Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sprouting Process
2.3. Aqueous Extraction
2.4. Total Starch, Damaged Starch, Fiber, and Glucose Content
2.5. Endogenous α-Amylase Activity
2.6. SDS-PAGE Analysis of Soluble Proteins
2.7. Soluble Proteins and Amino Acids/Small Peptides Content
2.8. Endogenous Protease Activity
2.9. Lipid Content and Composition
2.10. Lipid Peroxidation
2.11. Phytic Acid Content
2.12. Pepsin, Trypsin, and Chymotrypsin Activity
2.13. Tocols Extraction and Determination by HPLC–FLD
2.14. Extraction and Determination of Free and Bound Phenolic Compounds
2.15. Total Antioxidant Capacity (TAC) and Ferric Reducing Antioxidant Power (FRAP)
2.16. 1H(HR)-NMR Spectra Acquisition and Processing
2.17. Statistical Analysis
3. Results and Discussion
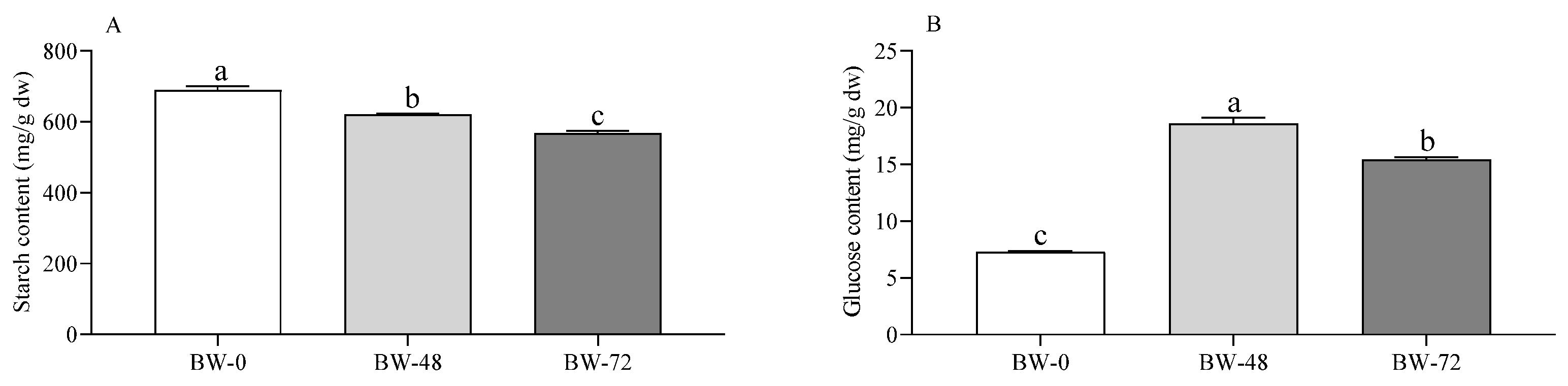
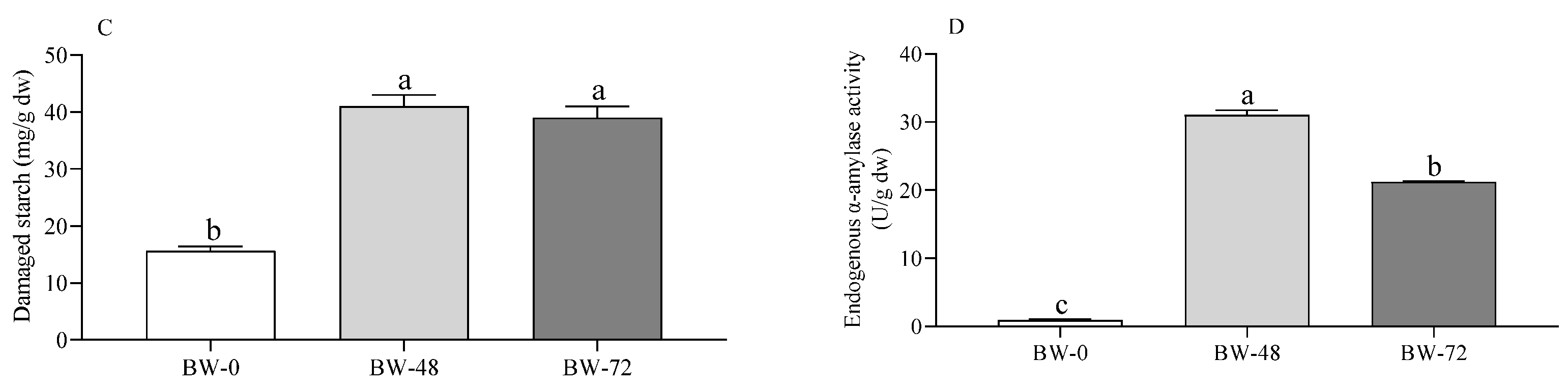
3.1. Carbohydrates
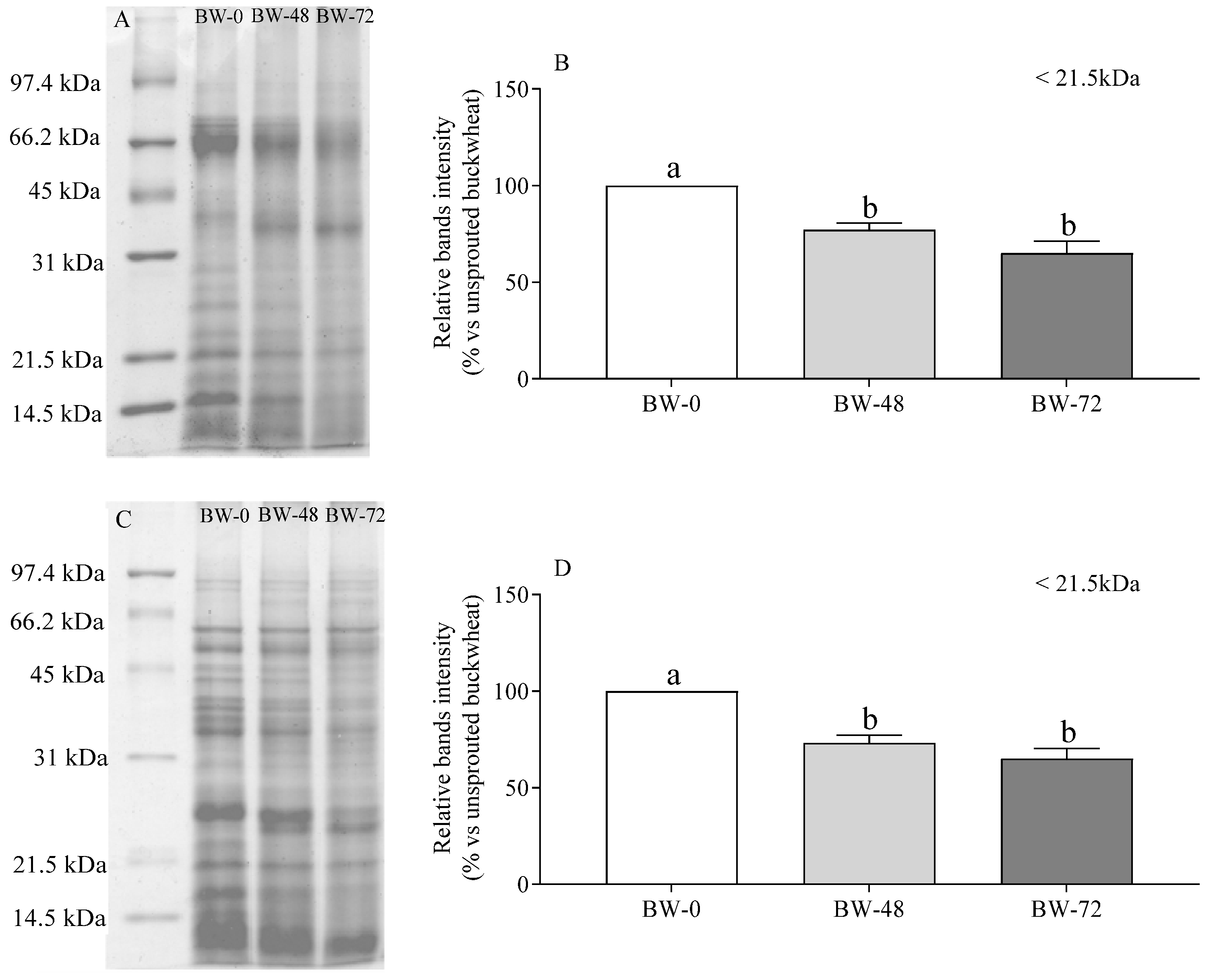
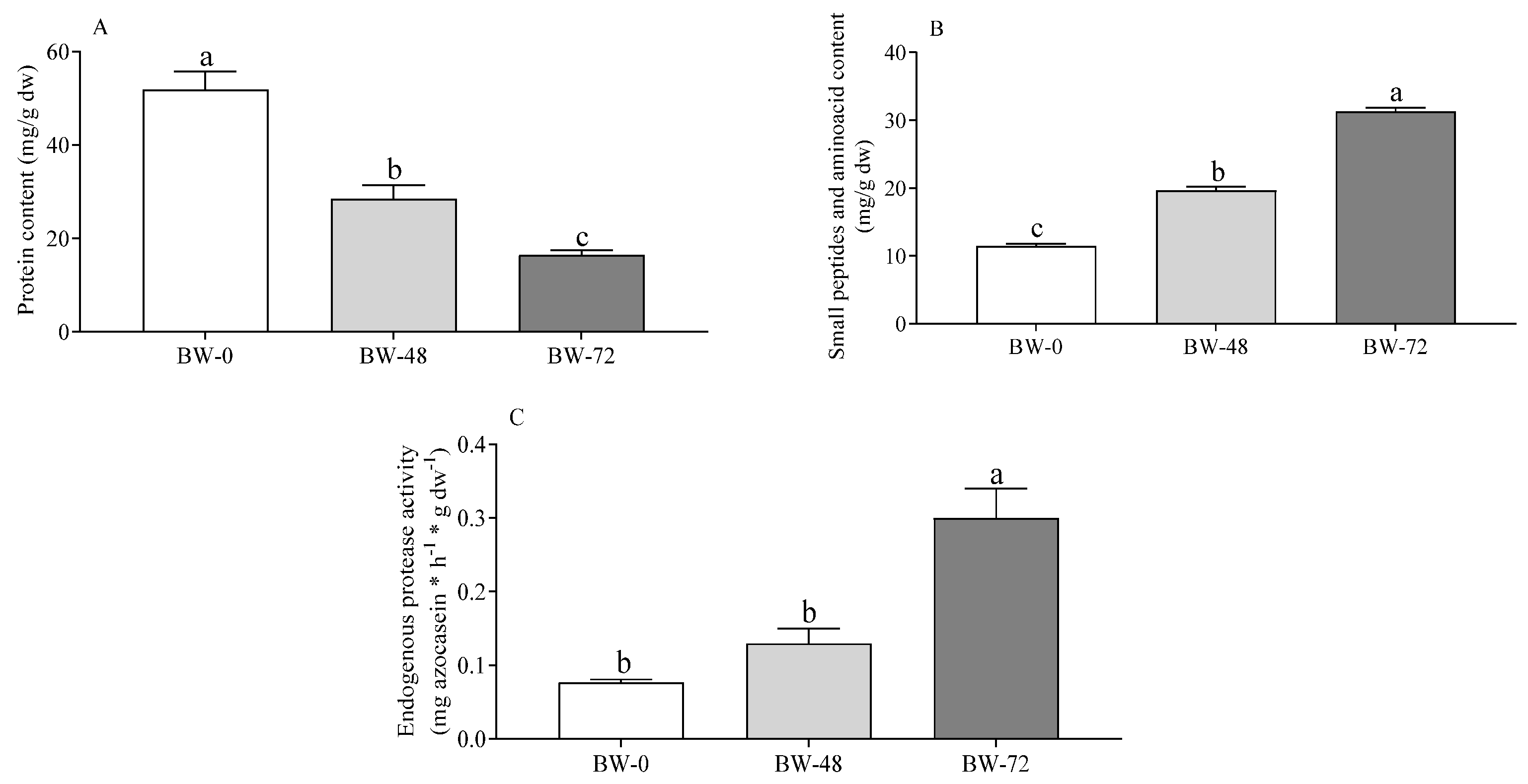
3.2. Proteins
3.3. Lipids
3.4. Bioactive Compounds
3.4.1. Tocols
3.4.2. Free and Bound Polyphenols
3.5. Antioxidant Capacity
3.6. Metabolome
3.7. Anti-Nutritional Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádová, D.; Barris, S.; Quinet, M. Different drought resistance mechanisms between two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2021, 172, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Gaberščik, A. Chapter twenty one—The Effect of Environmental Factors on Buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 273–281. [Google Scholar]
- Singh, M.; Malhotra, N.; Sharma, K. Buckwheat (Fagopyrum sp.) genetic resources: What can they contribute towards nutritional security of changing world? Genet. Resour. Crop Evol. 2020, 67, 1639–1658. [Google Scholar] [CrossRef]
- Sturza, A.; Păucean, A.; Chiș, M.S.; Mureșan, V.; Vodnar, D.C.; Man, S.M.; Urcan, A.C.; Rusu, I.E.; Fostoc, G.; Muste, S. Influence of Buckwheat and Buckwheat Sprouts Flours on the Nutritional and Textural Parameters of Wheat Buns. Appl. Sci. 2020, 10, 7969. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Technol. 2015, 44, 58–65. [Google Scholar] [CrossRef]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Saithalavi, K.M.; Bhasin, A.; Yaqoob, M. Impact of sprouting on physicochemical and nutritional properties of sorghum: A review. J. Food Meas. Charact. 2021, 15, 4190–4204. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J.; Zielińska-Dawidziak, M.; Tomczak, A.; Świeca, M. Starch and protein analysis in buckwheat (Fagopyrum esculentum Moench) sprouts enriched with probiotic yeast. LWT 2022, 168, 113903. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F. Analysis of Fatty Acid Composition in Sprouted Grains. Foods 2023, 12, 1853. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Properties and bread-baking performance of wheat flour composited with germinated pulse flours. Cereal Chem. 2020, 97, 459–471. [Google Scholar] [CrossRef]
- Sharanagat, V.S.; Nema, P.K. Bread preparation by partial replacement of wheat by germinated sorghum. Food Sci. Technol. Int. 2023, 29, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Loffi, C.; Chiarello, E.; Dellafiora, L.; Picone, G.; Antonelli, G.; Di Gregorio, C.; Capozzi, F.; Tedeschi, T.; Galaverna, G.; et al. Impact of a Shorter Brine Soaking Time on Nutrient Bioaccessibility and Peptide Formation in 30-Months-Ripened Parmigiano Reggiano Cheese. Molecules 2022, 27, 664. [Google Scholar] [CrossRef]
