Fermented Kamut Sprout Extract Decreases Cell Cytotoxicity and Increases the Anti-Oxidant and Anti-Inflammation Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Microorganisms
2.3. Fermentation of KaS Using Solid-State Fermentation Technology
2.4. Preparation of KaS Extracts
2.5. β-Glucan
2.6. Polyphenols
2.7. Anti-Oxidant Efficacy Test
2.8. Cell Cytotoxicity
2.9. Determination of the Anti-Inflammation Effect
2.9.1. Measurement of NO Values
2.9.2. mRNA Expression Levels of IL-6
2.9.3. mRNA Expression Levels of COX-2 and IL-1β
2.10. Statistical Analysis
3. Results
3.1. Optimization of Solid-State Fermentation (SsF)
3.2. Bioactive Substances in Fermented KaS
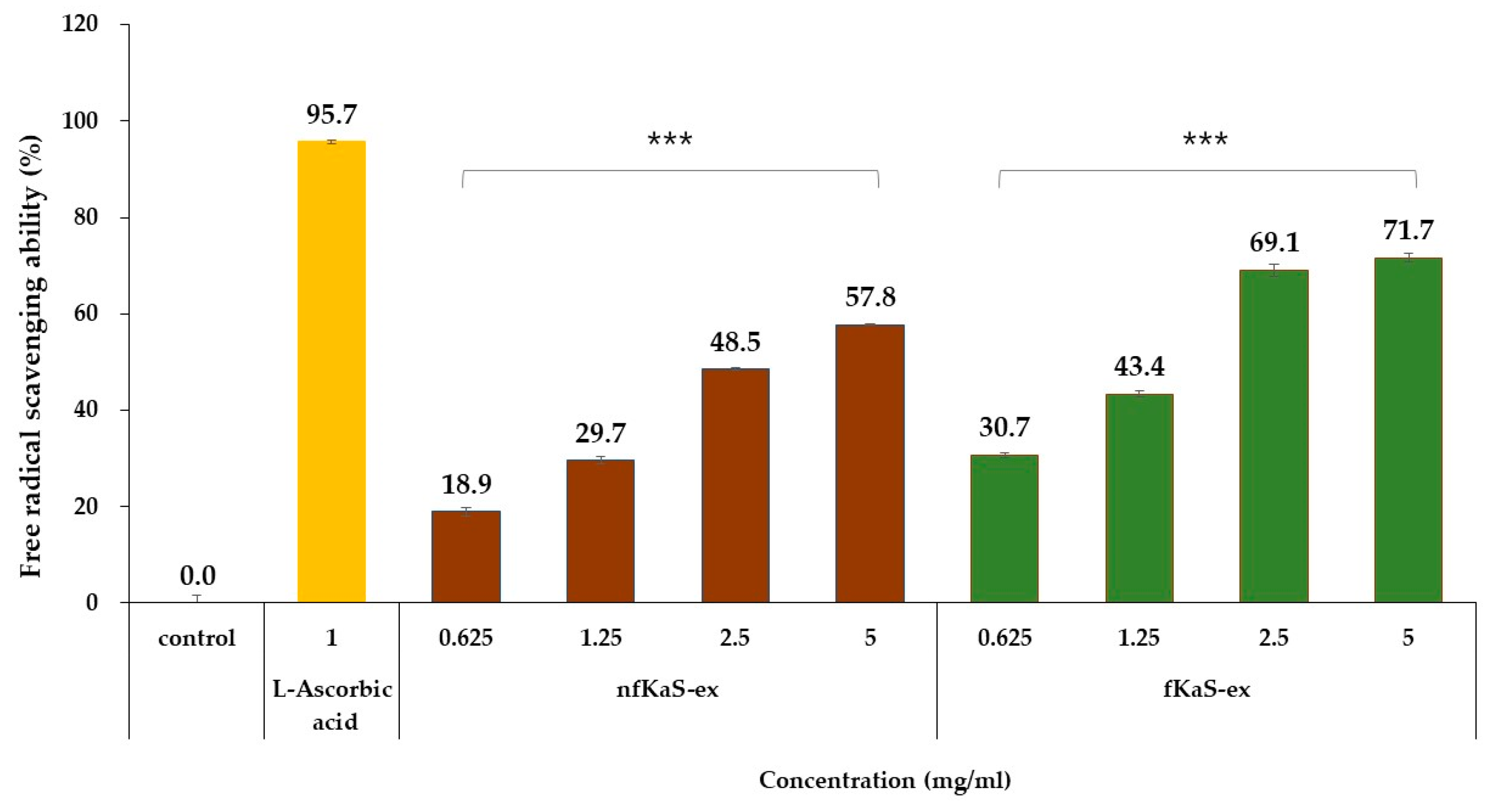
3.3. Anti-Oxidant Efficacy
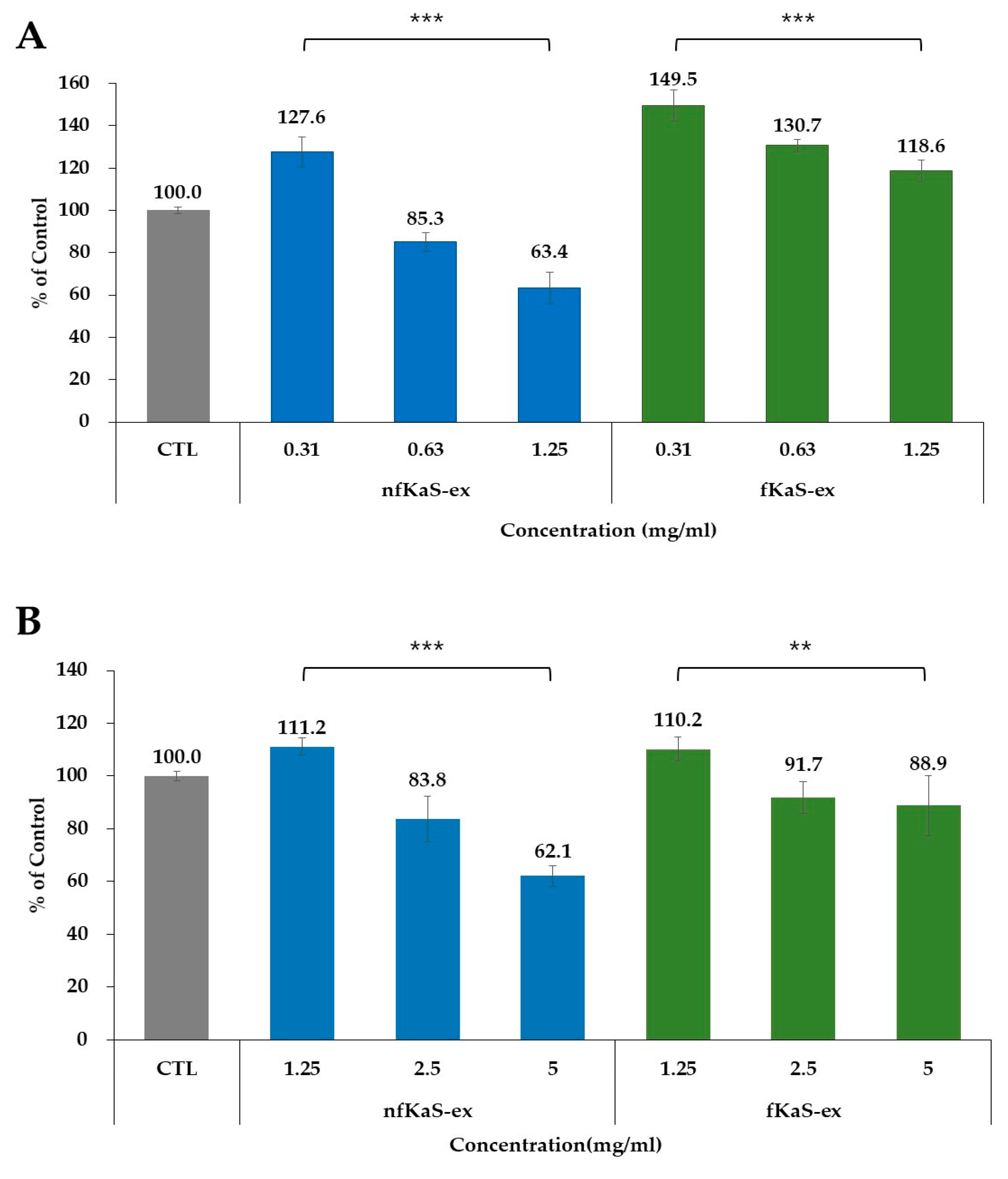
3.4. Cytotoxicity
3.5. Anti-Inflammatory Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piergiovanni, A.; Simeone, R.; Pasqualone, A. Oriental wheat, an underutilised tetraploid wheat species. A case study: Nutritional and technological traits of Kamut. Food 2009, 3, 33–38. [Google Scholar]
- Van Boxstael, F.; Aerts, H.; Linssen, S.; Latre, J.; Christiaens, A.; Haesaert, G.; Dierickx, I.; Brusselle, J.; De Keyzer, W. A comparison of the nutritional value of Einkorn, Emmer, Khorasan and modern wheat: Whole grains, processed in bread, and population-level intake implications. J. Sci. Food Agric. 2020, 100, 4108–4118. [Google Scholar] [CrossRef] [PubMed]
- Dapcevic-Hadnadev, T.; Stupar, A.; Stevanovic, D.; Skrobot, D.; Maravic, N.; Tomic, J.; Hadnadev, M. Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread. Foods 2022, 11, 3985. [Google Scholar] [CrossRef]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring Production in Kamut(R), Quinoa and Wheat Doughs Fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis: A SPME-GC/MS Study. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, A.M.H.; Seleem, H.A.; Galal, W.K. Assessment of Kamut® wheat quality. World Rev. Sci. Technol. Sustain. Dev. 2012, 9, 194–203. [Google Scholar] [CrossRef]
- Valli, V.; Danesi, F.; Gianotti, A.; Di Nunzio, M.; Taneyo Saa, D.L.; Bordoni, A. Antioxidative and anti-inflammatory effect of in vitro digested cookies baked using different types of flours and fermentation methods. Food Res. Int. 2016, 88, 256–262. [Google Scholar] [CrossRef]
