Smelling Peppers and Pout Submitted to Convective Drying: Mathematical Modeling, Thermodynamic Properties and Proximal Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Processing
2.2. Drying Kinetics
2.3. Effective Diffusivity
2.4. Thermodynamic Properties
2.5. Proximal Composition of in Natura and Dried Peppers
3. Results and Discussion
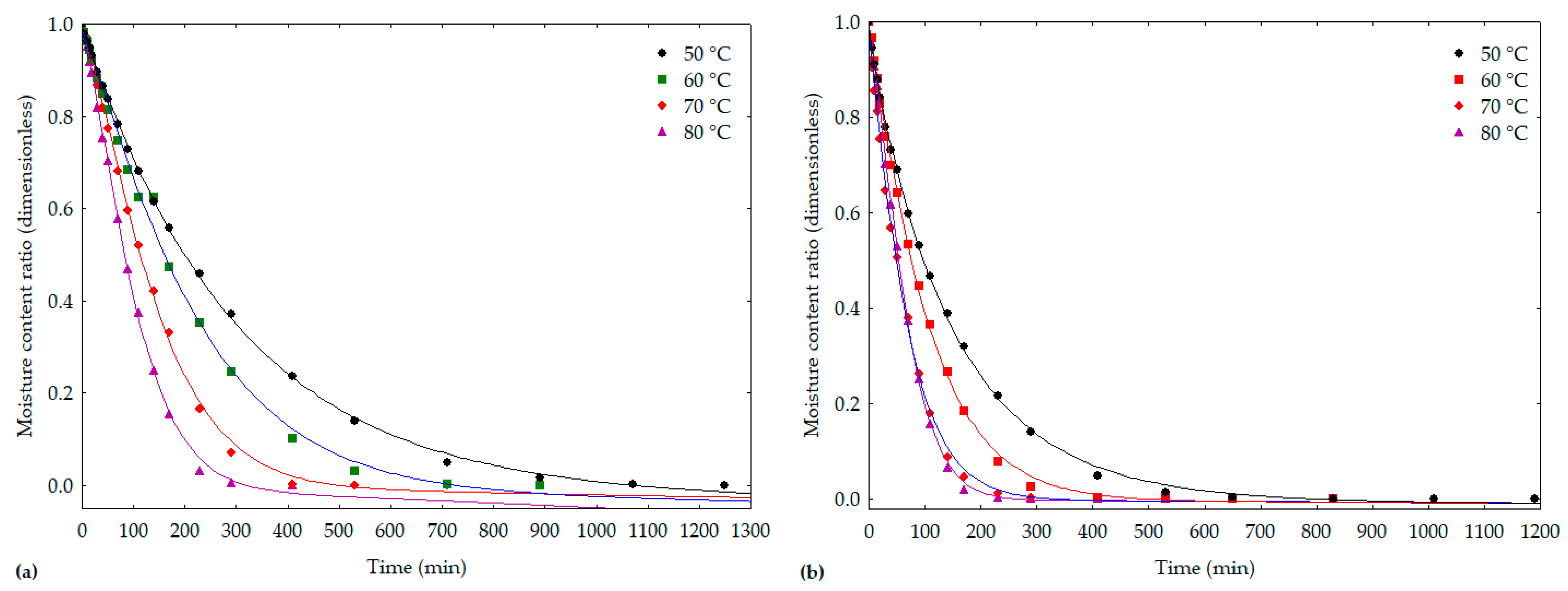
3.1. Drying Kinetics
3.2. Effective Diffusivity
3.3. Thermodynamic Properties
3.4. Proximal Composition of in Natura and Dried Peppers
4. Conclusions
5. Patents
- -
- Production of the biquinho-type red pepper (Capsicum chinense Jacq.) powder—BR 10 2021 014 391 6;
- -
- Green pepper (Capsicum chinense) powder—BR 10 2021 014 319 3.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrade, E.T.; Figueira, V.G.; Teixeira, L.P.; Taveira, J.H.S.; Borém, F.M. Determination of the hygroscopic equilibrium and isosteric heat of aji chili pepper. Rev. Bras. Eng. Agric. E Ambient. 2017, 21, 865–871. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Sergio, C.S.A.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; Ferraz, G.; Ragassi, R.M.; Reifschneider, O. Characterization and evaluation of selfed progenies of Biquinho salmon pepper. Hortic Bras. 2015, 33, 465–470. [Google Scholar] [CrossRef]
- Martinez, M.; Santos, C.P.; Verruma-Bernardi, M.R.; Carrilho, E.N.V.M.; Silva, P.P.M.; Spoto, M.H.F.; Ciarrocchi, I.R.; Fernando, F.C. Agronomic, physical–chemical and sensory evaluation of pepper hybrids (Capsicum chinense Jacquin). Sci. Hortic. 2021, 277, 109819. [Google Scholar] [CrossRef]
- Ribeiro, C.S.C.; Carvalho, S.I.C.; Heinrich, A.G.; Reifschneider, F.J.B. BRS Tui: A new Biquinho-type pepper cultivar released by Embrapa. Hortic Bras. 2018, 36, 526–528. [Google Scholar] [CrossRef]
- Maurya, V.K.; Gothandam, K.M.; Ranjan, V.; Shakya, A.; Pareek, S. Effect of drying methods (microwave vacuum, freeze, hot air and sun drying) on physical, chemical and nutritional attributes of five pepper (Capsicum annuum var. annuum) cultivars. J. Sci. Food Agric. 2018, 98, 3492–3500. [Google Scholar] [CrossRef]
- Diógenes, A.M.G.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Ferreira, J.P.L.; Silva, W.P.; Gomes, J.P.; Santos, F.S.; Castro, D.S.; Oliveira, M.N.; Santos, D.C.; et al. Mathematical Models to Describe the Foam Mat Drying Process of Cumbeba Pulp (Tacinga inamoena) and Product Quality. Foods 2022, 11, 1751. [Google Scholar] [CrossRef]
- Leite, D.D.F.; Queiroz, A.J.M.; Figueirêdo, R.M.F.; Lima, L.S.L. Mathematical drying kinetics modeling of jackfruit seeds (Artocarpus heterophyllus Lam.). Rev. Ciênc. Agron. 2019, 50, 361–369. [Google Scholar] [CrossRef]
