Effect of Soybean Protein Concentrate Preparation on Copy Numbers and Structural Characteristics of DNA from Genetically Modified Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
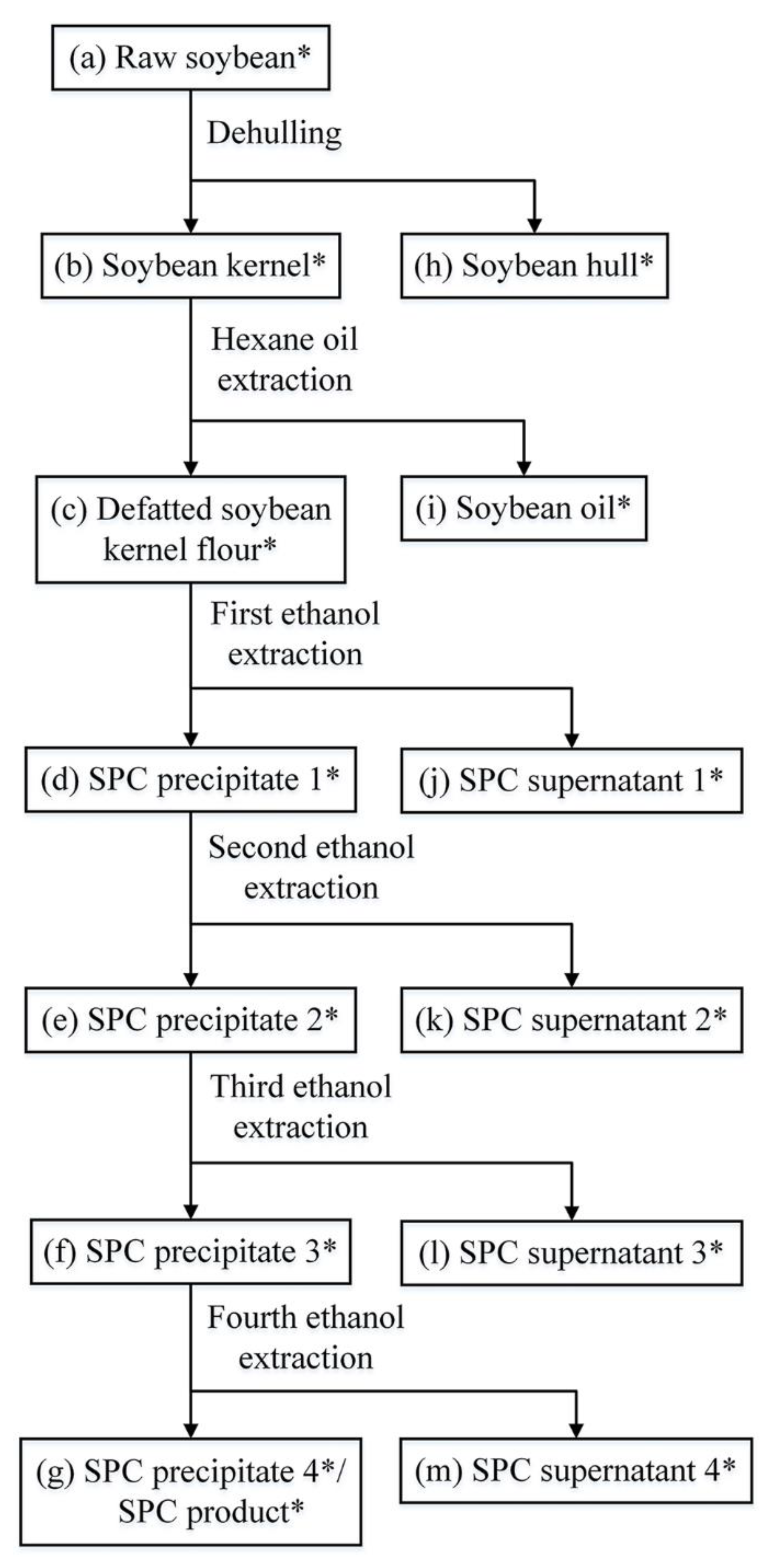
2.2. SPC Preparation
2.3. Genomic DNA Isolation