- Bordoni, A.; Danesi, F.; Di Nunzio, M.; Taccari, A.; Valli, V. Ancient wheat and health: A legend or the reality? A review on KAMUT khorasan wheat. Int. J. Food. Sci. Nutr. 2017, 68, 278–286. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Benedetti, S.; Primiterra, M.; Tagliamonte, M.C.; Carnevali, A.; Gianotti, A.; Bordoni, A.; Canestrari, F. Counteraction of oxidative damage in the rat liver by an ancient grain (Kamut brand khorasan wheat). Nutrition 2012, 28, 436–441. [Google Scholar] [CrossRef]
- Sofi, F.; Whittaker, A.; Cesari, F.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Marotti, I.; Dinelli, G.; Casini, A.; Abbate, R.; et al. Characterization of Khorasan wheat (Kamut) and impact of a replacement diet on cardiovascular risk factors: Cross-over dietary intervention study. Eur. J. Clin. Nutr. 2013, 67, 190–195. [Google Scholar] [CrossRef]
- Taneyo Saa, D.; Turroni, S.; Serrazanetti, D.I.; Rampelli, S.; Maccaferri, S.; Candela, M.; Severgnini, M.; Simonetti, E.; Brigidi, P.; Gianotti, A. Impact of Kamut® Khorasan on gut microbiota and metabolome in healthy volunteers. Food Res. Int. 2014, 63, 227–232. [Google Scholar] [CrossRef]
- Siervo, M.; Shannon, O.M.; Llewellyn, D.J.; Stephan, B.C.; Fontana, L. Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic. Biol. Med. 2021, 176, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Kucek, L.K.; Veenstra, L.D.; Amnuaycheewa, P.; Sorrells, M.E. A Grounded Guide to Gluten: How Modern Genotypes and Processing Impact Wheat Sensitivity. Compr. Rev. Food Sci. 2015, 14, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Márton, M.; Mándoki, Z.; Zs, C.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae 2010, 3, 81–117. [Google Scholar]
- Mechery, J.; Shaheen, A. Peripartum cardiomyopathy and pre-eclampsia: An infrequently associated obstetric emergency. J. Obs. Gynaecol. 2005, 25, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tsuji, G.; Hashimoto-Hachiya, A.; Furue, M. Galactomyces Ferment Filtrate Potentiates an Anti-Inflammaging System in Keratinocytes. J. Clin. Med. 2022, 11, 6338. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.H.; Yoo, J.S. Analysis of ceramides in cosmetics by reversed-phase liquid chromatography/electrospray ionization mass spectrometry with collision-induced dissociation. Rapid Commun. Mass Spectrom. 2003, 17, 64–75. [Google Scholar] [CrossRef]