- Freitas, C.; Souza, D.; Araújo, E.; Cavalheiro, M.; Oliveira, L.; Ramos, M. Anti-oxidative and proteolytic activities and protein profile of laticifer cells of Cryptostegia grandiflora, Plumeria rubra and Euphorbia tirucalli. Braz. J. Plant Physiol. 2009, 22, 11–22. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Stoffel, W.; Insull, W., Jr.; Ahrens, E.H., Jr. Gas-liquid chromatography of highly unsaturated fatty acid methyl esters. Proc. Soc. Exp. Biol. Med. 1958, 99, 238–241. [Google Scholar] [CrossRef]
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; van Deursen, D.; Verhoeven, A.J.; Bordoni, A. n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells. Br. J. Nutr. 2010, 103, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Situnayake, R.D.; Crump, B.J.; Zezulka, A.V.; Davis, M.; McConkey, B.; Thurnham, D.I. Measurement of conjugated diene lipids by derivative spectroscopy in heptane extracts of plasma. Ann. Clin. Biochem. 1990, 27 Pt 3, 258–266. [Google Scholar] [CrossRef]
- Urbinati, E.; Di Nunzio, M.; Picone, G.; Chiarello, E.; Bordoni, A.; Capozzi, F. The Effect of Balsamic Vinegar Dressing on Protein and Carbohydrate Digestibility is Dependent on the Food Matrix. Foods 2021, 10, 411. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Gómez-Caravaca, A.M.; Blasco, T.; Cvejić, J.; Caboni, M.F.; Verardo, V. Assessment of Lipid Quality in Commercial Omega-3 Supplements Sold in the French Market. Biomolecules 2022, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Caboni, M.F.; Pasini, F. Co-milling process of olives and oleaginous matrices with high nutritional value: A preliminary characterisation of the obtained oils. Int. J. Food Sci. Nutr. 2022, 73, 1057–1066. [Google Scholar] [CrossRef]
- Bombai, G.; Pasini, F.; Verardo, V.; Sevindik, O.; Di Foggia, M.; Tessarin, P.; Bregoli, A.M.; Caboni, M.F.; Rombolà, A.D. Monitoring of compositional changes during berry ripening in grape seed extracts of cv. Sangiovese (Vitis vinifera L.). J. Sci. Food Agric. 2017, 97, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Pasini, F.; Verardo, V.; Gómez-Caravaca, A.M.; Marconi, E.; Caboni, M.F. Distribution of Free and Bound Phenolic Compounds in Buckwheat Milling Fractions. Foods 2019, 8, 670. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Berardinelli, A.; Marconi, E.; Caboni, M.F. A chemometric approach to determine the phenolic compounds in different barley samples by two different stationary phases: A comparison between C18 and pentafluorophenyl core shell columns. J. Chromatogr. A 2014, 1355, 134–142. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Bordoni, A.; Aureli, F.; Cubadda, F.; Gianotti, A. Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread. Nutrients 2018, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Acquaviva, A.; Suktham, T.; Dennis, G.R.; Shalliker, R.A.; Soliven, A. Total Antioxidant Capacity with Peak Specificity via Reaction Flow Chromatography and the Ferric Reducing Antioxidant Power Assay. Food Anal. Methods 2020, 13, 608–616. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and Normalization Effects in NMR Spectroscopic Metabonomic Data Sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, J.; Zhang, J.; Liu, R. Changes in starch structures and in vitro digestion characteristics during maize (Zea mays L.) germination. Food Sci. Nutr. 2020, 8, 1700–1708. [Google Scholar] [CrossRef]
