Panicum decompositum, an Australian Native Grass, Has Strong Potential as a Novel Grain in the Modern Food Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Preparation and Physical Analysis of Grain
2.2. Milling
2.3. Chemical and Physical Analysis of Flour
2.4. Preparation and Quality Testing of Flatbread
2.5. Sensory Analysis of Flatbread
2.6. Statistical Analysis
3. Results
3.1. Physical Properties of Whole Grain
3.2. Milling Yield
3.3. Physical and Chemical Properties of Flour
3.4. Quality of Flatbreads
3.5. Sensory Analysis of Flatbreads
4. Discussion
4.1. Influence of Grain Size and Chemistry on Flour Properties
4.2. Use of Wholemeal versus White Flour from Native Millet
4.3. Consumer Acceptance of Native Millet Flour
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drake, A.; Keitel, C.; Pattison, A. The Use of Australian Native Grains as a Food: A Review of Research in a Global Grains Context. Rangel. J. 2021, 43, 223–233. [Google Scholar] [CrossRef]
- Kirk, R.L. Aboriginal Man Adapting: The Human Biology of Australian Aborigines (Research Monographs on Human Population Biology); Oxford University Press: Melbourne, FL, USA, 1983; ISBN 0198575327. [Google Scholar]
- O’Connell, J.F.; Latz, P.K.; Barnett, P. Traditional and Modern Plant Use among the Alyawara of Central Australia. Econ. Bot. 1983, 37, 80–109. [Google Scholar] [CrossRef]
- Cane, S. Australian Aboriginal Seed Grinding and Its Archaeological Record: A Case Study from the Western Desert. In Foraging and Farming: The Evolution of Plant Exploitation; Routledge: London, UK, 2014. [Google Scholar]
- Pattison, A.; McGee, K.; Birch, J.; Saunders, K.; Ashby, R.; Quinnell, R.; Bell-Anderson, K. What Do We Know about Threshing Traditional Grains in Australia? J. Ethnobiol. 2023. [Google Scholar]
- Chase, A.K. Domestication and Domiculture in Northern Australia: A Social Perspective. In Foraging and Farming: The Evolution of Plant Exploitation; Routledge: London, UK, 2014. [Google Scholar]
- O’Connell, J.F.; Hawkes, K. Food Choice and Foraging Sites among the Alyawara. J. Anthropol. Res. 1984, 40, 504–535. [Google Scholar] [CrossRef]
- Zeanah, D.W.; Codding, B.F.; Bird, D.W.; Bliege Bird, R.; Veth, P.M. Diesel and Damper: Changes in Seed Use and Mobility Patterns Following Contact amongst the Martu of Western Australia. J. Anthropol. Archaeol. 2015, 39, 51–62. [Google Scholar] [CrossRef]
- FAO. OECD-FAO Agricultural Outlook 2020–2029; OECD: Paris, France, 2020; ISBN 9789264317673. [Google Scholar]
- Zarnkow, M.; Keßler, M.; Burberg, F.; Back, W.; Arendt, E.K.; Kreisz, S. The Use of Response Surface Methodology to Optimise Malting Conditions of Proso Millet (Panicum miliaceum L.) as a Raw Material for Gluten-Free Foods. J. Inst. Brew. 2007, 113, 280–292. [Google Scholar] [CrossRef]
- Kurek, M.A.; Karp, S.; Wyrwisz, J.; Niu, Y. Physicochemical Properties of Dietary Fibers Extracted from Gluten-Free Sources: Quinoa (Chenopodium quinoa), Amaranth (Amaranthus caudatus) and Millet (Panicum miliaceum). Food Hydrocoll. 2018, 85, 321–330. [Google Scholar] [CrossRef]
- Shewry, P. What Is Gluten—Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Jauhar, P.P. Morphological Studies in Some Species of Panicum L. Bull. Bot. Surv. India 1966, 8, 183–188. [Google Scholar]
- Sridhar, R.; Lakshminarayana, G. Contents of Total Lipids and Lipid Classes and Composition of Fatty Acids in Small Millets: Foxtail (Setaria italica), Proso (Panicum miliaceum), and Finger (Eleusine coracana). Cereal Chem. 1994, 71, 355–359. [Google Scholar]
- Revankar, J.; Ankita Shamnani, D.K.; Podili, K. Antioxidant Activities of Pearl Millet (Pennisetum glaucum) and Little Millet (Panicum sumatrense) in Different in Vitro Models. Int. J. Bioassays 2018, 7, 5595–5601. [Google Scholar]
- Wheat Quality Australia. Wheat Qualification Guidelines. Available online: https://wheatquality.com.au/ (accessed on 4 April 2023).
