Effect of Carrier Agents on Quality Parameters of Spray-Dried Encapsulated Diosgenin Powder and the Optimization of Process Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Preparation
2.2. Yield
2.3. Microcapsule Characterization
2.3.1. Encapsulation Efficiency (EE, %)
2.3.2. Moisture Content (MC, %)
2.3.3. Antioxidant Activity (AA,%)
2.3.4. Hygroscopicity (HG, %)
2.3.5. Solubility (SB, %)
2.3.6. Powder Morphology
2.3.7. X-ray Diffraction
2.4. Experimental Design
3. Results
3.1. Model Fitting
3.2. Yield
3.3. Powder Characterization
3.3.1. Encapsulation Efficiency (EE)
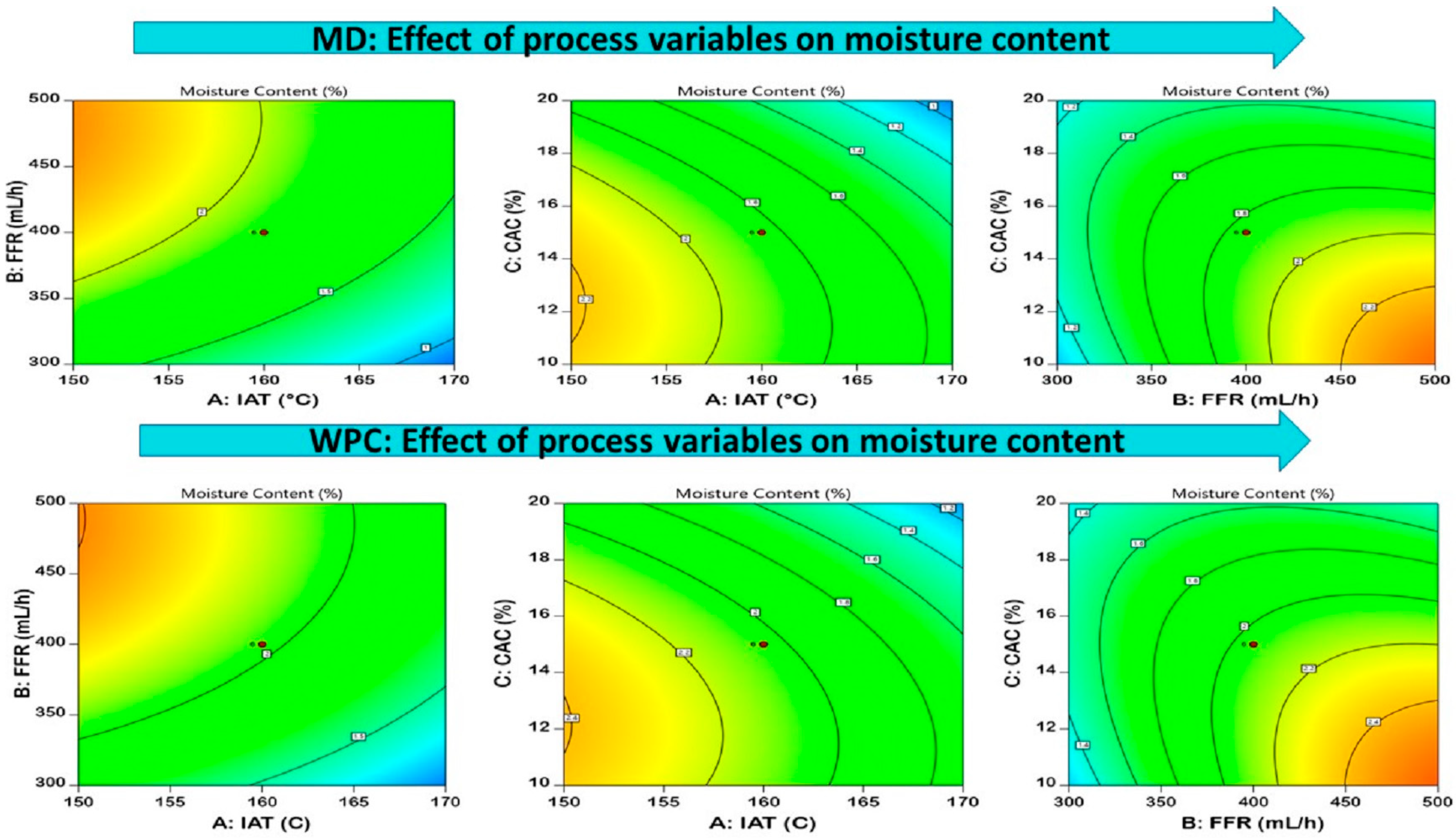
3.3.2. Moisture Content
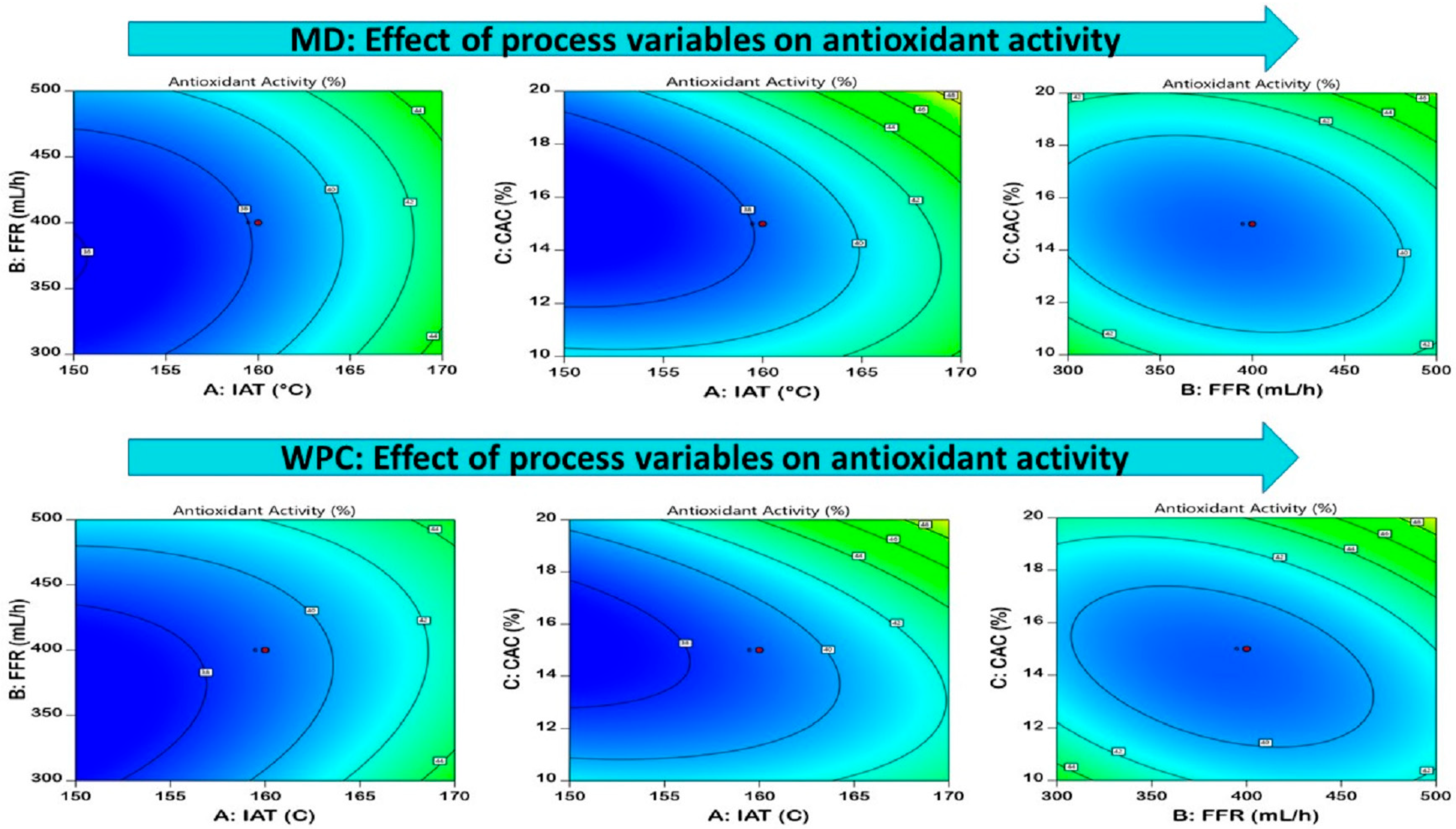
3.3.3. Antioxidant Activity
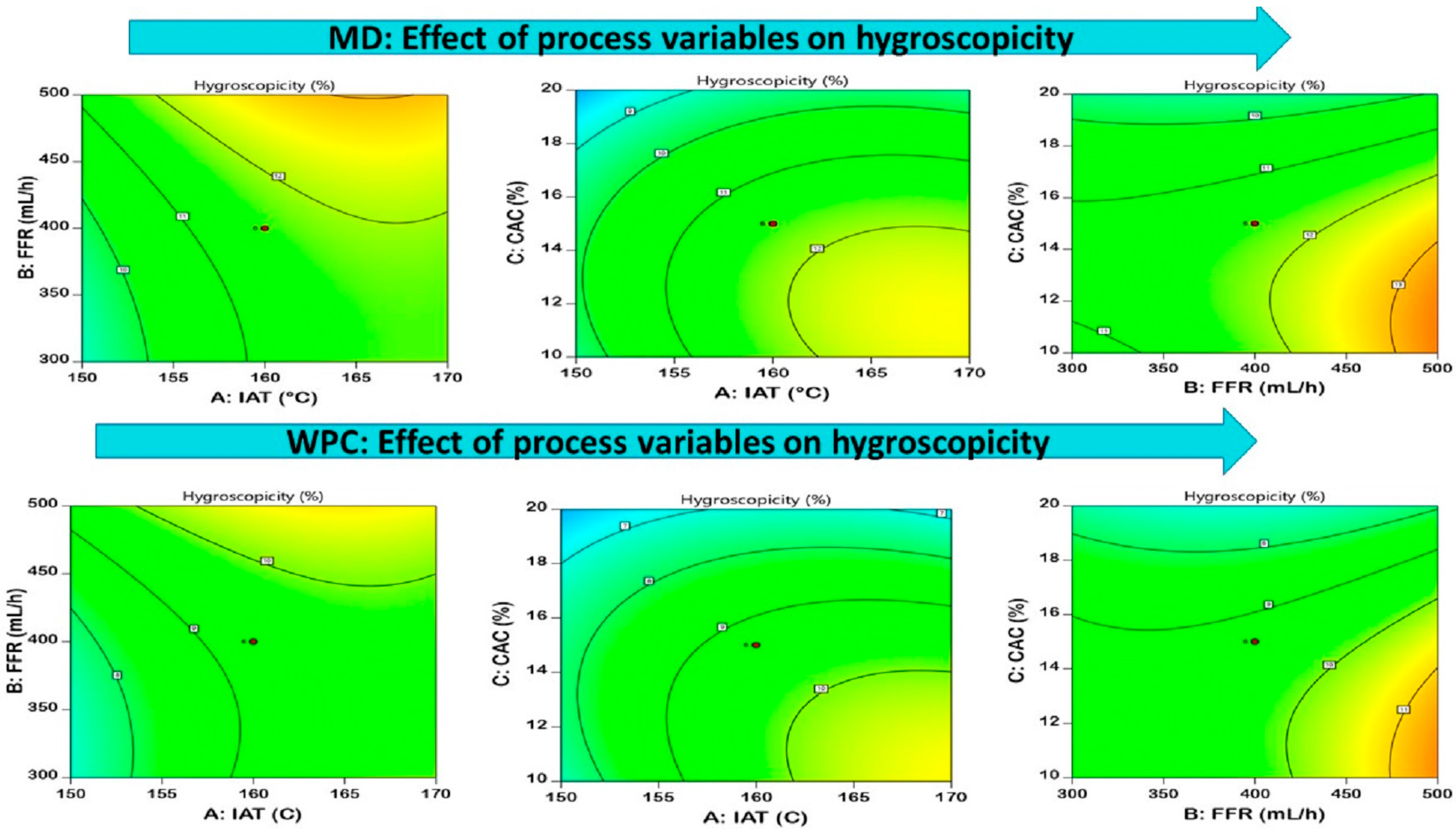
3.3.4. Hygroscopicity
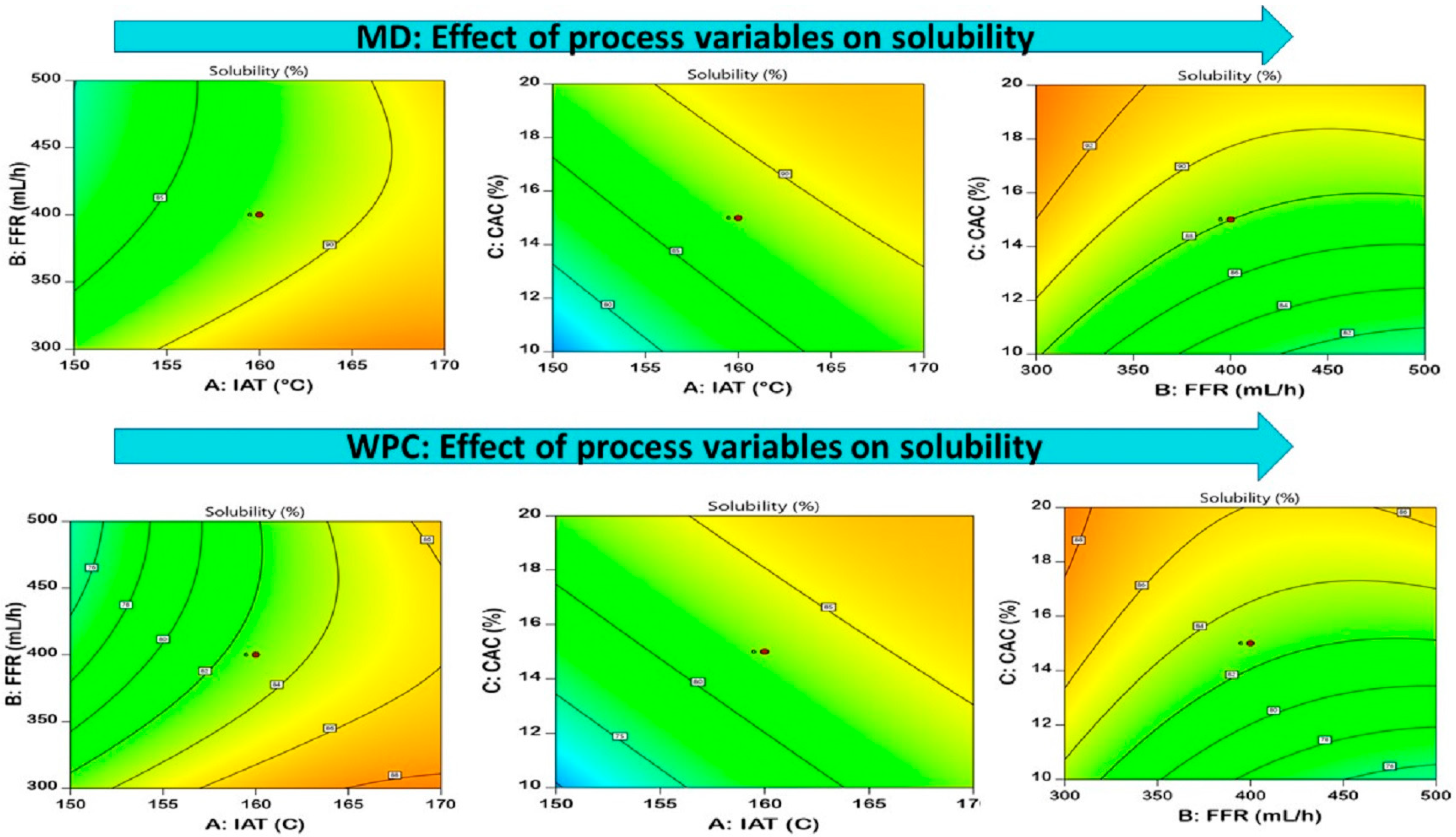
3.3.5. Solubility
3.4. Optimizations of Carrier Agent Concentration, IAT, and FFR
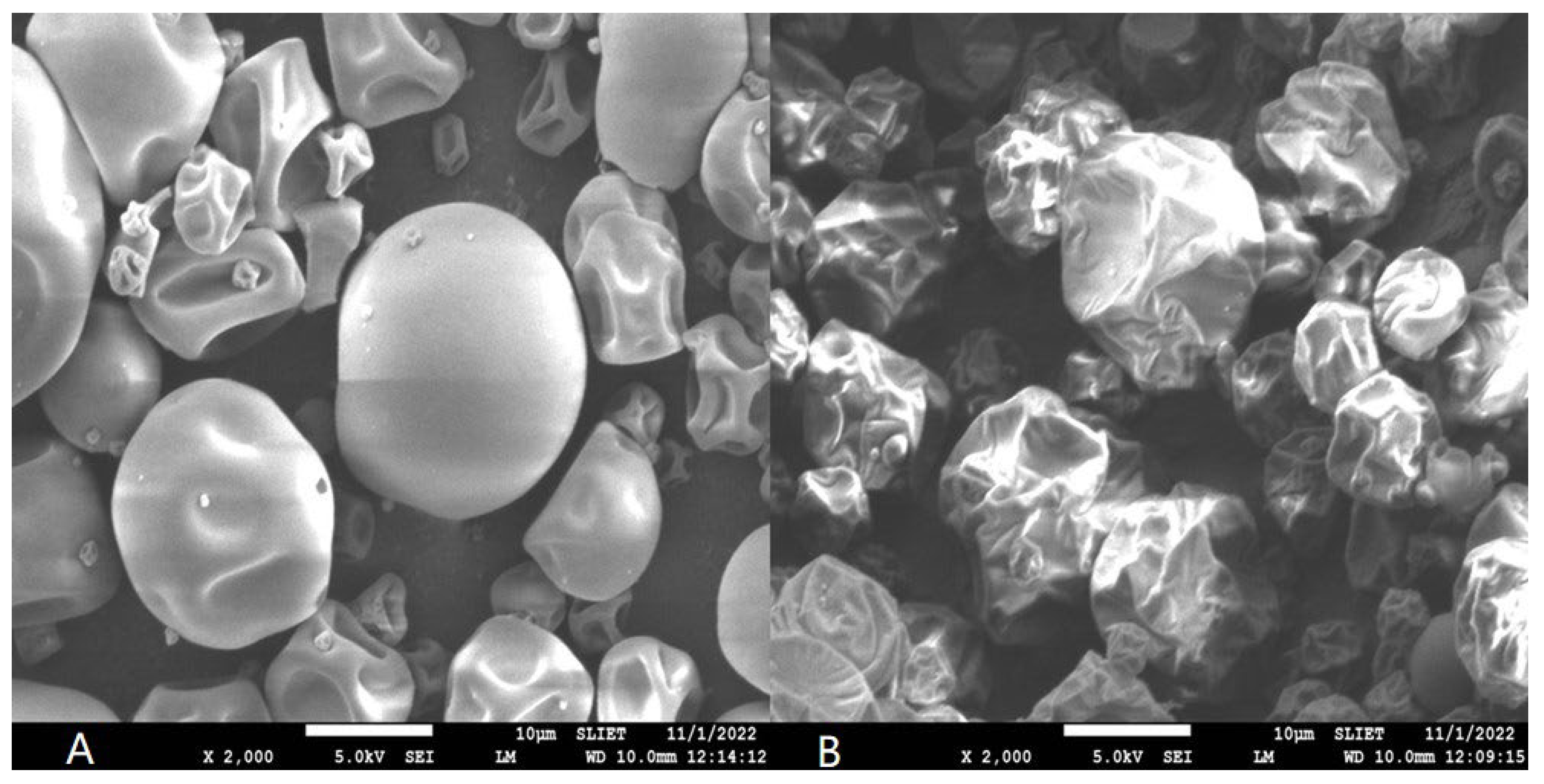
3.4.1. Powder Morphology
3.4.2. X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arya, P.; Kumar, P. Encapsulated diosgenin powder production using binary carrier: Process optimization and powder characterization. Food Hydrocoll. Health 2023, 3, 100134. [Google Scholar] [CrossRef]
- Arya, P.; Munshi, M.; Kumar, P. Diosgenin: Chemistry, extraction, quantification and health benefits. Food Chem. Adv. 2023, 2, 100170. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Diosgenin: An ingress towards solving puzzle for diabetes treatment. J. Food Biochem. 2022, 46, e14390. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Kumar, P. Characterization of spray dried diosgenin from fenugreek using binary blend of carrier agents. Appl. Food Res. 2022, 2, 100054. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Comparison of ultrasound and microwave assisted extraction of diosgenin from Trigonella foenum graceum seed. Ultrason. Sonochem. 2021, 74, 105572. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.W.; Bhandari, B. Spray drying for food powder production. In Handbook of Food Powders; Woodhead Publishing: Cambridge, UK, 2013; pp. 29–56. [Google Scholar]
- Jamil Ur Rahman, U.; Pozarlik, A.K.; Brem, G. Experimental analysis of spray drying in a process intensified counter flow dryer. Dry. Technol. 2022, 40, 3128–3148. [Google Scholar] [CrossRef]
- Sobulska, M.; Zbicinski, I. Advances in spray drying of sugar-rich products. Dry. Technol. 2021, 39, 1774–1799. [Google Scholar] [CrossRef]
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef]