- Chinma, C.E.; Abu, J.O.; Adedeji, O.E.; Aburime, L.C.; Joseph, D.G.; Agunloye, G.F.; Adebo, J.A.; Oyeyinka, S.A.; Njobeh, P.B.; Adebo, O.A. Nutritional composition, bioactivity, starch characteristics, thermal and microstructural properties of germinated pigeon pea flour. Food Biosci. 2022, 49, 101900. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Ussenov, Y.A.; Akildinova, A.; Kuanbaevich, B.A.; Serikovna, K.A.; Gabdullin, M.; Dosbolayev, M.; Daniyarov, T.; Ramazanov, T. The Effect of Non-Thermal Atmospheric Pressure Plasma Treatment of Wheat Seeds on Germination Parameters and α-Amylase Enzyme Activity. IEEE Trans. Plasma Sci. 2022, 50, 330–340. [Google Scholar] [CrossRef]
- Marti, A.; Caramanico, R.; Bottega, G.; Pagani, M.A. Cooking behavior of rice pasta: Effect of thermal treatments and extrusion conditions. LWT—Food Sci. Technol. 2013, 54, 229–235. [Google Scholar] [CrossRef]
- Marchini, M.; Marti, A.; Folli, C.; Prandi, B.; Ganino, T.; Conte, P.; Fadda, C.; Mattarozzi, M.; Carini, E. Sprouting of Sorghum (Sorghum bicolor [L.] Moench): Effect of Drying Treatment on Protein and Starch Features. Foods 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Gujral, H.S. Modifying the dough mixing behavior, protein & starch digestibility and antinutritional profile of minor millets by sprouting. Int. J. Biol. Macromol. 2020, 153, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Qi, L.; Xu, C.; Shen, Y.; Wang, H.; Zhang, H. Understanding how the cooking methods affected structures and digestibility of native and heat-moisture treated rice starches. J. Cereal Sci. 2020, 95, 103085. [Google Scholar] [CrossRef]
- Li, F.; Guan, X.; Li, C. Effects of degree of milling on the starch digestibility of cooked rice during (in vitro) small intestine digestion. Int. J. Biol. Macromol. 2021, 188, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pulido, I.J.; Rico, D.; Martinez-Villaluenga, C.; Pérez-Jiménez, J.; Luis, D.D.; Martín-Diana, A.B. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods 2022, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.F.; Kise, M.; Wang, M.F.; Ito, Y.; Yang, M.D.; Aoto, H.; Yoshihara, R.; Yokoyama, J.; Kunii, D.; Yamamoto, S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol. 2008, 54, 163–168. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat proteins and peptides: Biological functions and food applications. Trends Food Sci. Technol. 2021, 110, 155–167. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Mazza, G.; Oomah, B.D. Buckwheat; Elsevier: Amsterdam, The Netherlands, 2003; Volume 2, pp. 692–699. [Google Scholar]
- Guo, X.; Xiong, Y.L. Characteristics and functional properties of buckwheat protein–sugar Schiff base complexes. LWT—Food Sci. Technol. 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Loffi, C.; Montalbano, S.; Chiarello, E.; Dellafiora, L.; Picone, G.; Antonelli, G.; Tedeschi, T.; Buschini, A.; Capozzi, F.; et al. Cleaning the Label of Cured Meat; Effect of the Replacement of Nitrates/Nitrites on Nutrients Bioaccessibility, Peptides Formation, and Cellular Toxicity of In Vitro Digested Salami. Int. J. Mol. Sci. 2022, 23, 12555. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.; Castro-Rosas, J.; Gomez-Aldapa, C.; Mora-Escobedo, R.; Rojas-León, A.; Rodriguez, M.; Cortés, R.; Alma Delia, R.-G. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2018, 35, 177–200. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kokalj, D.; Vidrih, R.; Meglič, V. Milling fractions fatty acid composition of common (Fagopyrum esculentum Moench) and tartary (Fagopyrum tataricum (L.) Gaertn) buckwheat. J. Stored Prod. Res. 2020, 85, 101551. [Google Scholar] [CrossRef]