- Kurian, J.V. A New Polymer Platform for the Future—Sorona® from Corn Derived 1,3-Propanediol. J. Polym. Environ. 2005, 13, 159–167. [Google Scholar] [CrossRef]
- Waksman, S.A.; Reilly, H.C.; Johnstone, D.B. Isolation of Streptomycin-producing Strains of Streptomyces griseus. J. Bacteriol. 1946, 52, 393–397. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Chelule, P.; Mokoena, M.; Gqaleni, N. Advantages of traditional lactic acid bacteria fermentation of food in Africa. Appl. Microbiol. Biotechnol. 2010, 2, 1160–1167. [Google Scholar]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ahn, J.-M.; Kwon, O.J.; Kim, S.H. β-Glucan Contents and Anti-wrinkling Effects of Brown Rice Phellinus linteus Mycelium Extracts Fermented with Lactobacillus plantarum. Asian J. Beauty Cosmetol. 2016, 14, 127–137. [Google Scholar] [CrossRef]
- Teleky, B.E.; Martau, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 17535. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Han, J.-W.; Choi, J.-H.; Shin, K.-O. Quality Characteristics and Antioxidant Activity of White Bread Added with Germinated Kamut (Triticum turanicum Jakubz) Powder. J. East Asian Soc. Diet Life 2020, 30, 345–354. [Google Scholar] [CrossRef]
- Balestra, F.; Laghi, L.; Taneyo Saa, D.; Gianotti, A.; Rocculi, P.; Pinnavaia, G. Physico-chemical and metabolomic characterization of KAMUT(R) Khorasan and durum wheat fermented dough. Food Chem. 2015, 187, 451–459. [Google Scholar] [CrossRef]
- Skrobot, D.; Dapcevic-Hadnadev, T.; Tomic, J.; Maravic, N.; Popovic, N.; Jovanov, P.; Hadnadev, M. Techno-Functional Performance of Emmer, Spelt and Khorasan in Spontaneously Fermented Sourdough Bread. Foods 2022, 11, 3927. [Google Scholar] [CrossRef]
- Holker, U.; Lenz, J. Solid-state fermentation--are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Onyema, V.O.; Amadi, O.C.; Moneke, A.N.; Agu, R.C. A Brief Review: Saccharomyces cerevisiae Biodiversity Potential and Promising Cell Factories for Exploitation in Biotechnology and Industry Processes—West African Natural Yeasts Contribution. Food Chem. Adv. 2023, 2, 100162. