C.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Hollow magnetic-fluorescent nanoparticles for dual-modality virus detection. Biosens. Bioelectron. 2020, 170, 112680. [Google Scholar] [CrossRef] [PubMed]
- Surynek, M.; Spanhel, L.; Lapcik, L.; Mrazek, J. Tuning the photocatalytic properties of sol–gel-derived single, coupled, and alloyed ZnO–TiO2 nanoparticles. Res. Chem. Intermed. 2019, 45, 4193–4204. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Bacteriophage | Definition, Life Cycle, & Research | Britannica. Available online: https://www.britannica.com/science/bacteriophage (accessed on 3 January 2022).
- Rahimzadeh, G.; Saeedi, M.; Moosazadeh, M.; Hashemi, S.M.H.; Babaei, A.; Rezai, M.S.; Kamel, K.; Asare-Addo, K.; Nokhodchi, A. Encapsulation of bacteriophage cocktail into chitosan for the treatment of bacterial diarrhea. Sci. Rep. 2021, 11, 15603. [Google Scholar] [CrossRef]
- Huff, W.; Huff, G.; Rath, N.; Donoghue, A. Method of administration affects the ability of bacteriophage to prevent colibacillosis in 1-day-old broiler chickens. Poult. Sci. 2013, 92, 930–934. [Google Scholar] [CrossRef]
- Kaikabo, A.A.; Mohammed, A.S.; Abas, F. Chitosan Nanoparticles as Carriers for the Delivery of ΦKAZ14 Bacteriophage for Oral Biological Control of Colibacillosis in Chickens. Molecules 2016, 21, 256. [Google Scholar] [CrossRef]
- Camelo-Silva, C.; Verruck, S.; Ambrosi, A.; Di Luccio, M. Innovation and Trends in Probiotic Microencapsulation by Emulsification Techniques. Food Eng. Rev. 2022, 14, 462–490. [Google Scholar] [CrossRef]
- Fujiu, K.B.; Kobayashi, I.; Uemura, K.; Nakajima, M. Temperature effect on microchannel oil-in-water emulsification. Microfluid. Nanofluid. 2011, 10, 773–783. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2010, 7, 2297–2316. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Calabrese, V.; Courtenay, J.C.; Edler, K.J.; Scott, J.L. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr. Opin. Green Sustain. Chem. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Comunian, T.A.; Anthero, A.G.D.S.; Bezerra, E.O.; Moraes, I.C.F.; Hubinger, M.D. Encapsulation of Pomegranate Seed Oil by Emulsification Followed by Spray Drying: Evaluation of Different Biopolymers and Their Effect on Particle Properties. Food Bioprocess Technol. 2019, 13, 53–66. [Google Scholar] [CrossRef]
- Dinkgreve, M.; Velikov, K.P.; Bonn, D. Stability of LAPONITE®-stabilized high internal phase Pickering emulsions under shear. Phys. Chem. Chem. Phys. 2016, 18, 22973–22977. [Google Scholar] [CrossRef]
- Ganley, W.J.; van Duijneveldt, J.S. Controlling the Rheology of Montmorillonite Stabilized Oil-in-Water Emulsions. Langmuir 2017, 33, 1679–1686. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef]
- Dini, C.; Islan, G.A.; de Urraza, P.J.; Castro, G.R. Novel Biopolymer Matrices for Microencapsulation of Phages: Enhanced Protection Against Acidity and Protease Activity. Macromol. Biosci. 2012, 12, 1200–1208. [Google Scholar] [CrossRef]
- Kim, S.-G.; Giri, S.S.; Jo, S.-J.; Kang, J.-W.; Lee, S.-B.; Jung, W.-J.; Lee, Y.-M.; Kim, H.-J.; Kim, J.-H.; Park, S.-C. Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation. Antibiotics 2022, 11, 1264. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated Probiotics: Potential Techniques and Coating Materials for Non-Dairy Food Applications. Appl. Sci. 2022, 12, 10005. [Google Scholar] [CrossRef]
- Safiah Sabrina Hassan, Intan Nabihah Ahmad Fadzil, Anida Yusoff, and Khalilah Abdul Khalil. A Review on Microencap-sulation in Improving Probiotic Stability for Beverages Application. 2020. Available online: https://myjms.mohe.gov.my/index.php/SL/article/view/7900/5169 (accessed on 23 January 2023).