- Simonelli, C.; Galassi, L.; Cormegna, M.; Bianchi, P. Chemical, Physical, Textural and Sensory Evaluation on Italian Rice Varieties. Univers. J. Agric. Res. 2017, 5, 104–112. [Google Scholar] [CrossRef]
- AACC International. AACC International Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2009. [Google Scholar]
- Miskelly, D.; Suter, D. Assessing and Managing Wheat-Flour Quality Before, During and After Milling. In Cereal Grains: Assessing and Managing Quality: Second Edition; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Khoddami, A.; Truong, H.H.; Liu, S.Y.; Roberts, T.H.; Selle, P.H. Concentrations of Specific Phenolic Compounds in Six Red Sorghums Influence Nutrient Utilisation in Broiler Chickens. Anim. Feed. Sci. Technol. 2015, 210, 190–199. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical Characteristics of Nigella Seed (Nigella sativa L.) Oil as Affected by Different Extraction Methods. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Yun, S.H.; Quail, K. RVA Pasting Properties of Australian Wheat Starches. Starch/Staerke 1999, 51, 274–280. [Google Scholar] [CrossRef]
- Gujral, H.S.; Gaur, S. Effects of Barley Flour, Wet Gluten and Liquid Shortening on the Texture and Storage Characteristics of Chapati. J. Texture Stud. 2002, 33, 461–469. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Chiu, M.-C.M. Ancient Whole Grain Gluten-Free Flatbreads. Food Nutr. Sci. 2014, 5, 1717–1724. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, P.; Shukla, G.; Wadhwa, N. Quality Attributes and Acceptability of Bread Made from Wheat and Amorphophallus paeoniifolius Flour. J. Food Sci. Technol. 2015, 52, 7472–7478. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of Nutritional Quality and Sensory Acceptability of Biscuits Obtained from Native, Fermented, and Malted Pearl Millet (Pennisetum glaucum) Flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Alencar, N.M.M.; de Morais, E.C.; Steel, C.J.; Bolini, H.M.A. Sensory Characterisation of Gluten-Free Bread with Addition of Quinoa, Amaranth Flour and Sweeteners as an Alternative for Coeliac Patients. Int. J. Food Sci. Technol. 2017, 52, 872–879. [Google Scholar] [CrossRef]
- Brito, I.L.; de Souza, E.L.; Felex, S.S.S.; Madruga, M.S.; Yamashita, F.; Magnani, M. Nutritional and Sensory Characteristics of Gluten-Free Quinoa (Chenopodium quinoa Willd)-Based Cookies Development Using an Experimental Mixture Design. J. Food Sci. Technol. 2015, 52, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Sandri, L.T.B.; Santos, F.G.; Fratelli, C.; Capriles, V.D. Development of Gluten-Free Bread Formulations Containing Whole Chia Flour with Acceptable Sensory Properties. Food Sci. Nutr. 2017, 5, 1021–1028. [Google Scholar] [CrossRef]