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Duan, H.; Qiao, N.; Wang, G.; Zhao, J.; Zhai, Q.; Tian, F.; Chen, W. Latilactobacillus sakei: A candidate probiotic with a key role in food fermentations and health promotion. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Kang, K.K.; Yoo, Y.-B.; Kim, B.H.; Bae, S.-H. Dietary Fiber and β-Glucan Contents of Sparassis crispa Fruit Fermented with Lactobacillus brevis and Monascus pilosus. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1740–1746. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of beta-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Jung, S.H.; Kim, J.S.; Choi, J. Toxic potentiality of bio-oils, from biomass pyrolysis, in cultured cells and Caenorhabditis elegans. Environ. Toxicol. 2014, 29, 1409–1419. [Google Scholar] [CrossRef]
- Gao, J.; Chen, F.; Fang, H.; Mi, J.; Qi, Q.; Yang, M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol. Res. 2020, 53, 48. [Google Scholar] [CrossRef]
- Ashida, M.; Bito, T.; Budiyanto, A.; Ichihashi, M.; Ueda, M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp. Dermatol. 2003, 12, 445–452. [Google Scholar] [CrossRef]
- de Mello Andrade, J.M.; Fasolo, D. Chapter 20—Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 253–265. [Google Scholar] [CrossRef]
- Dalvi, R.R.; Bowie, W.C. Toxicology of solanine: An overview. Vet. Hum. Toxicol. 1983, 25, 13–15. [Google Scholar]
- van Buul, V.J.; Brouns, F.J.P.H. Health effects of wheat lectins: A review. J. Cereal Sci. 2014, 59, 112–117. [Google Scholar] [CrossRef]
- Balčiūnaitė-Murzienė, G.; Dzikaras, M. Wheat Germ Agglutinin—From Toxicity to Biomedical Applications. Appl. Sci. 2021, 11, 884. [Google Scholar] [CrossRef]
- Kehinde, A.; Tope. Fermented sprouted and unsprouted maize for ogi production. J. Adv. Res. 2013, 1, 428–434. [Google Scholar]
- Lee, Y.G.; Seo, J.H. Production of 2,3-butanediol from glucose and cassava hydrolysates by metabolically engineered industrial polyploid Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, X.; Zhu, L.; Liang, R.; Mao, Y.; Yang, X.; Niu, L.; Zhang, Y.; Dong, P. The biological effect of a beef-derived Latilactobacillus sakei on beef steaks during chilled storage. Food Sci. Nutr. 2023, 11, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, D. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. [Google Scholar]