- Liliana, S.C.; Vladimir, V.C.; Serna-Cock, L.; Vallejo-Castillo, V. Probiotic encapsulation. Afr. J. Microbiol. Res. 2013, 7, 4743–4753. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, X.; Baxi, S.; Chambers, J.R.; Sabour, P.M.; Wang, Q. Whey protein improves survival and release characteristics of bacteriophage Felix O1 encapsulated in alginate microspheres. Food Res. Int. 2013, 52, 460–466. [Google Scholar] [CrossRef]
- Yin, H.; Li, J.; Huang, H.; Wang, Y.; Qian, X.; Ren, J.; Xue, F.; Dai, J.; Tang, F. Microencapsulated phages show prolonged stability in gastrointestinal environments and high therapeutic efficiency to treat Escherichia coli O157:H7 infection. Vet. Res. 2021, 52, 118. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.M.; Gajic, I.M.S.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants 2022, 11, 297. [Google Scholar] [CrossRef]
- Albadran, H.A.; Monteagudo-Mera, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of chitosan-coated agar-gelatin particles for probiotic delivery and targeted release in the gastrointestinal tract. Appl. Microbiol. Biotechnol. 2020, 104, 5749–5757. [Google Scholar] [CrossRef]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier Science: Amsterdam, The Netherlands, 2020; Available online: https://www.sciencedirect.com/science/article/pii/B9780128166628000217 (accessed on 10 January 2023).
- Mandal, A.; Bera, A.; Ojha, K.; Kumar, T. Characterization of Surfactant Stabilized Nanoemulsion and Its Use in Enhanced Oil Recovery. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2014, 5, 123–127. [Google Scholar] [CrossRef]
- Oprea, I.; Fărcaș, A.C.; Leopold, L.F.; Diaconeasa, Z.; Coman, C.; Socaci, S.A. Nano-Encapsulation of Citrus Essential Oils: Methods and Applications of Interest for the Food Sector. Polymers 2022, 14, 4505. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2017, 8, 39–47. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M. Nanoemulsions in Food Industry. In Some New Aspects of Colloidal Systems in Foods; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, properties, and applications of solid self-emulsifying delivery systems (S-SEDS) in the food and pharmaceutical industries. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Jasmina, H.; Džana, O.; Alisa, E.; Edina, V.; Ognjenka, R. Preparation of Nanoemulsions by high-energy and lowenergy emulsification methods. In CMBEBIH 2017: Proceedings of the International Conference on Medical and Biological Engineering 2017; Springer Verlag: Berlin/Heidelberg, Germany, 2017; Volume 62, pp. 317–322. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Moghaddasi, F.; Housaindokht, M.R.; Darroudi, M.; Bozorgmehr, M.R.; Sadeghi, A. Synthesis of nano curcumin using black pepper oil by O/W Nanoemulsion Technique and investigation of their biological activities. LWT 2018, 92, 92–100. [Google Scholar] [CrossRef]
- Dey, T.K.; Ghosh, S.; Ghosh, M.; Koley, H.; Dhar, P. Comparative study of gastrointestinal absorption of EPA & DHA rich fish oil from nano and conventional emulsion formulation in rats. Food Res. Int. 2012, 49, 72–79. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.M.; Choi, W.I. Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.K.; Muzammil, M.S.; Dhandayuthabani, R.; Kumari, V.S. Development of nanoemulsion of Alginate/Aloe vera for oral delivery of insulin. Mater. Today Proc. 2021, 36, 357–363. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Garcia, J.; Giralt, E.; Teixidó, M. Using peptides to increase transport across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 355–366. [Google Scholar] [CrossRef]
- Meng, Q.; Long, P.; Zhou, J.; Ho, C.-T.; Zou, X.; Chen, B.; Zhang, L. Improved absorption of β-carotene by encapsulation in an oil-in-water nanoemulsion containing tea polyphenols in the aqueous phase. Food Res. Int. 2019, 116, 731–736. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-Drying Microencapsulation of Polyphenol Bioactives: A Comparative Study Using Different Natural Fibre Polymers as Encapsulants. Food Bioprocess Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2022, 1–33. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Kemp, I.C.; Wadley, R.; Hartwig, T.; Cocchini, U.; See-Toh, Y.; Gorringe, L.; Fordham, K.; Ricard, F. Experimental Study of Spray Drying and Atomization with a Two-Fluid Nozzle to Produce Inhalable Particles. Dry. Technol. 2013, 31, 930–941. [Google Scholar] [CrossRef]
- Spray Drying Basics—Spray Drying Nozzles. Available online: https://spraydryingnozzles.com/spray-drying-basics/ (accessed on 16 January 2023).