- Latz, P.K.; Green, J.; Institute for Aboriginal Development. Bushfires & Bushtucker: Aboriginal Plant Use in Central Australia; Latz, P.K., Ed.; IAD Press: Alice Springs, NT, Australia, 1995; ISBN 0949659835. [Google Scholar]
- Barnes, P.J. The Influence of Wheat Endosperm on Flour Colour Grade. J. Cereal Sci. 1986, 4, 143–155. [Google Scholar] [CrossRef]
- Hidalgo, A.; Fongaro, L.; Brandolini, A. Colour Screening of Whole Meal Flours and Discrimination of Seven Triticum Subspecies. J. Cereal Sci. 2017, 77, 9–16. [Google Scholar] [CrossRef]
- Miskelly, D.M. Flour Components Affecting Paste and Noodle Colour. J. Sci. Food Agric. 1984, 35, 463–471. [Google Scholar] [CrossRef]
- Kulp, K. Handbook of Cereal Science and Technology, Revised and Expanded; Kulp, K., Ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429116605. [Google Scholar]
- Ortolan, F.; Steel, C.J. Protein Characteristics That Affect the Quality of Vital Wheat Gluten to Be Used in Baking: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 369–381. [Google Scholar] [CrossRef]
- Lagrain, B.; Wilderjans, E.; Glorieux, C.; Delcour, J.A. Importance of Gluten and Starch for Structural and Textural Properties of Crumb from Fresh and Stored Bread. Food Biophys. 2012, 7, 173–181. [Google Scholar] [CrossRef]
- Gao, P.; Quilichini, T.D.; Zhai, C.; Qin, L.; Nilsen, K.T.; Li, Q.; Sharpe, A.G.; Kochian, L.V.; Zou, J.; Reddy, A.S.N.; et al. Alternative Splicing Dynamics and Evolutionary Divergence during Embryogenesis in Wheat Species. Plant Biotechnol. J. 2021, 19, 1624–1643. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of Grain Quality in Wheat—A Review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Cauvain, S.P. Breadmaking: Improving Quality, 2nd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; ISBN 9780857095695. [Google Scholar]
- Cappelli, A.; Guerrini, L.; Parenti, A.; Palladino, G.; Cini, E. Effects of Wheat Tempering and Stone Rotational Speed on Particle Size, Dough Rheology and Bread Characteristics for a Stone-Milled Weak Flour. J. Cereal Sci. 2020, 91, 102879. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Cookie Making Behavior of Wheat-Barley Flour Blends and Effects on Antioxidant Properties. LWT 2014, 55, 301–307. [Google Scholar] [CrossRef]
- Koletta, P.; Irakli, M.; Papageorgiou, M.; Skendi, A. Physicochemical and Technological Properties of Highly Enriched Wheat Breads with Wholegrain Non Wheat Flours. J. Cereal Sci. 2014, 60, 561–568. [Google Scholar] [CrossRef]
- Frost, D.J.; Adhikari, K.; Lewis, D.S. Effect of Barley Flour on the Physical and Sensory Characteristics of Chocolate Chip Cookies. J. Food Sci. Technol. 2011, 48, 569–576. [Google Scholar] [CrossRef]
- Torbica, A.; Belović, M.; Tomić, J. Novel Breads of Non-Wheat Flours. Food Chem. 2019, 282, 134–140. [Google Scholar] [CrossRef]