- Leite, D.D.F.; Queiroz, A.J.M.; Figueiredo, R.M.F.; Santos, F.S.; Silva, S.N.; Santos, D.C. Mathematical modeling and thermodynamic properties in the drying of citron watermelon seeds. Rev. Bras. Eng. Agric. E Ambient. 2022, 26, 67–74. [Google Scholar] [CrossRef]
- Corrêa, P.C.; Oliveira, G.H.H.; Oliveira, A.P.L.R.; Botelho, F.M.; Goneli, A.L.D. Thermodynamic properties of drying process and water absorption of rice grains. CYTA J. Food 2017, 15, 204–210. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rate of Air Drying Shelled Corn in Thin-Layers. Master’s Thesis, Purdue University, West Lafayette, Indiana, 1949. [Google Scholar]
- Santos, F.S.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Lima, A.R.C.; Lima, T.L.B. The temperature effect in okra drying process: A kinetic study on powders physical properties. Aust. J. Crop Sci. 2021, 15, 649–660. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of The Association of Official Analytical Chemists, 20th ed.; Method 934.01; AOAC: Arlington, TX, USA, 2016. [Google Scholar]
- Paiva, Y.F.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Ferreira, J.P.L.; Santos, F.S.; Reis, C.G.; Amadeu, L.T.S.; Lima, A.G.B.; Gomes, J.P.; Silva, W.P.; et al. Tropical Red Fruit Blends: The Effect of Combination of Additives on Foaming, Drying and Thermodynamic Properties. Processes 2023, 11, 888. [Google Scholar] [CrossRef]
- Henderson, S.M. Progress in developing the thin layer drying equation. Trans. ASAE 1974, 17, 1167–1168. [Google Scholar] [CrossRef]
- Sharaf-Eldeen, Y.I.; Blaisdell, J.L.; Hamdy, M.Y. A model for ear corn drying. Trans. ASAE 1980, 23, 1261–1265. [Google Scholar] [CrossRef]
- Silva, W.A.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Santos, F.S.; Wanderley, R.O.S.; Paiva, Y.F.; Moura, H.V.; Sousa, E.P. Foam-mat drying of prickly pear and acerola mixed pulp: Kinetics and thermodynamic properties. Sylwan 2021, 165, 539–556. [Google Scholar]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Wanderley, R.O.S.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Santos, F.S.; Paiva, Y.F.; Ferreira, J.P.L.; Lima, A.G.B.; Gomes, J.P.; Costa, C.C.; Silva, W.P.; et al. The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds. Foods 2023, 12, 286. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 1st ed.; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Guiné, R.P.F.; Henrriques, F.; João Barroca, M. Mass transfer coefficients for the drying of pumpkin (Cucurbita moschata) and dried product quality. Food Bioprocess Technol. 2012, 5, 176–183. [Google Scholar] [CrossRef]
- Doymaz, İ. Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transf. 2017, 53, 25–35. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Brazil Ministry of Health; National Health Surveillance Agency. RDC Resolution N° 360 of December 23, 2003; Provides for technical regulation on nutritional labeling of packaged foods; Official Gazette of the Federative Republic of Brazil: Brasilia, Brazil, 2003; Section 1; pp. 33–34. [Google Scholar]