- Singh Banjare, I.; Gandhi, K.; Sao, K.; Sharma, R. Spray-dried whey protein concentrate-iron complex: Preparation and physicochemical characterization. Food Technol. Biotechnol. 2019, 57, 331–340. [Google Scholar] [CrossRef]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Cano-Higuita, D.M.; Malacrida, C.R.; Telis, V.R.N. Stability of curcumin microencapsulated by spray and freeze drying in binary and ternary matrices of maltodextrin, gum arabic and modified starch. J. Food Process. Preserv. 2015, 39, 2049–2060. [Google Scholar] [CrossRef]
- Balasubramani, P.; Palaniswamy, P.T.; Visvanathan, R.; Thirupathi, V.; Subbarayan, A.; Maran, J.P. Microencapsulation of garlic oleoresin using maltodextrin as wall material by spray drying technology. Int. J. Biol. Macromol. 2015, 72, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ganesan, P.; Baharin, B.S.; Kwak, H.S. Characteristics, stability, and release of peanut sprout extracts in powdered microcapsules by spray drying. Dry. Technol. 2015, 33, 1991–2001. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Microencapsulation of garcinia fruit extract by spray drying and its effect on bread quality. J. Sci. Food Agric. 2014, 94, 1116–1123. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Production of encapsulated (25R)-Spirost-5-en-3β-ol powder with composite coating material and its characterization. Steroids 2023, 194, 109218. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, X.; Huang, W.; Gong, G.; Li, D.; He, Y.; Zhao, Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis CH Wright in rodents. J. Ethnopharmacol. 2009, 126, 543–550. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ferdinand, V.; Siow, L.F. Effect of drying methods on yield, physicochemical properties, and total polyphenol content of chamomile extract powder. Front. Pharmacol. 2022, 13, 1003209. [Google Scholar] [CrossRef]
- Liew, S.Y.; Mohd Zin, Z.; Mohd Maidin, N.M.; Mamat, H.; Zainol, M.K. Effect of the different encapsulation methods on the physicochemical and biological properties of Clitoriaternatea flowers microencapsulated in gelatine. Food Res. 2020, 4, 1098–1108. [Google Scholar] [CrossRef]
- Chapagain, B.; Wiesman, Z. Variation in diosgenin level in seed kernels among different provenances of Balanites aegyptiaca Del (Zygophyllaceae) and its correlation with oil content. Afr. J. Biotechnol. 2005, 4, 1209–1213. [Google Scholar]