- Dhingra, S.; Jood, S. Organoleptic and Nutritional Evaluation of Wheat Breads Supplemented with Soybean and Barley Flour. Food Chem. 2002, 77, 479–488. [Google Scholar] [CrossRef]
- Noor Aziah, A.; Komathi, C.A. Acceptability Attributes of Crackers Made from Different Types of Composite Flour. Int. Food Res. J. 2009, 16, 479–482. [Google Scholar]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; de Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory Characteristics of Wholegrain and Bran-Rich Cereal Foods—A Review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Holt, S.H.A. Australian Aboriginal Plant Foods: A Consideration of Their Nutritional Composition and Health Implications. Nutr. Res. Rev. 1998, 11, 5–23. [Google Scholar] [CrossRef]
- Bell, T.L.; Pattison, A.; Claudia, K.; Khoddami, A.; Cross, R. Native Grasses as a Traditional and Emerging Source of Food. Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2022, 31, 3–6. [Google Scholar]
- Sydney Institute of Agriculture. Native Grains from Paddock to Plate: Study of the Economic, Environmental and Social Sustainability of an Ancient System in a Modern Context; Sydney Institute of Agriculture: Eveleigh, NSW, Australia, 2020. [Google Scholar]
- Garden, D.L.; Dowling, P.M.; Eddy, D.A.; Nicol, H.I. The Influence of Climate, Soil, and Management on the Composition of Native Grass Pastures on the Central, Southern, and Monaro Tablelands of New South Wales. Aust. J. Agric. Res. 2001, 52, 925–936. [Google Scholar] [CrossRef]
- Shete, M.; Rutten, M.; Schoneveld, G.C.; Zewude, E. Land-Use Changes by Large-Scale Plantations and Their Effects on Soil Organic Carbon, Micronutrients and Bulk Density: Empirical Evidence from Ethiopia. Agric. Hum. Values 2016, 33, 689–704. [Google Scholar] [CrossRef]
- Gorman, J.T.; Bentivoglio, M.; Brady, C.; Wurm, P.; Vemuri, S.; Sultanbawa, Y. Complexities in Developing Australian Aboriginal Enterprises Based on Natural Resources. Rangel. J. 2020, 42, 113. [Google Scholar] [CrossRef]
- Bodle, K.; Brimble, M.; Weaven, S.; Frazer, L.; Blue, L. Critical Success Factors in Managing Sustainable Indigenous Businesses in Australia. Pac. Account. Rev. 2018, 30, 35–51. [Google Scholar] [CrossRef]
- Wrigley, C.; Batey, I.; Miskelly, D. Grain Quality: The Future Is With the Consumer, the Scientist and the Technologist. In Cereal Grains: Assessing and Managing Quality: Second Edition; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
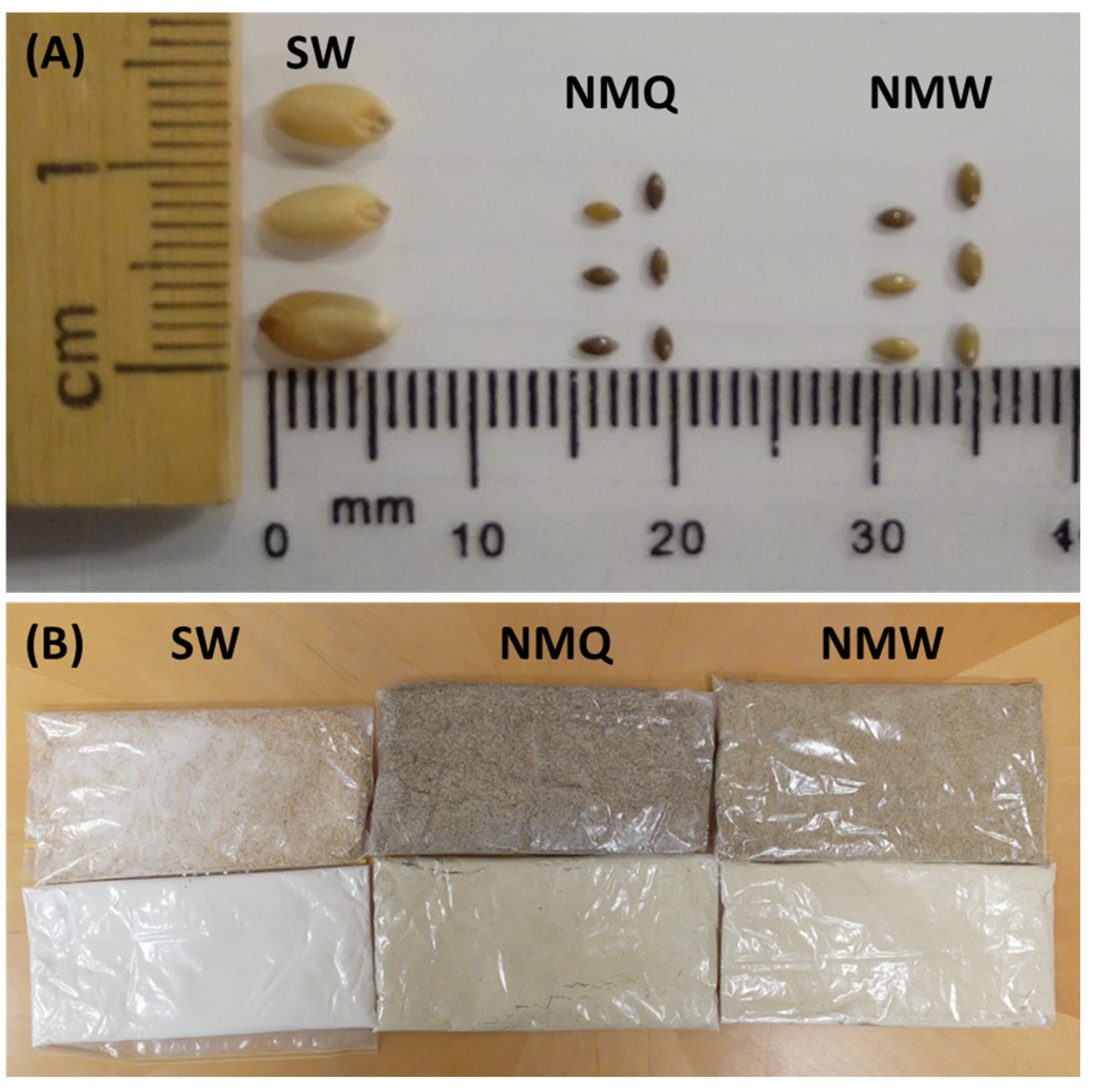



| Grain | Grain Length/Width (mm) | TKW (g) | Test Weight (kg hL−1) | Moisture Content (%) |
|---|---|---|---|---|
| SW | 6/4 | 38.6 ± 1.2 a | 80.5 ± 0.3 a | 9.5 ± 0.02 a |
| NMQ | 2/1 | 0.9 ± 0.1 b | 76.7 ± 1.3 b | 13.2 ± 0.01 b |
| NMW | 2/1 | 1.2 ± 0.1 b | 77.3 ± 0.4 b | 13.5 ± 0.06 b |
| Flour | Protein (%) | Ash (%) |