- Watt, B.; Merrill, A.L. Composition of Foods: Raw, Processed, Prepared; Consumer and Food Economics Research; Agriculture Handbook, 8; Divison/Agricultural Service: Washington, DC, USA, 1963; p. 198. [Google Scholar]
- Reis, R.C.; Barbosa, L.S.; Lima, M.L.; Reis, J.S.; Devilla, I.A.; Ascheri, D.P.R. Mathematical modeling of the drying of Cumari pepper from Pará. Rev. Bras. De Eng. Agrícola E Ambient. 2011, 15, 347–353. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Sobolik, V.; Montejano-Gaitán, J.G.; Abdulla, G.; Allaf, K. Impact of Swell-Drying Process on Water Activity and Drying Kinetics of Moroccan Pepper (Capsicum annum). Dry. Technol. 2015, 33, 131–142. [Google Scholar] [CrossRef]
- Silva, H.W.; Vale, L.S.R.; Silva, C.F.; Souza, R.C.; Soares, R.S. Drying kinetics and physiological quality of ‘Cabacinha’ pepper seeds during storage. Rev. Bras. Eng. Agric. E Ambient. 2018, 22, 292–297. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.M.; Mujumdar, A.S.; Qian, J.Y.; Zhang, Q.; Yang, X.H.; Liu, Y.H.; Gao, Z.J.; Xiao, H.W. Effect of high-humidity hot air impingement blanching (HHAIB) on drying and quality of red pepper (Capsicum annuum L.). Food Chem. 2017, 220, 145–152. [Google Scholar] [CrossRef]
- Gandolfi, O.R.R.; Gonçalves, G.R.F.; Bonomo, R.C.F.; Fontan, R.C.I. Sorption equilibrium and kinetics of thin-layer drying of green bell peppers. Emir. J. Food Agric. 2018, 30, 137–143. [Google Scholar] [CrossRef]
- Morm, E.; Ma, K.; Horn, S.; Debaste, F.; Haut, B. Experimental Characterization of the Drying of Kampot Red Pepper (Piper nigrum L.). Foods 2020, 9, 1532. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent drying of food products: A critical review. J. Food Eng. 2014, 121, 48–57. [Google Scholar] [CrossRef]
- Tekin, Z.H.; Baslar, M. The effect of ultrasound-assisted vacuum drying on the drying rate and quality of red peppers. J. Therm. Anal. Calorim. 2018, 132, 1131–1143. [Google Scholar] [CrossRef]
- Souza, J.L.F.; Oliveira, D.E.C.; Plácido, G.R.; Egea, M.B.; Caliari, M.; Silva, M.A.P. Thermodynamic and nutritional properties and drying kinetics of pequi (Caryocar brasiliense Cambess) mesocarp. Rev. Bras. Eng. Agric. E Ambient. 2019, 23, 655–661. [Google Scholar] [CrossRef]
- Madamba, P.S. Thin layer drying models for osmotically pré-dried young coconut. Dry. Technol. 2003, 21, 1759–1780. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Kumar, C.; Joardder, M.U.H.; Karim, M.A. Determination of appropriate effective diffusivity for different food materials. Dry. Technol. 2016, 35, 335–346. [Google Scholar] [CrossRef]