- Nguyen, Q.D.; Dang, T.T.; Nguyen, T.V.L.; Nguyen, T.T.D.; Nguyen, N.N. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- Chang, L.S.; Ooi, Y.W.; Pui, L.P. Production of enzymatic hydrolysed spray-dried honeydew melon (Cucumis melo L.) powder. J. Agric. Food Res. 2022, 10, 100364. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Bhusari, S.N.; Muzaffar, K.; Kumar, P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]
- Sasikumar, R.; Das, M.; Sahu, J.K.; Deka, S.C. Qualitative properties of spray-dried blood fruit (Haemato carpusvalidus) powder and its sorption isotherms. J. Food Process Eng. 2020, 43, e13373. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Eun, J.B. Optimization of spray drying process parameters for production of Japanese apricot (Prunus mume Sieb. et Zucc.) juice powder. Food Sci. Biotechnol. 2021, 30, 1075–1086. [Google Scholar] [CrossRef]
- Sarabandi, K.H.; Peighambardoust, S.H.; Sadeghi Mahoonak, A.R.; Samaei, S.P. Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J. Food Sci. Technol. 2018, 55, 3098–3109. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Suhag, Y.; Nayik, G.A.; Karabagias, I.K.; Nanda, V. Development and characterization of a nutritionally rich spray-dried honey powder. Foods 2021, 10, 162. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res. Int. 2012, 48, 478–483. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Kalajahi, S.E.M.; Ghandiha, S. Optimization of spray drying parameters for encapsulation of nettle (Urtica dioica L.) extract. LWT-Food Sci.Technol. 2022, 158, 113149. [Google Scholar]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Wardhani, D.H.; Wardana, I.N.; Ulya, H.N.; Cahyono, H.; Kumoro, A.C.; Aryanti, N. The effect of spray-drying inlet conditions on iron encapsulation using hydrolysed glucomannan as a matrix. Food Bioprod. Process. 2020, 123, 72–79. [Google Scholar] [CrossRef]
- Phisut, N. Spray drying technique of fruit juice powder: Some factors influencing the properties of product. Int. Food Res. J. 2012, 19, 1297–1306. [Google Scholar]
- Du, J.; Ge, Z.Z.; Xu, Z.; Zou, B.; Zhang, Y.; Li, C.M. Comparison of the efficiency of five different drying carriers on the spray drying of persimmon pulp powders. Dry. Technol. 2014, 32, 1157–1166. [Google Scholar] [CrossRef]
- Yang, H.I.; Ameer, K.; Chung, Y.B.; Min, S.G.; Eun, J.B. Optimization of spray drying process for recovery of onion–stevia leaf hot water extract powder using response surface methodology. Food Sci. Nutr. 2023, 11, 1770–1784. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A.; Ozkan, C.; Karacan, M. Effect of vegetable proteins on physical characteristics of spray-dried tomato powders. Food Sci. Technol. Int. 2016, 22, 516–524. [Google Scholar] [CrossRef]