- Faraoni, P.; Sereni, E.; Gnerucci, A.; Cialdai, F.; Monici, M.; Ranaldi, F. Glyoxylate cycle activity in Pinus pinea seeds during germination in altered gravity conditions. Plant Physiol. Biochem. 2019, 139, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2017, 7, 1. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of Reactive Oxygen Species and Mitochondria in Seed Germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Chitarrini, G.; Nobili, C.; Pinzari, F.; Antonini, A.; De Rossi, P.; Del Fiore, A.; Procacci, S.; Tolaini, V.; Scala, V.; Scarpari, M.; et al. Buckwheat achenes antioxidant profile modulates Aspergillus flavus growth and aflatoxin production. Int. J. Food Microbiol. 2014, 189, 1–10. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Soliven, A. Lipophilic bioactive compounds in the oils recovered from cereal by-products. J. Sci. Food Agric. 2016, 96, 3256–3265. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and Esterified Tocopherols, Tocotrienols and Other Extractable and Non-Extractable Tocochromanol-Related Molecules: Compendium of Knowledge, Future Perspectives and Recommendations for Chromatographic Techniques, Tools, and Approaches Used for Tocochromanol Determination. Molecules 2022, 27, 6560. [Google Scholar]
- Žilić, S.; Basić, Z.; Šukalović, V.; Maksimović, V.; Simic, M.; Filipović, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, T.; Zhou, Y.; Tang, W.; Xiao, Y.; Meng, X.; Fang, X. Relationships between antioxidant compounds and antioxidant activities of tartary buckwheat during germination. J. Food Sci. Technol. 2015, 52, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, S.; Yao, L.; Wang, L.; Li, C. Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis. Antioxidants 2019, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Ling, A.; Li, X.; Hu, X.; Ma, Z.; Wu, K.; Zhang, H.; Hao, M.; Wei, S. Dynamic changes in polyphenol compounds, antioxidant activity, and PAL gene expression in different tissues of buckwheat during germination. J. Sci. Food Agric. 2018, 98, 5723–5730. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-L.; Wang, Y.-H.; Wang, T.-Y.; Zhu, Y.; Wu, P.; Li, L.-J. Comparative metabolomic profiling of secondary metabolites in different tissues of Euryale ferox and functional characterization of phenylalanine ammonia-lyase. Ind. Crops Prod. 2023, 195, 116450. [Google Scholar] [CrossRef]
- Ren, S.-C.; Sun, J.-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods 2014, 7, 298–304. [Google Scholar] [CrossRef]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; Lopez de Dicastillo, C. Evaluation of Polyphenol Content and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef]