- Martins, E.A.S.; Lage, E.Z.; Goneli, A.L.D.; Hartmann Filho, C.P.; Lopes, J.G. Timbó leaf drying kinetics (Serjania marginata Casar). Rev. Bras. Eng. Agric. E Ambient. 2015, 19, 238–244. [Google Scholar] [CrossRef]
- Zogzas, N.P.; Maroulis, Z.B.; Marinos-Kouris, D. Moisture diffusivity data compilation in foodstuffs. Dry. Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
- Kumar, V.; Shrivastavaa, S.L. Vacuum-assisted microwave drying characteristics of green bell pepper. Int. J. Food Stud. 2017, 6, 67–81. [Google Scholar] [CrossRef]
- Vega, A.; Fito, P.; Andrés, A.; Lemus, R. Mathematical modeling of hot-air drying kinetics of red bell pepper (var. Lamuyo). J. Food Eng. 2007, 79, 1460–1466. [Google Scholar] [CrossRef]
- Kaleemullah, S.; Kailappan, R. Modelling of thin-layer drying kinetics of red chillies. J. Food Eng. 2006, 76, 531–537. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the Thin-Layer Drying of Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef]
- Rodovalho, R.S.; Silva, H.W.; Silva, I.L.; Rossetto, C.A.V. Drying kinetics of goat peppercorns. Glob. Sci. Technol. 2015, 8, 128–142. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidante capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Reis, R.C.; Castro, V.C.; Devilla, I.A.; Oliveira, C.A.; Barbosa, L.S.; Rodovalho, R. Effect of drying temperature on the nutritional and antioxidante qualities of cumari peppers from pará (Capsicum chinense Jacqui). Braz. J. Chem. Eng. 2013, 30, 337–343. [Google Scholar] [CrossRef]
- Pinar, H.; Çetin, N.; Ciftci, B.; Karaman, K.; Kaplan, M. Biochemical composition, drying kinetics and chromatic parameters of red pepper as affected by cultivars and drying methods. J. Food Compost. Anal. 2021, 102, 103976. [Google Scholar] [CrossRef]
- Faustino, J.M.F.; Barroca, M.J.; Guiné, R.P.F. Study of the drying kinetics of green bell pepper and chemical characterization. Food Bioprod. Process. 2007, 85, 163–170. [Google Scholar] [CrossRef]

| Equation | Model Designation | Mathematical Model | References |
|---|---|---|---|
| (2) | Two terms | MR = a·exp(−k0·t) + b exp(−k1·t) | [15] |
| (3) | Henderson and Pabis | MR = a·exp(−k·t) | [16] |
| (4) | Henderson and Pabis modified | MR = a·exp(−k·t) + b·exp(−k0·t) + c·exp(−k1·t) | [17] |
| (5) | Logarithmic | MR = a·exp(−k·t) + c | [8] |
| (6) | Logistic | MR = a0/(1a·exp(k·t)) | [18] |
| (7) | Midilli | MR = a·exp(−k·tn) + b.t | [19] |
| (8) | Newton | MR = exp(−k·t) | [10] |
| (9) | Page | MR = exp(−k·tn) | [12] |
| (10) | Thompson | [13] | |
| (11) | Verna | MR = a·exp(−k·t) + (1 − a) exp(−k2·t) | [20] |
| Pepper | Temperature (°C) | Drying Time (min) | Water Content (% w.b.) | Water Content (% d.b.) |
|---|---|---|---|---|
| Smelling | 50 | 1250 ± 0.00 | 6.34 ± 0.24 | 6.77 ± 0.27 |
| 60 | 890 ± 0.00 | 7.33 ± 0.49 | 7.91 ± 0.57 | |