- Salimi, A.; Maghsoudlou, Y.; Jafari, S.M. Effect of emulsion stability and spray drying conditions on physicochemical characteristics of encapsulated powders. Lat. Am. Appl. Res. Int. J. 2018, 48, 95–100. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food Bioprod. Process. 2014, 92, 252–258. [Google Scholar] [CrossRef]
- Widyaningsih, T.D.; Akbar, S.M.; Wijayanti, N. Optimization of maltodextrin concentration, drying temperature and drying time on total flavonoid content and antioxidant activity of black garlic (Allium sativum L.) aqueous extract powder using response surface methodology. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012035. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Stipkovits, L.; Rachman, A.; Tu, J.; Brennan, M.A.; Brennan, C.S. Whey protein-blackcurrant concentrate particles obtained by spray-drying and freeze-drying for delivering structural and health benefits of cookies. Innov. Food Sci. Emerg. Technol. 2021, 68, 102606. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried beetroot juice concentrate. Food Biosci. 2016, 14, 21–27. [Google Scholar] [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.; Beristain, C.I.; Lobato-Calleros, C.; Rodriguez-Huezo, M.E.; Vernon Carter, E.J. Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. J. Food Eng. 2006, 77, 753–760. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W. Characterization of spray-dried soy sauce powders using maltodextrins as carrier. J. Food Eng. 2012, 109, 399–405. [Google Scholar] [CrossRef]
- Pui, L.P.; Karim, R.; Yusof, Y.A.; Wong, C.W.; Ghazali, H.M. Optimization of spray-drying parameters for the production of ‘Cempedak’ (Artocarpus integer) fruit powder. J. Food Meas. Charact. 2020, 14, 3238–3249. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Chen, X.D. On measurement of food powder reconstitution properties. Dry. Technol. 2007, 26, 3–14. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Li, Y. Effect of spray-drying and freeze-drying on the properties of soybean hydrolysates. J. Chem. 2020, 2020, 9201457. [Google Scholar] [CrossRef]
- Caliskan, G.; Nur Dirim, S. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food Bioprod. Process. 2013, 91, 539–548. [Google Scholar] [CrossRef]