- Johnsen, P.R.; Pinna, C.; Mattio, L.; Strube, M.B.; Di Nunzio, M.; Iametti, S.; Dallavalle, S.; Pinto, A.; Frøkiær, H. Investigation of the Effects of Monomeric and Dimeric Stilbenoids on Bacteria-Induced Cytokines and LPS-Induced ROS Formation in Bone Marrow-Derived Dendritic Cells. Int. J. Mol. Sci. 2023, 24, 2731. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Merendino, N.; Molinari, R.; Costantini, L.; Mazzucato, A.; Pucci, A.; Bonafaccia, F.; Esti, M.; Ceccantoni, B.; Papeschi, C.; Bonafaccia, G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014, 5, 1017–1026. [Google Scholar] [CrossRef]
- Meschini, R.; Filippi, S.; Molinari, R.; Costantini, L.; Bonafaccia, G.; Merendino, N. Pasta containing tartary buckwheat sprouts prevents DNA damage in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2015, 66, 574–578. [Google Scholar] [CrossRef]
- Kil, Y.S.; Han, A.R.; Hong, M.J.; Kim, J.B.; Park, P.H.; Choi, H.; Nam, J.W. 1H NMR-Based Chemometrics to Gain Insights Into the Bran of Radiation-Induced Colored Wheat Mutant. Front. Nutr. 2021, 8, 806744. [Google Scholar] [CrossRef]
- Ciampa, A.; Danesi, F.; Picone, G. NMR-Based Metabolomics for a More Holistic and Sustainable Research in Food Quality Assessment: A Narrative Review. Appl. Sci. 2023, 13, 372. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.e.; Li, Z.; Liu, Q.; Zhao, X.; Yang, H. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019, 286, 87–97. [Google Scholar] [CrossRef]
- Farag, M.A.; Sharaf El-Din, M.G.; Selim, M.A.; Owis, A.I.; Abouzid, S.F.; Porzel, A.; Wessjohann, L.A.; Otify, A. Nuclear Magnetic Resonance Metabolomics Approach for the Analysis of Major Legume Sprouts Coupled to Chemometrics. Molecules 2021, 26, 761. [Google Scholar] [CrossRef]
- Nam, K.H. Glucose Isomerase: Functions, Structures, and Applications. Appl. Sci. 2022, 12, 428. [Google Scholar] [CrossRef]
- Chiarello, E.; Di Nunzio, M.; Picone, G.; Antonelli, G.; Capozzi, F.; Bordoni, A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients 2022, 14, 517. [Google Scholar] [CrossRef] [PubMed]
- Selegato, D.M.; Pilon, A.C.; Carnevale Neto, F. Plant Metabolomics Using NMR Spectroscopy. In NMR-Based Metabolomics: Methods and Protocols; Gowda, G.A.N., Raftery, D., Eds.; Springer: New York, NY, USA, 2019; pp. 345–362. [Google Scholar]
- Bouajila, A.; Ammar, H.; Chahine, M.; Khouja, M.; Hamdi, Z.; Khechini, J.; Salem, A.-F.Z.M.; Ghorbel, A.; López, S. Changes in phytase activity, phosphorus and phytate contents during grain germination of barley (Hordeum vulgare L.) cultivars. Agrofor. Syst. 2020, 94, 1151–1159. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can sprouting reduce phytate and improve the nutritional composition and nutrient bioaccessibility in cereals and legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Paukkonen, I.; Gómez-Gallego, C.; Kolehmainen, M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Modgil, R.; Sood, P. Effect of Roasting and Germination on Carbohydrates and Anti-nutritional Constituents of Indigenous and Exotic Cultivars of Pseudo-cereal (Chenopodium). J. Life Sci. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adedeji, O.E.; Etim, I.I.; Aniaka, G.I.; Mathew, E.O.; Ekeh, U.B.; Anumba, N.L. Physicochemical, nutritional, and sensory properties of chips produced from germinated African yam bean (Sphenostylis stenocarpa). LWT 2021, 136, 110330. [Google Scholar] [CrossRef]
- Azeez, S.; Chinma, C.; Bassey, S.; Eze, R.; Makinde, A.; Sakariyah, A.; Okubanjo, S.; Danbaba, N.; Adebo, O. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT 2021, 154, 112734. [Google Scholar] [CrossRef]