| 70 | 710 ± 0.00 | 6.47 ± 0.26 | 6.92 ± 0.29 | |
| 80 | 410 ± 0.00 | 4.48 ± 0.19 | 4.70 ± 0.23 | |
| Pout | 50 | 1190 ± 0.00 | 5.65 ± 0.10 | 6.33 ± 0.14 |
| 60 | 830 ± 0.00 | 5.02 ± 0.27 | 5.29 ± 0.30 | |
| 70 | 650 ± 0.00 | 4.80 ± 0.22 | 5.04 ± 0.24 | |
| 80 | 530 ± 0.00 | 4.32 ± 0.12 | 4.52 ± 0.19 |
| Model | Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two Term | T (°C) | a | k0 | b | k1 | R2 | MSD | χ2 | ||||
| 50 | 0.5428 | 0.0035 | 0.4583 | 0.0035 | 0.9989 | 0.0118 | 0.0002 | |||||
| 60 | 0.5102 | 0.0046 | 0.5081 | 0.0046 | 0.9927 | 0.0302 | 0.0012 | |||||
| 70 | 0.5237 | 0.0068 | 0.5237 | 0.0068 | 0.9915 | 0.0339 | 0.0015 | |||||
| 80 | 0.5318 | 0.0098 | 0.5318 | 0.0098 | 0.9876 | 0.0403 | 0.0022 | |||||
| Henderson and Pabis | T (°C) | a | k | R2 | MSD | χ2 | ||||||
| 50 | 1.0012 | 0.0035 | 0.9989 | 0.0118 | 0.0002 | |||||||
| 60 | 1.0183 | 0.0046 | 0.9927 | 0.0302 | 0.0010 | |||||||
| 70 | 1.0475 | 0.0068 | 0.9915 | 0.0339 | 0.0013 | |||||||
| 80 | 1.0588 | 0.0097 | 0.9876 | 0.0402 | 0.0018 | |||||||
| Henderson and Pabis Modified | T (°C) | a | k | b | k0 | c | k1 | R2 | MSD | χ2 | ||
| 50 | 0.3365 | 0.0035 | 0.3320 | 0.0035 | 0.3326 | 0.0035 | 0.9989 | 0.0118 | 0.0002 | |||
| 60 | 0.3338 | 0.0046 | 0.3347 | 0.0046 | 0.3498 | 0.0046 | 0.9927 | 0.0302 | 0.0013 | |||
| 70 | 0.3490 | 0.0068 | 0.3490 | 0.0068 | 0.3490 | 0.0068 | 0.9915 | 0.0339 | 0.0017 | |||
| 80 | 0.3529 | 0.0097 | 0.3529 | 0.0097 | 0.3529 | 0.0097 | 0.9876 | 0.0402 | 0.0026 | |||
| Logarithmic | T (°C) | a | k | c | R2 | MSD | χ2 | |||||
| 50 | 1.0268 | 0.0033 | −0.0305 | 0.9997 | 0.0064 | 0.00005 | ||||||
| 60 | 1.0753 | 0.0040 | −0.0656 | 0.9955 | 0.0236 | 0.00066 | ||||||
| 70 | 1.1000 | 0.0061 | −0.0611 | 0.9946 | 0.0272 | 0.00089 | ||||||
| 80 | 1.1442 | 0.0080 | −0.0999 | 0.9934 | 0.0294 | 0.00106 | ||||||
| Logistic | T (°C) | a0 | a | k | R2 | MSD | χ2 | |||||
| 50 | 0.0415 | 0.0415 | 0.0035 | 0.9989 | 0.0118 | 0.0002 | ||||||
| 60 | 0.0714 | 0.0701 | 0.0046 | 0.9927 | 00302 | 0.0011 | ||||||
| 70 | 0.0766 | 0.0732 | 0.0068 | 0.9915 | 0.0339 | 0.0014 | ||||||
| 80 | 0.1837 | 0.1735 | 0.0097 | 0.9876 | 0.0402 | 0.0020 | ||||||
| Midilli | T (°C) | a | k | n | b | R2 | MSD | χ2 | ||||
| 50 | 0.9938 | 0.0031 | 1.0200 | −0.00002 | 0.9996 | 0.0070 | 0.0001 | |||||
| 60 | 0.9839 | 0.0018 | 1.1691 | −0.00003 | 0.9967 | 0.0176 | 0.0005 | |||||
| 70 | 0.9923 | 0.0015 | 1.2874 | −0.00002 | 0.9990 | 0.0117 | 0.0002 | |||||
| 80 | 0.9895 | 0.0018 | 1.3451 | −0.00005 | 0.9992 | 0.0106 | 0.0001 | |||||
| Newton | T (°C) | k | R2 | MSD | χ2 | |||||||
| 50 | 0.0035 | 0.9989 | 0.0119 | 0.0001 | ||||||||
| 60 | 0.0045 | 0.9919 | 0.0318 | 0.0011 | ||||||||
| 70 | 0.0064 | 0.9871 | 0.0419 | 0.0019 | ||||||||
| 80 | 0.0089 | 0.9810 | 0.0499 | 0.0027 | ||||||||