- Farahani, Z.K.; Mousavi, M.; Ardebili, M.S.; Bakhoda, H. The influence of sodium alginate and sodium alginate/WPI as coating material on microcapsules of Jujube extract produced by spray dryer. J. Food Process. Preserv. 2022, 46, e17175. [Google Scholar]
- Both, E.; Boom, R.; Schutyser, M. Particle morphology and powder properties during spray drying of maltodextrin and whey protein mixtures. Powder Technol. 2020, 363, 519–524. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Rivas, J.C.; Cabral, L.M.C.; Rocha-Leão, M.H. Stability of bioactive compounds of microencapsulated mango and passion fruit mixed pulp. Int. J. Fruit Sci. 2020, 20, S94–S110. [Google Scholar] [CrossRef]
- Rivas, J.C.; Cabral, L.M.C.; Rocha-Leão, M.H.M.D. Microencapsulation of guava pulp using prebiotic wall material. Brazil. J. Food Technol. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A.; Oliveira, C.R.D. Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein–inulin blends as carriers. Int. J. Food. Sci. Technol. 2014, 49, 1522–1529. [Google Scholar] [CrossRef]



| Inlet Air Temperature (IAT, A °C) | Feed Flow Rate (FFR, B mL/h) | Carrier Agent Concentration (CAC, C %) | ||||
|---|---|---|---|---|---|---|
| Runs | Coded | Real | Coded | Real | Coded | Real |
| 1 | −1 | 150 | −1 | 300 | −1 | 10 |
| 2 | +1 | 170 | −1 | 300 | −1 | 10 |
| 3 | −1 | 150 | +1 | 500 | −1 | 10 |
| 4 | +1 | 170 | +1 | 500 | −1 | 10 |
| 5 | −1 | 150 | −1 | 300 | +1 | 20 |
| 6 | +1 | 170 | −1 | 300 | +1 | 20 |
| 7 | −1 | 150 | +1 | 500 | +1 | 20 |
| 8 | +1 | 170 | +1 | 500 | +1 | 20 |
| 9 | −1.682 | 143 * | 0 | 400 | 0 | 15 |
| 10 | +1.682 | 177 * | 0 | 400 | 0 | 15 |
| 11 | 0 | 160 | −1.682 | 232 * | 0 | 15 |
| 12 | 0 | 160 | +1.682 | 568 * | 0 | 15 |
| 13 | 0 | 160 | 0 | 400 | −1.682 | 6.6 |
| 14 | 0 | 160 | 0 | 400 | +1.682 | 23.4 |
| 15 | 0 | 160 | 0 | 400 | 0 | 15 |
| 16 | 0 | 160 | 0 | 400 | 0 | 15 |
| 17 | 0 | 160 | 0 | 400 | 0 | 15 |
| 18 | 0 | 160 | 0 | 400 | 0 | 15 |
| 19 | 0 | 160 | 0 | 400 | 0 | 15 |
| 20 | 0 | 160 | 0 | 400 | 0 | 15 |
| Run | MD | WPC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A- IAT (°C) | B- FFR (mL/h) | C- CAC (%) | Yield (%) | EE (%) | MC (%) | AA (%) | HG (%) | SB (%) | Yield (%) | EE (%) | MC (%) | AA (%) | HG (%) | SB (%) | |
| 1 | 150 | 300 | 10 | 50.42 | 60.52 | 1.85 | 37.57 | 11.84 | 87.64 | 49.04 | 51.27 | 2.56 | 37.34 | 5.92 | 70.92 |
| 2 | 160 | 400 | 15 | 48.64 | 60.45 | 0.65 | 47.94 | 11.44 | 91.12 | 44.57 | 60.05 | 1.45 | 43.59 | 6.25 | 76.96 |