- Singh, A.; Bobade, H.; Sharma, S.; Singh, B.; Gupta, A. Enhancement of Digestibility of Nutrients (In vitro), Antioxidant Potential and Functional Attributes of Wheat Flour through Grain Germination. Plant Foods Hum. Nutr. 2021, 76, 118–124. [Google Scholar] [CrossRef] [PubMed]
| FAME | BW-0 | BW-48 | BW-72 |
|---|---|---|---|
| C14:0 | 0.07 ± 0.01a | 0.05 ± 0.00a | 0.03 ± 0.04a |
| C16:0 | 3.96 ± 0.02a | 3.30 ± 0.18b | 2.81 ± 0.34c |
| C16:1 n-7 | 0.07 ± 0.10a | 0.05 ± 0.00a | 0.04 ± 0.06a |
| C17:0 | 0.03 ± 0.04a | 0.04 ± 0.00a | 0.02 ± 0.03a |
| C18:0 | 0.21 ± 0.07a | 0.08 ± 0.03b | 0.04 ± 0.05c |
| C18:1 n-9 | 8.09 ± 0.39a | 6.59 ± 0.36a | 4.72 ± 0.97b |
| C18:2 n-6 | 8.79 ± 0.60a | 9.14 ± 0.59a | 7.63 ± 1.25a |
| C18:3 n-3 | 0.52 ± 0.02b | 0.68 ± 0.04a | 0.65 ± 0.04a |
| C20:0 | 0.32 ± 0.06a | 0.28 ± 0.01a | 0.22 ± 0.09a |
| C20:1 n-9 | 0.68 ± 0.09a | 0.59 ± 0.01a | 0.39 ± 0.07b |
| C22:0 | 0.46 ± 0.19a | 0.31 ± 0.01a | 0.27 ± 0.11a |
| ΣSFA | 5.04 ± 0.27a | 4.06 ± 0.17b | 3.39 ± 0.42b |
| ΣMUFA | 8.84 ± 0.38a | 7.23 ± 0.37a | 5.15 ± 0.98b |
| ΣPUFA | 9.31 ± 0.62a | 9.82 ± 0.63a | 8.28 ± 1.3a |
| Σn-6/Σn-3 | 16.74 ± 0.49a | 13.46 ± 0.12b | 11.65 ± 1.15b |
| Total | 23.20 ± 1.27a | 21.11 ± 1.17ab | 16.81 ± 2.70b |
| UI | 120.65 ± 0.50c | 130.47 ± 0.65b | 132.97 ± 0.16a |
| PI | 43.35 ± 0.44c | 50.57 ± 0.39b | 53.92 ± 0.55a |
| CD | 100.00 ± 2.65c | 174.03 ± 20.29b | 252.00 ± 20.40a |
| Tocols 1 | BW-0 | BW-48 | BW-72 |
|---|---|---|---|
| α-tocopherol | 2.11 ± 0.10a | 2.70 ± 0.32a | 2.48 ± 0.12a |
| γ-tocopherol | 56.07 ± 0.16a | 55.31 ± 5.03a | 44.55 ± 1.61a |
| δ-tocopherol | 3.79 ± 0.03a | 3.52 ± 0.05b | 2.95 ± 0.03c |
| Total tocols | 61.97 ± 0.26a | 61.53 ± 5.2a | 49.98 ± 2.10a |
| Compounds | [M − H]− | MS Fragments | Q. T. | Free Phenolic Compounds | Anova | Bound Phenolic Compounds | Anova | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW-0 | BW-48 | BW-72 | BW-0 | BW-48 | BW-72 | ||||||
| Phenolic acids | |||||||||||
| Protocatechuic-4-O-glucoside acid | 315 | 153 | 315→153 | 30.25 ± 1.01c | 59.31 ± 0.73b | 82.90 ± 4.24a | p < 0.05 | 11.92 ± 0.09a | 3.39 ± 0.61b | 2.93 ± 0.10b | p < 0.05 |
| Caffeic acid hexose | 341 | 251 | 341→251 | 1.88 ± 0.22a | 2.15 ± 0.03a | 2.09 ± 0.25a | n.s. | n.d. | n.d. | n.d. | |
| Caffeic acid hexose | 341 | 251 | 341→251 | n.d.c | 5.82 ± 0.85b | 13.30 ± 0.56a | p < 0.05 | n.d. | n.d. | n.d. | |
| p-Coumaric acid | 163 | 119 | 163→119 | 3.25 ± 0.60b | 8.76 ± 0.52a | 9.08 ± 0.92a | p < 0.05 | n.d.b | 0.25 ± 0.04b | 3.28 ± 0.59a | p < 0.05 |
| Swertiamacroside | 487 | 451, 179 | 487→179 | 0.93 ± 0.15b | 3.95 ± 0.67a | 4.44 ± 0.62a | p < 0.05 | 22.99 ± 1.64a | 10.37 ± 0.67b | 15.48 ± 1.22b | p < 0.05 |
| Ferulic acid | 193 | 178 | 193→178 | n.d.c | 2.49 ± 0.15b | 3.84 ± 0.49a | p < 0.05 | n.d.b | 0.32 ± 0.03b | 3.67 ± 0.37a | p < 0.05 |
| Total phenolic acids | 36.31 ± 1.53c | 82.48 ± 1.43b | 115.65 ± 2.82a | p < 0.05 | 34.90 ± 1.55a | 14.34 ± 1.27c | 25.36 ± 1.54b | p < 0.05 | |||
| Flavan-3-ols | |||||||||||