| Page | T (°C) | k | n | R2 | MSD | χ2 | ||||||
| 50 | 0.0030 | 1.0309 | 0.9991 | 0.0106 | 0.0001 | |||||||
| 60 | 0.0021 | 1.1445 | 0.9959 | 0.0227 | 0.0006 | |||||||
| 70 | 0.0017 | 1.2758 | 0.9987 | 0.0131 | 0.0002 | |||||||
| 80 | 0.0020 | 1.3329 | 0.9987 | 0.0131 | 0.0002 | |||||||
| Thompson | T (°C) | a | b | R2 | MSD | χ2 | ||||||
| 50 | −8206.30 | 5.3873 | 0.9989 | 0.0119 | 0.0002 | |||||||
| 60 | −4878.02 | 4.6680 | 0.9919 | 0.0318 | 0.0011 | |||||||
| 70 | −2551.27 | 4.0268 | 0.9871 | 0.0419 | 0.0020 | |||||||
| 80 | −4143.82 | 6.0725 | 0.9809 | 0.0499 | 0.0028 | |||||||
| Verna | T (°C) | a | k | k1 | R2 | MSD | χ2 | |||||
| 50 | −0.0019 | 0.1944 | 0.0035 | 0.9989 | 0.0118 | 0.0002 | ||||||
| 60 | −0.1863 | 0.0186 | 0.0055 | 0.9952 | 0.0245 | 0.0007 | ||||||
| 70 | −7.1194 | 0.0130 | 0.0118 | 0.9987 | 0.0133 | 0.0002 | ||||||
| 80 | −14.5638 | 0.0184 | 0.0175 | 0.9984 | 0.0143 | 0.0003 | ||||||
| Model | Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Two Term | T (°C) | a | k0 | b | k1 | R2 | MSD | χ2 | |||
| 50 | 0.0453 | 0.1071 | 0.9538 | 0.0065 | 0.9997 | 0.0063 | 0.00005 | ||||
| 60 | 0.5715 | 0.0096 | 0.4427 | 0.0096 | 0.9984 | 0.0147 | 0.00028 | ||||
| 70 | 0.5017 | 0.0149 | 0.5017 | 0.0148 | 0.9974 | 0.0183 | 0.00043 | ||||
| 80 | 0.5294 | 0.0152 | 0.5294 | 0.0152 | 0.9912 | 0.0356 | 0.00165 | ||||
| Henderson and Pabis | T (°C) | a | k | R2 | MSD | χ2 | |||||
| 50 | 0.9742 | 0.0067 | 0.9993 | 0.0098 | 0.0001 | ||||||
| 60 | 1.0141 | 0.0096 | 0.9984 | 0.0147 | 0.0002 | ||||||
| 70 | 1.0028 | 0.0148 | 0.9974 | 0.0183 | 0.0004 | ||||||
| 80 | 1.0587 | 0.0152 | 0.9912 | 0.0356 | 0.0014 | ||||||
| Henderson and Pabis Modified | T (°C) | a | k | b | k0 | c | k1 | R2 | MSD | χ2 | |
| 50 | 0.3246 | 0.0062 | 0.3246 | 0.0062 | 0.3263 | 0.0081 | 0.9993 | 0.0098 | 0.0001 | ||
| 60 | 0.3382 | 0.0096 | 0.3382 | 0.0096 | 0.3378 | 0.0096 | 0.9984 | 0.0147 | 0.0003 | ||
| 70 | 0.3342 | 0.0148 | 0.3342 | 0.0148 | 0.3343 | 0.0148 | 0.9974 | 0.0183 | 0.0005 | ||
| 80 | 0.3530 | 0.0152 | 0.3530 | 0.0152 | 0.3530 | 0.0152 | 0.9912 | 0.0356 | 0.0020 | ||
| Logarithmic | T (°C) | a | k | c | R2 | MSD | χ2 | ||||
| 50 | 0.9773 | 0.0067 | −0.0039 | 0.9993 | 0.0097 | 0.0001 | |||||
| 60 | 1.0270 | 0.0092 | −0.0159 | 0.9988 | 0.0127 | 0.0002 | |||||
| 70 | 1.0142 | 0.0143 | −0.0147 | 0.9978 | 0.0167 | 0.0003 | |||||
| 80 | 1.0851 | 0.0142 | −0.0327 | 0.9929 | 0.0319 | 0.0012 | |||||
| Logistic | T (°C) | a0 | a | k | R2 | MSD | χ2 | ||||
| 50 | 0.1219 | 0.1251 | 0.0067 | 0.9993 | 0.0098 | 0.0001 | |||||
| 60 | 0.1489 | 0.1468 | 0.0096 | 0.9984 | 0.0147 | 0.0003 | |||||
| 70 | 0.1035 | 0.1032 | 0.0148 | 0.9974 | 0.0183 | 0.0004 | |||||
| 80 | 0.1153 | 0.1089 | 0.0152 | 0.9912 | 0.0356 | 0.0015 | |||||
| Midilli | T (°C) | a | k | n | b | R2 | MSD | χ2 | |||