| 3 | 170 | 500 | 20 | 67.75 | 62.16 | 0.75 | 52.71 | 9.76 | 94.12 | 52.34 | 65.17 | 2.06 | 38.44 | 9.38 | 82.92 |
| 4 | 160 | 400 | 15 | 69.43 | 54.29 | 1.47 | 37.77 | 8.04 | 92.4 | 53.63 | 65.18 | 2.05 | 38.74 | 9.15 | 83.48 |
| 5 | 150 | 500 | 10 | 37.48 | 58.37 | 1.75 | 46.42 | 11.09 | 78.88 | 53.94 | 64.28 | 1.95 | 39.42 | 9.19 | 82.32 |
| 6 | 160 | 400 | 6.6 | 50.23 | 58.79 | 1.88 | 39.59 | 11.78 | 88.76 | 53.78 | 63.35 | 2.09 | 39.08 | 9.6 | 83.12 |
| 7 | 150 | 300 | 20 | 51.77 | 58.21 | 1.74 | 38.85 | 11.20 | 87.44 | 82.25 | 88.60 | 0.98 | 52.26 | 4.82 | 85.81 |
| 8 | 170 | 300 | 20 | 34.12 | 46.78 | 2.58 | 42.76 | 11.27 | 71.52 | 74.53 | 83.23 | 0.97 | 49.28 | 7.24 | 88.12 |
| 9 | 170 | 500 | 10 | 80.65 | 59.91 | 0.79 | 51.19 | 6.64 | 91.23 | 50.53 | 67.78 | 0.82 | 48.05 | 10.53 | 86.26 |
| 10 | 150 | 500 | 20 | 59.65 | 50.49 | 0.97 | 47.89 | 11.58 | 91.6 | 71.68 | 63.71 | 1.60 | 38.40 | 6.32 | 87.18 |
| 11 | 160 | 568 | 15 | 39.76 | 58.51 | 2.06 | 45.41 | 13.52 | 86.8 | 69.46 | 86.41 | 0.96 | 53.95 | 7.09 | 89.28 |
| 12 | 160 | 232 | 15 | 51.34 | 55.29 | 1.86 | 38.42 | 11.68 | 88.24 | 59.12 | 69.11 | 0.85 | 44.28 | 9.69 | 91.72 |
| 13 | 160 | 400 | 15 | 42.98 | 83.33 | 1.85 | 45.72 | 14.42 | 87.48 | 39.45 | 58.41 | 1.98 | 47.51 | 8.92 | 73.8 |
| 14 | 160 | 400 | 23.4 | 51.76 | 56.47 | 1.84 | 37.10 | 11.34 | 87.72 | 53.55 | 64.61 | 2.09 | 38.30 | 9.37 | 82.12 |
| 15 | 160 | 400 | 15 | 72.67 | 53.73 | 0.75 | 48.24 | 9.78 | 93.12 | 63.65 | 70.35 | 1.79 | 46.64 | 6.94 | 82.42 |
| 16 | 177 | 400 | 15 | 57.33 | 81.58 | 0.65 | 43.63 | 11.17 | 96.64 | 36.76 | 55.49 | 2.76 | 43.21 | 9.66 | 65.48 |
| 17 | 143 | 400 | 15 | 47.99 | 78.08 | 2.39 | 36.63 | 7.65 | 75.84 | 52.79 | 63.12 | 2.03 | 39.46 | 9.22 | 83.04 |
| 18 | 160 | 400 | 15 | 51.85 | 67.43 | 1.87 | 37.41 | 11.57 | 88.36 | 61.37 | 72.96 | 1.17 | 46.13 | 9.28 | 86.88 |
| 19 | 160 | 400 | 15 | 42.74 | 58.16 | 1.27 | 42.97 | 8.23 | 81.68 | 41.87 | 61.08 | 2.25 | 43.59 | 11.99 | 81.8 |
| 20 | 170 | 300 | 10 | 61.57 | 65.19 | 1.58 | 45.19 | 8.75 | 87.72 | 44.87 | 63.78 | 2.06 | 46.57 | 12.64 | 82.64 |
| MD | WPC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (%) | EE (%) | MC (%) | AA (%) | HG (%) | SB (%) | Yield (%) | EE (%) | MC (%) | AA (%) | HG (%) | SB (%) | |