| Catechin-glucoside | 451 | 289 | 451→289 | 118.76 ± 2.96c | 202.06 ± 28.76b | 356.54 ± 0.70a | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin | 289 | 203 | 289→203 | 0.87 ± 0.05c | 36.42 ± 2.88b | 60.88 ± 5.11a | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer A | 561 | 543, 435, 425, 407, 289, 271 | 561→289 | 38.70 ± 1.04a | 2.12 ± 0.06c | 6.78 ± 0.23b | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin-glucoside | 451 | 289 | 451→289 | 95.00 ± 0.88b | 117.91 ± 2.69a | 35.68 ± 3.91c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin | 289 | 244 | 289→244 | 59.42 ± 1.13a | 20.65 ± 0.21b | 16.25 ± 1.36c | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin-glucoside | 451 | 289 | 451→289 | 17.66 ± 2.96b | 38.20 ± 6.74a | 29.02 ± 2.10ab | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer B | 561 | 543, 435, 425, 407, 289, 271 | 561→289 | 124.29 ± 0.46a | 55.97 ± 1.6b | 21.66 ± 1.41c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epiafzelchin-epicatechin | 833 | 561, 543, 289, 271 | 833→561 | 143.23 ± 9.48a | 140.22 ± 0.02a | 125.91 ± 2.01a | n.s | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer C | 561 | 543, 435, 425, 407, 289, 271 | 561→289 | 45.04 ± 8.17a | 10.89 ± 2.09b | 13.65 ± 0.63b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin-gallate | 441 | 289, 169 | 441→169 | 9.62 ± 1.12b | 20.15 ± 1.38a | 10.18 ± 0.04b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epicatechin-O-methyl gallate | 727 | 561, 455, 289, 271 | 727→289 | 125.94 ± 3.21a | 128.91 ± 1.63a | 119.97 ± 5.65a | n.s. | n.d. | n.d. | n.d. | |
| (-)-Epicatechin-3-(3″-O-methyl) gallate | 455 | 289, 183 | 4555→183 | 149.20 ± 1.76a | 48.49 ± 7.36b | 43.41 ± 0.79b | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer D | 561 | 543, 435, 425, 407, 289, 271 | 561→289 | 32.32 ± 2.90a | 8.32 ± 0.23b | 0.67 ± 0.03c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epicatechin-O-dimethyl gallate | 741 | 469, 319, 271 | 741→469 | 78.74 ± 4.91a | 32.42 ± 0.97b | 34.20 ± 1.14b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin-O-3,4-dimethyl gallate | 469 | 319, 271, 125 | 469→271 | 126.39 ± 4.50a | 25.58 ± 0.79b | 19.49 ± 1.08b | p < 0.05 | n.d. | n.d. | n.d. | |
| Total flavan-3-ol | 1165.17 ± 14.29a | 888.32 ± 37.45b | 894.29 ± 13.16b | p < 0.05 | n.d. | n.d. | n.d. | ||||
| Flavonols | |||||||||||
| Quercitrin | 447 | 301, 179, 151 | 447→301 | 4.64 ± 0.50b | 3.20 ± 0.13b | 8.61 ± 1.41a | p < 0.05 | n.d. | n.d. | n.d. | |
| Rutin | 609 | 301 | 609→301 | 9.91 ± 0.84c | 15.59 ± 0.14b | 23.01 ± 1.15a | p < 0.05 | n.d. | n.d. | n.d. | |
| Quercetin | 301 | 178, 151 | 30→151 | 3.39 ± 0.15b | 21.37 ± 1.02a | 28.12 ± 2.93a | p < 0.05 | 4.29 ± 0.01a | n.d.b | n.d.b | p < 0.05 |
| Total flavonols | 17.93 ± 1.19c | 40.17 ± 1.29b | 59.74 ± 2.66a | p < 0.05 | 4.29 ± 0.01a | n.d.b | n.d.b | p < 0.05 | |||
| Flavones | |||||||||||
| Orientin | 447 | 357, 327 | 447→357 | 2.42 ± 0.18c | 16.27 ± 0.54b | 24.20 ± 1.52a | p < 0.05 | n.d. | n.d. | n.d. | |
| Isorientin | 447 | 357, 327 | 447→357 | 9.56 ± 1.73c | 403.42 ± 22.51b | 1419.45 ± 144.04a | p < 0.05 | n.d. | n.d. | n.d. | |
| Vitexin | 431 | 311 | 431→311 | 75.78 ± 4.48c | 1966.23 ± 246.04b | 5263.67 ± 352.42a | p < 0.05 | 15.60 ± 1.58c | 303.08 ± 8.66b | 1050.46 ± 63.59a | p < 0.05 |
| Total flavones | 87.76 ± 6.03a | 2385.92 ± 269.09b | 6707.32 ± 206.86a | p < 0.05 | 15.60 ± 1.58c | 303.08 ± 8.66b | 1050.46 ± 63.59a | p < 0.05 | |||
| Proanthocyanidins | |||||||||||
| Procyanidin B2-3-O-gallate | 729 | 577, 289 | 729→577 | 65.68 ± 4.47b | 67.15 ± 0.24b | 139.11 ± 4.20a | p < 0.05 | n.d. | n.d. | n.d. | |