| 50 | 0.9866 | 0.0088 | 0.9478 | −0.000008 | 0.9995 | 0.0081 | 0.0001 | ||||
| 60 | 0.9907 | 0.0059 | 1.0963 | −0.000008 | 0.9993 | 0.0100 | 0.0001 | ||||
| 70 | 0.9724 | 0.0086 | 1.1207 | −0.000006 | 0.9986 | 0.0134 | 0.0002 | ||||
| 80 | 0.9852 | 0.0033 | 1.3463 | −0.000007 | 0.9995 | 0.0089 | 0.0001 | ||||
| Newton | T (°C) | k | R2 | MSD | χ2 | ||||||
| 50 | 0.0071 | 0.9981 | 0.0155 | 0.0003 | |||||||
| 60 | 0.0094 | 0.9981 | 0.0161 | 0.0003 | |||||||
| 70 | 0.0148 | 0.9974 | 0.0184 | 0.0004 | |||||||
| 80 | 0.0141 | 0.9867 | 0.0436 | 0.0020 | |||||||
| Page | T (°C) | k | n | R2 | MSD | χ2 | |||||
| 50 | 0.0100 | 0.9271 | 0.9993 | 0.0098 | 0.0001 | ||||||
| 60 | 0.0066 | 1.0776 | 0.9991 | 0.0108 | 0.0001 | ||||||
| 70 | 0.0114 | 1.0618 | 0.9981 | 0.0158 | 0.0003 | ||||||
| 80 | 0.0039 | 1.3065 | 0.9992 | 0.0104 | 0.0001 | ||||||
| Thompson | T (°C) | a | b | R2 | MSD | χ2 | |||||
| 50 | −35.1148 | 0.5118 | 0.9985 | 0.0138 | 0.0002 | ||||||
| 60 | −2247.7001 | 4.5874 | 0.9981 | 0.0161 | 0.0003 | ||||||
| 70 | −4120.3012 | 7.7995 | 0.9974 | 0.0184 | 0.0004 | ||||||
| 80 | −4282.2716 | 7.7620 | 0.9867 | 0.0436 | 0.0022 | ||||||
| Verna | T (°C) | a | k | k1 | R2 | MSD | χ2 | ||||
| 50 | −6.3939 | 0.0074 | 0.0074 | 0.9981 | 0.0156 | 0.0003 | |||||
| 60 | −3.4090 | 0.0059 | 0.0066 | 0.9994 | 0.0087 | 0.0001 | |||||
| 70 | −4.7678 | 0.0096 | 0.0104 | 0.9987 | 0.0128 | 0.0002 | |||||
| 80 | −2.8776 | 0.0075 | 0.0089 | 0.9931 | 0.0315 | 0.0013 | |||||
| Varieties | T (°C) | Deff (m2 s−1) | R2 |
|---|---|---|---|
| Smelling | 50 | 5.75 × 10−10 | 0.9650 |
| 60 | 7.34 × 10−10 | 0.9424 | |
| 70 | 10.62 × 10−10 | 0.9276 | |
| 80 | 15.16 × 10−10 | 0.9126 | |
| Pout | 50 | 3.47 × 10−10 | 0.9753 |
| 60 | 4.67 × 10−10 | 0.9646 | |
| 70 | 7.29 × 10−10 | 0.9663 | |
| 80 | 8.59 × 10−10 | 0.9386 |
| Peppers | Deff0 (m2·s−1) | Ea (kJ·mol−1) | R2 |
|---|---|---|---|
| Smelling | 5.67 × 10−5 | 31.01 | 0.9875 |
| Pout | 2.56 × 10−5 | 30.11 | 0.9777 |
| Peppers | T (°C) | ΔH (kJ·mol−1) | ΔS (kJ mol−1·K−1) | ΔG (kJ·mol−1) |
|---|---|---|---|---|
| Smelling | 50 | 28.3243 | −0.3269 | 133.9500 |
| 60 | 28.2412 | −0.3271 | 137.2199 | |
| 70 | 28.1581 | −0.3274 | 140.4923 | |
| 80 | 28.0749 | −0.3276 | 143.7671 | |
| Pout | 50 | 27.4223 | −0.3335 | 135.1890 |
| 60 | 27.3392 | −0.3337 | 138.5252 | |
| 70 | 27.2561 | −0.3340 | 141.8638 | |
| 80 | 27.1729 | −0.3342 | 145.2049 |
| Parameters | Peppers | In Natura | Drying Temperatures (°C) | |||
|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | |||
| Water content (%) | Smelling | 91.88 ± 0.16 aA | 9.34 ± 0.14 bB | 9.06 ± 0.03 bB | 8.92 ± 0.05 aB | 7.93 ± 0.04 aC |
| Pout | 83.30 ± 0.72 bA | 11.95 ± 0.02 aB | 10.41 ± 0.04 aC | 7.25 ± 0.12 bD | 6.56 ± 0.10 bE | |