- Van Deventer, H.; Houben, R.; Koldeweij, R. New Atomization Nozzle for Spray Drying. Dry. Technol. 2013, 31, 891–897. [Google Scholar] [CrossRef]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Stepanic, E.M.; Tibaldo, A.M.; Pavón, Y.L.; Santiago, L.G. Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Res. Int. 2019, 119, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.C.S.; da Silva, M.F.d.G.F.; Fernandes, J.B.; Forim, M.R. Evaluation of the microencapsulation of orange essential oil in biopolymers by using a spray-drying process. Sci. Rep. 2020, 10, 11799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, C.; Song, X.; Zeng, J.; Zhang, L.; Gong, P. Spray drying co-encapsulation of lactic acid bacteria and lipids: A review. Trends Food Sci. Technol. 2022, 129, 134–143. [Google Scholar] [CrossRef]
- Martinić, A.; Kalušević, A.; Lević, S.; Nedović, V.; Cebin, A.V.; Karlović, S.; Špoljarić, I.; Mršić, G.; Žižek, K.; Komes, D. Microencapsulation of Dandelion (Taraxacum officinale L.) Leaf Extract by Spray Drying. Food Technol. Biotechnol. 2022, 60, 237–252. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Levic, S.; Kalusevic, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedovic, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, L.; Tian, H.; Luo, X.; Liu, S. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 115065. [Google Scholar] [CrossRef] [PubMed]
- Guowei, S.; Yang, X.; Li, C.; Huang, D.; Lei, Z.; He, C. Comprehensive optimization of composite cryoprotectant for Saccharomyces boulardii during freeze-drying and evaluation of its storage stability. Prep. Biochem. Biotechnol. 2019, 49, 846–857. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y. A novel method for the preparation of liposomes: Freeze drying of monophase solutions. J. Pharm. Sci. 2004, 93, 1403–1414. [Google Scholar] [CrossRef]
- Ghasemi, S.; Assadpour, E.; Kharazmi, M.S.; Jafarzadeh, S.; Zargar, M.; Jafari, S.M. Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions. Foods 2023, 12, 831. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Fawrin, H.; Siahaan, P. Simultant encapsulation of vitamin C and beta-carotene in sesame (Sesamum indicum L.) liposomes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012014. [Google Scholar] [CrossRef]
- Gomez, A.G.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef]
- El-Kader, A.A.; Abu Hashish, H. Encapsulation techniques of food bioproduct. Egypt. J. Chem. 2020, 63, 1881–1909. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Castañeda-Reyes, E.D.; Perea-Flores, M.D.J.; Davila-Ortiz, G.; Lee, Y.; de Mejia, E.G. Development, Characterization and Use of Liposomes as Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review. Int. J. Nanomed. 2020, 15, 7627–7650. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Tripathy, S.; Srivastav, P.P. Encapsulation of Centella asiatica leaf extract in liposome: Study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chem. Adv. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hamishehkar, H.; McClements, D.J.; Shahvalizadeh, R.; Barri, A. Encapsulation of Spirulina protein hydrolysates in liposomes: Impact on antioxidant activity and gastrointestinal behavior. Food Chem. 2023, 400, 133973. [Google Scholar] [CrossRef]
- Liposomes and Lipid Nanoparticles as Delivery Vehicles for Personalized Medicine. Available online: https://www.exeleadbiopharma.com/news/liposomes-and-lipid-nanoparticles-as-delivery-vehicles-for-personalized-medicine (accessed on 13 April 2023).