| Model | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| A | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| B | <0.0001 | 0.1924 | <0.0001 | 0.0035 | <0.0001 | <0.0001 | <0.0001 | 0.0882 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C | <0.0001 | <0.0001 | <0.0001 | 0.0032 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| AB | 0.3030 | 0.2245 | 0.9924 | 0.0655 | 0.1032 | 0.0005 | 0.0206 | 0.1820 | 0.0113 | <0.0001 | <0.0001 | <0.0001 |
| BC | 0.4099 | 0.0008 | 0.0673 | 0.0023 | 0.0170 | <0.0001 | 0.1984 | 0.0333 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| CA | 0.0854 | 0.0019 | <0.0001 | 0.0002 | 0.0008 | 0.0009 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0007 |
| A2 | 0.0025 | 0.4475 | 0.0172 | 0.0002 | <0.0001 | <0.0001 | 0.4944 | 0.2142 | 0.7233 | 0.0002 | 0.0050 | 0.0002 |
| B2 | 0.1411 | 0.1852 | <0.0001 | <0.0001 | 0.0016 | <0.0001 | 0.4330 | 0.0004 | 0.1746 | <0.0001 | <0.0001 | <0.0001 |
| C2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0012 | 0.2823 | 0.0005 | <0.0001 | <0.0001 | 0.0002 | 0.0003 |
| R2 | 0.9973 | 0.9848 | 0.9921 | 0.9830 | 0.9847 | 0.9909 | 0.9961 | 0.9874 | 0.9910 | 0.9955 | 0.9906 | 0.9918 |
| ADJ R2 | 0.9948 | 0.9711 | 0.9850 | 0.9677 | 0.9710 | 0.9827 | 0.9925 | 0.9760 | 0.9829 | 0.9914 | 0.9821 | 0.9843 |
| CV | 1.64 | 2.67 | 4.71 | 2.09 | 3.14 | 0.9328 | 1.88 | 2.23 | 4.45 | 1.06 | 3.11 | 0.9681 |
| Lack of fit | 0.244 NS | 0.071 NS | 0.129 NS | 0.616 NS | 0.171 NS | 0.071 NS | 0.063 NS | 0.058 NS | 0.096 NS | 0.606 NS | 0.073 NS | 0.087 NS |
| Response Variables | Response Model Equations for MD as Carrier Agent | Response Model Equations for WPC as Carrier Agent |
|---|---|---|
| Yield (%) | +51.23 + 2.97 A − 3.98 B + 13.09 C + 0.3365 AB − 0.2665 AC + 0.5914 BC + 0.9261 A2 − 0.3689 B2 + 2.78 C2 | + 53.32 + 2.96 A – 3.92 B + 13.00 C +0.2168 AB − 0.3017AC + 0.4197 BC + 0.7555 A2 − 0.3790 B2 + 2.75 C2 |
| Encapsulation Efficiency (%) | +59.33 + 6.26 A + 0.6211 B + 7.86 C − 0.7515 AB + 2.74 AC + 2.43 BC − 0.3419 A2 -0.6154 B2 + 3.97 C2 | + 64.25 + 6.25 A − 0.7625 B + 8.06 C − 0.6995 AB + 2.79 AC + 2.63 BC − 0.5636 A2 + 0.9693 B2 + 3.47 C2 |
| Moisture content (%) | +1.84 − 0.3724 A + 0.3549 B − 0.2660 C − 0.0003 AB − 0.0523 AC − 0.3273 BC − 0.0541 A2 − 0.2059 B2 − 0.1989 C2 | + 2.05 − 3610 A + 0.3610 B − 0.2659 C − 0.0099 AB − 0.0396 AC − 0.3224 BC -0.0625 A2 − 0.2084 B2 − 0.1988 C2 |
| Antioxidant activity (%) | +38.17 + 3.26 A + 0.9271 B + 0.9436 C − 0.6605 AB + 1.30 AC + 1.83 BC + 1.39 A2 + 2.24 B2 + 3.70 C2 | + 38.92 + 2.77 A + 0. 8724 B + 1.30 C − 0.9536 AB + 1.67 AC + 2.22 BC + 0.9461 A2 + 2.25 B2 + 3.83 C2 |