- Greene, N.; Aleo, M.D.; Louise-May, S.; Price, D.A.; Will, Y. Using an in vitro cytotoxicity assay to aid in compound selection for in vivo safety studies. Bioorg. Med. Chem. 2010, 20, 5308–5312. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Canli, M.; Celik, E.E.; Kocadagli, T.; Kanmaz, E.O.; Gokmen, V. Formation of Bioactive Tyrosine Derivatives during Sprouting and Fermenting of Selected Whole Grains. J. Agric. Food Chem. 2021, 69, 12517–12526. [Google Scholar] [CrossRef]




| Fermentation Condition | Fermentation Time (Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| Inoculation | 1st | 2nd | 3rd | Inoculation | 4th | 5th | 6th | |
| (1) | S. cerevisiae |  | La. sakei |  | ||||
| - | - | Sampling | - | - | Sampling | |||
| (2) | S. cerevisiae + La. sakei |  | ||||||
| - | - | Sampling | - | - | - | Sampling | ||
| (3) | La. sakei |  | S. Cerevisiae |  | ||||
| - | - | Sampling | - | - | Sampling | |||
| Sample | β-Glucan Content (mg/g dw) | Total Polyphenol Content (mg/g dw) |
|---|---|---|
| nfKaS-ex 1 | 34.61 ± 0.05 | 25.2 ± 0.02 |
| pre-fKaS-ex 2 | 38.45 ± 0.04 | 25.7 ± 0.04 |
| fKaS-ex 3 | 46.88 ± 0.03 | 26.3 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ki, H.; Baek, J.-S.; Hawkes, H.-J.K.; Kim, Y.S.; Hwang, K.Y. Fermented Kamut Sprout Extract Decreases Cell Cytotoxicity and Increases the Anti-Oxidant and Anti-Inflammation Effect. Foods 2023, 12, 2107. https://doi.org/10.3390/foods12112107
Ki H, Baek J-S, Hawkes H-JK, Kim YS, Hwang KY. Fermented Kamut Sprout Extract Decreases Cell Cytotoxicity and Increases the Anti-Oxidant and Anti-Inflammation Effect. Foods. 2023; 12(11):2107. https://doi.org/10.3390/foods12112107
Chicago/Turabian StyleKi, Hosam, Jun-Seok Baek, Hye-Jin Kim Hawkes, Young Soo Kim, and Kwang Yeon Hwang. 2023. "Fermented Kamut Sprout Extract Decreases Cell Cytotoxicity and Increases the Anti-Oxidant and Anti-Inflammation Effect" Foods 12, no. 11: 2107. https://doi.org/10.3390/foods12112107
APA StyleKi, H., Baek, J.-S., Hawkes, H.-J. K., Kim, Y. S., & Hwang, K. Y. (2023). Fermented Kamut Sprout Extract Decreases Cell Cytotoxicity and Increases the Anti-Oxidant and Anti-Inflammation Effect. Foods, 12(11), 2107. https://doi.org/10.3390/foods12112107