- da Silva, G.H.R.; de Moura, L.D.; de Carvalho, F.V.; Geronimo, G.; Mendonça, T.C.; de Lima, F.F.; de Paula, E. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules 2021, 26, 6929. [Google Scholar] [CrossRef]
- Azar, F.A.N.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, W.; Li, Y.; Liu, G. Influence of different solid lipids on the properties of a novel nanostructured lipid carrier containing Antarctic krill oil. Int. J. Food Sci. Technol. 2022, 57, 2886–2895. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; McClements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Pavani, M.; Singha, P.; Dash, D.R.; Asaithambi, N.; Singh, S.K. Novel encapsulation approaches for phytosterols and their importance in food products: A review. J. Food Process. Eng. 2022, 45, e14041. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Tordera, F.; Fabra, M.J.; Martínez-Sanz, M.; Lopez-Rubio, A. Coaxial electrospraying of biopolymers as a strategy to improve protection of bioactive food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 2–11. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of bioactive compounds using competitive emerging techniques: Electrospraying, nano spray drying, and electrostatic spray drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Zadbashkhanshir, K.; Fadaei, V.; Fahimdanesh, M. Canola meal phenolic compounds electrosprayed into capsules to increase the oxidative stability of canola oil. Chem. Biol. Technol. Agric. 2023, 10, 4. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Choudhary, P.; Moses, J.; Anandharamakrishnan, C. Emulsion electrospraying and spray drying of whey protein nano and microparticles with curcumin. Food Hydrocoll. Health 2023, 3, 100122. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Bellan, L.M.; Craighead, H.G. Applications of controlled electrospinning systems. Polym. Adv. Technol. 2011, 22, 304–309. [Google Scholar] [CrossRef]
- Du, Z.; Lv, H.; Wang, C.; He, D.; Xu, E.; Jin, Z.; Yuan, C.; Guo, L.; Wu, Z.; Liu, P.; et al. Organic solvent-free starch-based green electrospun nanofiber mats for curcumin encapsulation and delivery. Int. J. Biol. Macromol. 2023, 232, 123497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Khan, M.A.; Chen, K.; Zhang, L.; Chen, X. Electrospinning of Natural Biopolymers for Innovative Food Applications: A Review. Food Bioprocess Technol. 2022, 16, 704–725. [Google Scholar] [CrossRef]
- Pires, J.B.; Fonseca, L.M.; Siebeneichler, T.J.; Crizel, R.L.; dos Santos, F.N.; Hackbart, H.C.D.S.; Kringel, D.H.; Meinhart, A.D.; Zavareze, E.D.R.; Dias, A.R.G. Curcumin encapsulation in capsules and fibers of potato starch by electrospraying and electrospinning: Thermal resistance and antioxidant activity. Food Res. Int. 2022, 162, 112111. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Futur. Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Mudalip, S.A.; Khatiman, M.; Hashim, N.; Man, R.C.; Arshad, Z. A short review on encapsulation of bioactive compounds using different drying techniques. Mater. Today Proc. 2021, 42, 288–296. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Barroso, L.; Viegas, C.; Vieira, J.; Ferreira-Pêgo, C.; Costa, J.; Fonte, P. Lipid-based carriers for food ingredients delivery. J. Food Eng. 2021, 295, 110451. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Plati, F.; Paraskevopoulou, A. Micro- and Nano-encapsulation as Tools for Essential Oils Advantages’ Exploitation in Food Applications: The Case of Oregano Essential Oil. Food Bioprocess Technol. 2022, 15, 949–977. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, M.; Xu, X.; Liu, X.; Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: A review. Front. Nutr. 2022, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).