| Hygroscopicity (%) | +11.58 + 1.08A + 0.8256 B − 1.14 C − 0.2119 AB − 0.3377 AC − 0.5550 BC − 0.7454 A2 + 0.3790 B2 − 1.01 C2 | + 9.33 + 0.9763 A + 0.7538 B − 1.30 C − 0.3410 AB − 0.6908 AC − 0.5502 BC − 0.6574 A2 + 0.6012 B2 − 0.9175 C2 |
| Solubility (%) | +88.01 + 4.27 A − 2.46 B + 4.17 C + 1.44 AB − 2.20 AC + 1.35 BC − 1.43 A2 + 1.52 B2 − 0.9598 C2 | + 82.82 + 4.41 A − 2.55 B + 4.15 C + 1.62 AB − 2.23 AC + 1.54 BC − 1.33A2 + 1.60 B2 − 1.01 C2 |
| Upper and Lower Limits of Variables | Predicted and Experimental Values for the Optimized Variable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aim | MD | WPC | MD | WPC | ||||||||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | Pred. Value | Exptl. Value | Variation (%) | Pred. Value | Exptl. Value | Variation (%) | |||
| Factors | A | In range | 150 | 170 | 150 | 170 | 170 | 170 | ||||
| B | In range | 300 | 500 | 300 | 500 | 500 | 500 | |||||
| C | In range | 10 | 20 | 10 | 20 | 20 | 20 | |||||
| Responses | Yield (%) | Max. | 34.12 | 80.65 | 36.76 | 82.25 | 51.23 | 50.21 | 1.09 | 75.35 | 74.54 | 1.06 |
| EE (%) | Max. | 46.78 | 83.33 | 51.27 | 88.60 | 59.33 | 60.01 | 1.15 | 84.05 | 83.23 | 0.97 | |
| MC (%) | Min. | 0.65 | 2.58 | 0.821 | 2.763 | 1.84 | 1.76 | 4.18 | 0.88 | 0.92 | 4.55 | |
| AA (%) | Max. | 36.63 | 52.71 | 37.34 | 53.95 | 38.17 | 38.36 | 0.50 | 49.55 | 49.28 | 0.55 | |
| HG (%) | Min. | 6.64 | 14.42 | 4.82 | 12.64 | 11.57 | 11.89 | 2.80 | 7.48 | 7.25 | 3.06 | |
| SB (%) | Max. | 71.52 | 96.64 | 65.48 | 91.72 | 88.01 | 88.19 | 0.19 | 87.81 | 88.12 | 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arya, P.; Kumar, P. Effect of Carrier Agents on Quality Parameters of Spray-Dried Encapsulated Diosgenin Powder and the Optimization of Process Parameters. Foods 2023, 12, 2330. https://doi.org/10.3390/foods12122330
Arya P, Kumar P. Effect of Carrier Agents on Quality Parameters of Spray-Dried Encapsulated Diosgenin Powder and the Optimization of Process Parameters. Foods. 2023; 12(12):2330. https://doi.org/10.3390/foods12122330
Chicago/Turabian StyleArya, Prajya, and Pradyuman Kumar. 2023. "Effect of Carrier Agents on Quality Parameters of Spray-Dried Encapsulated Diosgenin Powder and the Optimization of Process Parameters" Foods 12, no. 12: 2330. https://doi.org/10.3390/foods12122330
APA StyleArya, P., & Kumar, P. (2023). Effect of Carrier Agents on Quality Parameters of Spray-Dried Encapsulated Diosgenin Powder and the Optimization of Process Parameters. Foods, 12(12), 2330. https://doi.org/10.3390/foods12122330