|---|---|---|
| SW | 12.1 ± 0.2 b | 1.6 ± 0.01 c |
| NMQ | 13.7 ± 0.4 a | 6.7 ± 0.06 b |
| NMW | 13.6 ± 0.1 a | 7.2 ± 0.01 a |
| Flour | Moisture Content (%) | L* Value | a* Value | b* Value |
|---|---|---|---|---|
| SW | 11.5 | 91.81 ± 0.20 a | 0.16 ± 0.08 ab | 7.74 ± 0.19 b |
| 13.5 | 91.31 ± 1.27 a | 0.14 ± 0.01 abc | 7.57 ± 0.14 b | |
| 15.5 | 92.50 ± 0.23 a | 0.06 ± 0.06 abc | 7.74 ± 0.27 b | |
| NMQ | 11.5 | 76.24 ± 0.38 d | 0.25 ± 0.12 ab | 17.76 ± 0.12 a |
| 13.5 | 77.75 ± 0.23 cd | -0.14 ± 0.12 bcd | 17.84 ± 0.26 a | |
| 15.5 | 79.27 ± 0.52 bc | -0.60 ± 0.08 d | 18.14 ± 0.01 a | |
| NMW | 11.5 | 78.57 ± 1.37 bcd | 0.28 ± 0.23 ab | 17.52 ± 0.22 a |
| 13.5 | 77.87 ± 0.98 bcd | 0.48 ± 0.16 a | 17.56 ± 0.76 a | |
| 15.5 | 80.86 ± 0.30 b | -0.34 ± 0.06 cd | 18.05 ± 0.07 a |
| Flour | Peak Viscosity cP | Holding Strength cP | Breakdown cP | Setback cP | Final Viscosity cP | Peak Time (min) |
|---|---|---|---|---|---|---|
| SW | 195.92 ± 3.25 a | 112.17 ± 5.21 a | 83.75 ± 2.34 a | 113.16 ± 2.51 a | 225.33 ± 4.46 a | 5.62 ± 0.04 a |
| NMQ | 58.32 ± 1.40 b | 43.53 ± 1.07 b | 14.78 ± 0.38 b | 71.58 ± 2.21 b | 115.11 ± 3.20 b | 5.35 ± 0.04 b |
| NMW | 53.97 ± 1.00 b | 40.47 ± 0.93 b | 13.50 ± 0.30 b | 67.69 ± 1.96 b | 108.16 ± 2.77 b | 5.33 ± 0.07 b |
| Flour Mix | L* Value | a* Value | b* Value |
|---|---|---|---|
| Top of flatbread | |||
| 100 SW | 55.42 ± 1.63 a | 4.91 ± 0.55 a | 16.19 ± 0.47 a |
| 25:75 NM:SW | 49.21 ± 1.90 c | 3.58 ± 0.50 b | 12.23 ± 0.34 b |
| 50:50 NM:SW | 52.75 ± 1.76 b | 2.35 ± 0.24 c | 11.21 ± 0.85 c |
| Bottom of flatbread | |||
| 100 SW | 58.07 ± 2.26 a | 4.12 ± 0.21 a | 16.63 ± 0.70 a |
| 25:75 NM:SW | 45.80 ± 1.73 b | 3.66 ± 0.19 b | 11.39 ± 0.56 b |
| 50:50 NM:SW | 42.57 ± 1.85 c | 3.45 ± 0.18 c | 9.55 ± 0.72 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenifer, J.; Bell, T.L.; Khoddami, A.; Pattison, A.L. Panicum decompositum, an Australian Native Grass, Has Strong Potential as a Novel Grain in the Modern Food Market. Foods 2023, 12, 2048. https://doi.org/10.3390/foods12102048
Jenifer J, Bell TL, Khoddami A, Pattison AL. Panicum decompositum, an Australian Native Grass, Has Strong Potential as a Novel Grain in the Modern Food Market. Foods. 2023; 12(10):2048. https://doi.org/10.3390/foods12102048
Chicago/Turabian StyleJenifer, Jenifer, Tina L. Bell, Ali Khoddami, and Angela L. Pattison. 2023. "Panicum decompositum, an Australian Native Grass, Has Strong Potential as a Novel Grain in the Modern Food Market" Foods 12, no. 10: 2048. https://doi.org/10.3390/foods12102048
APA StyleJenifer, J., Bell, T. L., Khoddami, A., & Pattison, A. L. (2023). Panicum decompositum, an Australian Native Grass, Has Strong Potential as a Novel Grain in the Modern Food Market. Foods, 12(10), 2048. https://doi.org/10.3390/foods12102048