| Procyanidin B2 | 577 | 425, 407, 289 | 577→425 | 48.03 ± 0.39c | 176.24 ± 5.77b | 491.72 ± 20.12a | p < 0.05 | n.d. | n.d. | n.d. | |
| Total proanthocyanidins | 113.71 ± 4.86c | 243.39 ± 6.01b | 630.83 ± 24.31a | p < 0.05 | n.d. | n.d. | n.d. | ||||
| Total phenols compounds | 1420.89 ± 3.06c | 3640.28 ± 234.93b | 8407.83 ± 163.85a | p < 0.05 | 54.79 ± 3.12c | 317.42 ± 9.93b | 1075.82 ± 62.05a | p < 0.05 | |||
| BW-0 | BW-48 | BW-72 | |
|---|---|---|---|
| TAC | 26.21 ± 4.23b | 31.09 ± 2.81b | 46.99 ± 3.21a |
| FRAP | 9.91 ± 0.45c | 13.63 ± 0.20b | 17.98 ± 0.40a |
| Metabolites | ppm (δ) | BW-0 | BW-48 | BW-72 |
|---|---|---|---|---|
| Tryptophan | 7.735 (d), 7.754 (d), 7.265 (t), 7.201 (t) | 90 ± 1.25b | 106 ± 12.7b | 130 ± 12.7a |
| Phenylalanine | 7.431 (m), 7.379 (m), 7.344 (d) | 719 ± 53a | 772 ± 50a | 737 ± 26a |
| Tyrosine | 7.194 (d) | 269 ± 51.4a | 280 ± 10a | 272 ± 20a |
| Glutamine | 2.475 (m), 2.155 (m) | 1305 ± 32b | 1870 ± 211b | 3876 ± 159a |
| Alanine | 1.480 (d) | 570 ± 14b | 770 ± 12a | 719 ± 50a |
| Valine | 1.042 (d), 0.978 (d) | 213 ± 21b | 384 ± 38a | 344 ± 68a |
| Isoleucine | 1.010 (d), 0.950 (t) | 183 ± 11b | 284 ± 36a | 237 ± 63a |
| Sucrose | 5.412 (d) | 872 ± 202a | 1451 ± 796a | 1536 ± 771a |
| Glucose | 5.237 (d) | 300 ± 20.2b | 2476 ± 202a | 2320 ± 202a |
| Fructose | 4.107 (d) | 753 ± 397b | 1044 ± 329ab | 1581 ± 605a |
| Acetate | 1.949 (s) | 291 ± 32b | 310 ± 32ab | 330 ± 27a |
| Lactate | 1.331 (d) | 329 ± 5b | 519 ± 55a | 497 ± 79a |
| GABA | 3.023 (t), 2.313 (t), 1.905 (m) | 987 ± 17a | 985 ± 29a | 955 ± 30.6a |
| NADP+ | 9.341 (s), 9.103 (d), 8.841 (d), 8.452 (s) | 24.5 ± 2.06a | 20.8 ± 2.65a | 20.4 ± 3.26a |
| Trigonelline | 9.090 (s), 8.840 (m) | 68 ± 4a | 52 ± 11ab | 47 ± 13b |
| BW-0 | BW-48 | BW-72 | |
|---|---|---|---|
| Phytic acid | 13.34 ± 0.27a | 12.09 ± 0.38ab | 11.38 ± 0.34b |
| w/o BW Extract | BW-0 | BW-48 | BW-72 | |
|---|---|---|---|---|
| Pepsin activity | 89.44 ± 4.81b | 92.41 ± 5.16b | 107.41 ± 20.50ab | 152.22 ± 27.76a |
| Trypsin activity | 180.37 ± 49.83a | 6.39 ± 2.03c | 65.56 ± 14.35bc | 82.96 ± 8.26b |
| Chymotrypsin activity | 540.28 ± 71.10a | 360.54 ± 62.22a | 372.78 ± 63.79a | 359.81 ± 46.65a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgonovi, S.M.; Chiarello, E.; Pasini, F.; Picone, G.; Marzocchi, S.; Capozzi, F.; Bordoni, A.; Barbiroli, A.; Marti, A.; Iametti, S.; et al. Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum). Foods 2023, 12, 2047. https://doi.org/10.3390/foods12102047
Borgonovi SM, Chiarello E, Pasini F, Picone G, Marzocchi S, Capozzi F, Bordoni A, Barbiroli A, Marti A, Iametti S, et al. Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum). Foods. 2023; 12(10):2047. https://doi.org/10.3390/foods12102047
Chicago/Turabian StyleBorgonovi, Sara Margherita, Elena Chiarello, Federica Pasini, Gianfranco Picone, Silvia Marzocchi, Francesco Capozzi, Alessandra Bordoni, Alberto Barbiroli, Alessandra Marti, Stefania Iametti, and et al. 2023. "Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum)" Foods 12, no. 10: 2047. https://doi.org/10.3390/foods12102047
APA StyleBorgonovi, S. M., Chiarello, E., Pasini, F., Picone, G., Marzocchi, S., Capozzi, F., Bordoni, A., Barbiroli, A., Marti, A., Iametti, S., & Di Nunzio, M. (2023). Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum). Foods, 12(10), 2047. https://doi.org/10.3390/foods12102047