| Lipids (% d.b.) | Smelling | 2.11 ± 0.20 aC | 3.21 ± 0.08 bB | 4.03 ± 0.27 bA | 3.95 ± 0.07 bA | 3.86 ± 0.03 bA |
| Pout | 2.10 ± 0.18 aD | 6,02 ± 0.11 aB | 4.58 ± 0.18 aC | 5.78 ± 0.24 aB | 7.70 ± 0.05 aA | |
| Proteins (% d.b.) | Smelling | 19.38 ± 0.58 aA | 14.87 ± 0.30 aC | 15.26 ± 0.21 aC | 18.40 ± 0.15 aB | 18.59 ± 0.35 aAB |
| Pout | 14.96 ± 0.39 bB | 14.79 ± 0.43 aB | 15.83 ± 0.18 aA | 15.34 ± 0.27 bAB | 15.39 ± 0.28 bAB | |
| Ashes (% d.b.) | Smelling | 6.83 ± 0.28 aA | 5.60 ± 0.18 aC | 6.30 ± 0.09 aB | 6.73 ± 0.09 aAB | 6.76 ± 0.14 aAB |
| Pout | 6.76 ± 0.47 aA | 5.37 ± 0.22 aC | 6.23 ± 0.06 aB | 6.47 ± 0.07 aAB | 6.21 ± 0.07 bB | |
| Carbohydrates (% d.b.) | Smelling | 71.67 ± 0.90 bC | 76.32 ± 0.38 aA | 74.41 ± 0.13 aB | 70.92 ± 0.30 bC | 70.80 ± 0.42 aC |
| Pout | 76.18 ± 0.88 aA | 73.82 ± 0.20 bB | 73.36 ± 0.34 bBC | 72.41 ± 0.21 aC | 70.71 ± 0.27 aD | |
| Energetic value (kcal 100 g−1 d.b.) | Smelling | 383.23 ± 1.59 aC | 393.65 ± 1.06 bAB | 394.97 ± 1.71 bA | 392.83 ± 0.33 bAB | 392.29 ± 0.71 bB |
| Pout | 383.43 ± 0.97 aE | 408.63 ± 1.00 aB | 398.00 ± 1.01 aD | 403.04 ± 0.95 aC | 413.65 ± 0.52 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, R.L.; Figueirêdo, R.M.F.d.; Queiroz, A.J.d.M.; Santos, F.S.d.; Lima, A.G.B.d.; Rego Junior, P.F.d.; Gomes, J.P.; Silva, W.P.d.; Paiva, Y.F.; Moura, H.V.; et al. Smelling Peppers and Pout Submitted to Convective Drying: Mathematical Modeling, Thermodynamic Properties and Proximal Composition. Foods 2023, 12, 2106. https://doi.org/10.3390/foods12112106
Moura RL, Figueirêdo RMFd, Queiroz AJdM, Santos FSd, Lima AGBd, Rego Junior PFd, Gomes JP, Silva WPd, Paiva YF, Moura HV, et al. Smelling Peppers and Pout Submitted to Convective Drying: Mathematical Modeling, Thermodynamic Properties and Proximal Composition. Foods. 2023; 12(11):2106. https://doi.org/10.3390/foods12112106
Chicago/Turabian StyleMoura, Rodrigo Leite, Rossana Maria Feitosa de Figueirêdo, Alexandre José de Melo Queiroz, Francislaine Suelia dos Santos, Antônio Gilson Barbosa de Lima, Pedro Francisco do Rego Junior, Josivanda Palmeira Gomes, Wilton Pereira da Silva, Yaroslávia Ferreira Paiva, Henrique Valentim Moura, and et al. 2023. "Smelling Peppers and Pout Submitted to Convective Drying: Mathematical Modeling, Thermodynamic Properties and Proximal Composition" Foods 12, no. 11: 2106. https://doi.org/10.3390/foods12112106
APA StyleMoura, R. L., Figueirêdo, R. M. F. d., Queiroz, A. J. d. M., Santos, F. S. d., Lima, A. G. B. d., Rego Junior, P. F. d., Gomes, J. P., Silva, W. P. d., Paiva, Y. F., Moura, H. V., Silva, E. T. d. V., Costa, C. C., & Gregório, M. G. (2023). Smelling Peppers and Pout Submitted to Convective Drying: Mathematical Modeling, Thermodynamic Properties and Proximal Composition. Foods, 12(11), 2106. https://doi.org/10.3390/foods12112106