2.4. The Absolute Quantification of DNA by Droplet Digital PCR (ddPCR)
2.5. Atomic Force Microscopy (AFM) Tests
2.6. Circular Dichroism (CD) Tests
2.7. Fluorescence Spectrometry Tests
2.8. Statistical Analysis
3. Results and Discussion
3.1. Variations in Copy Numbers of the Lectin and cp4 Epsps Targets during SPC Preparation
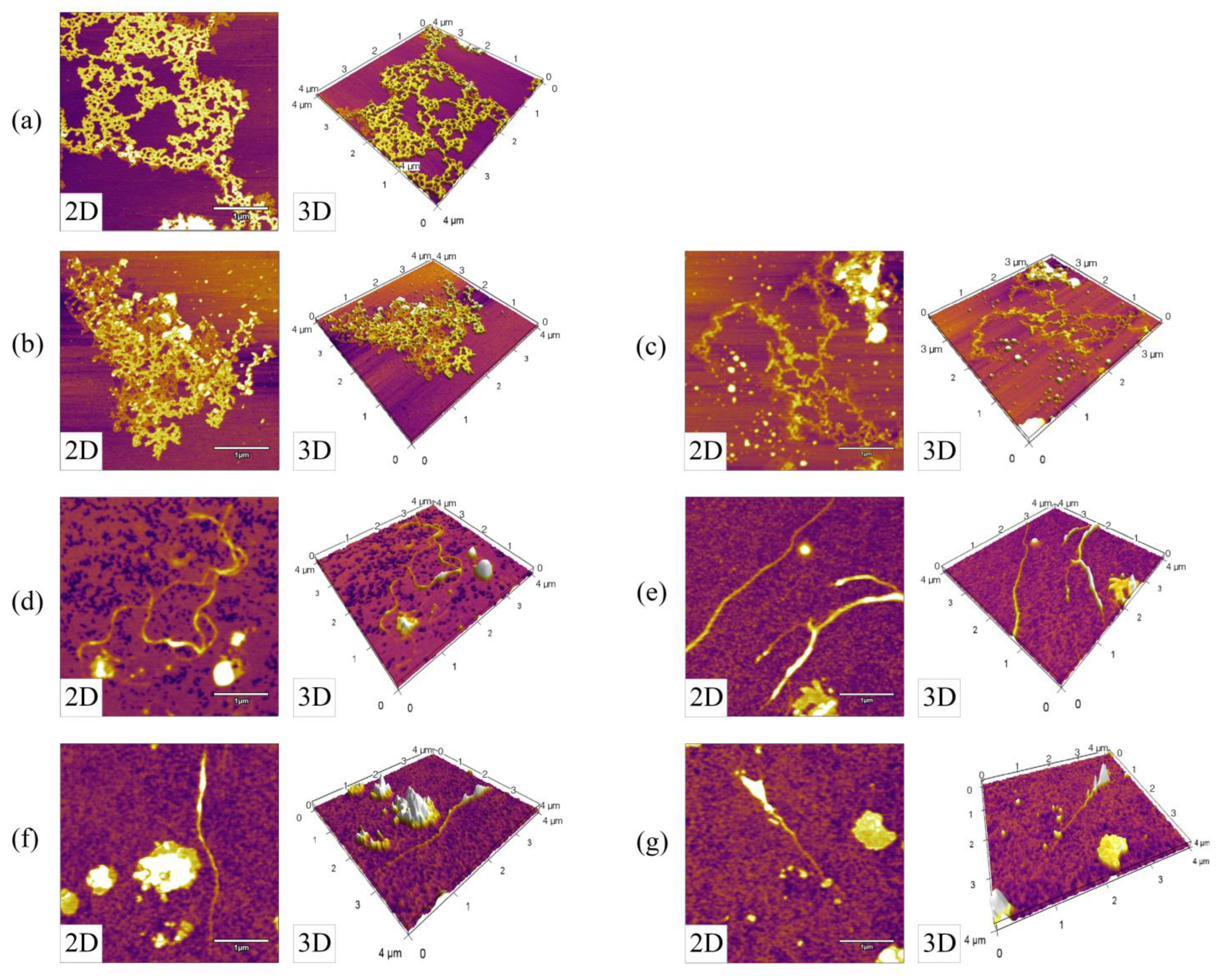
3.2. Variations in the Nanotopography of DNA during SPC Preparation
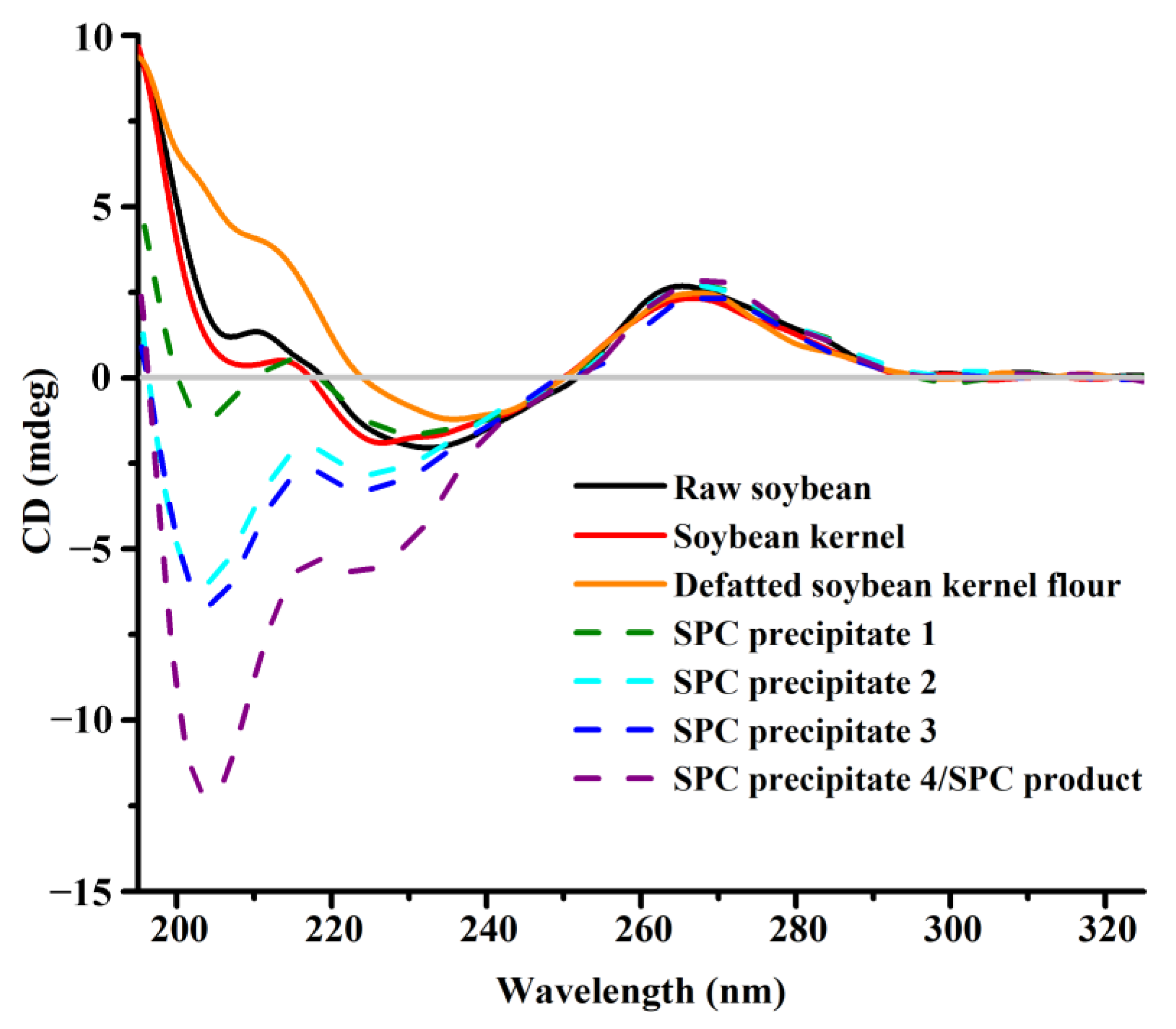
3.3. Variations in the Conformation of DNA during SPC Preparation
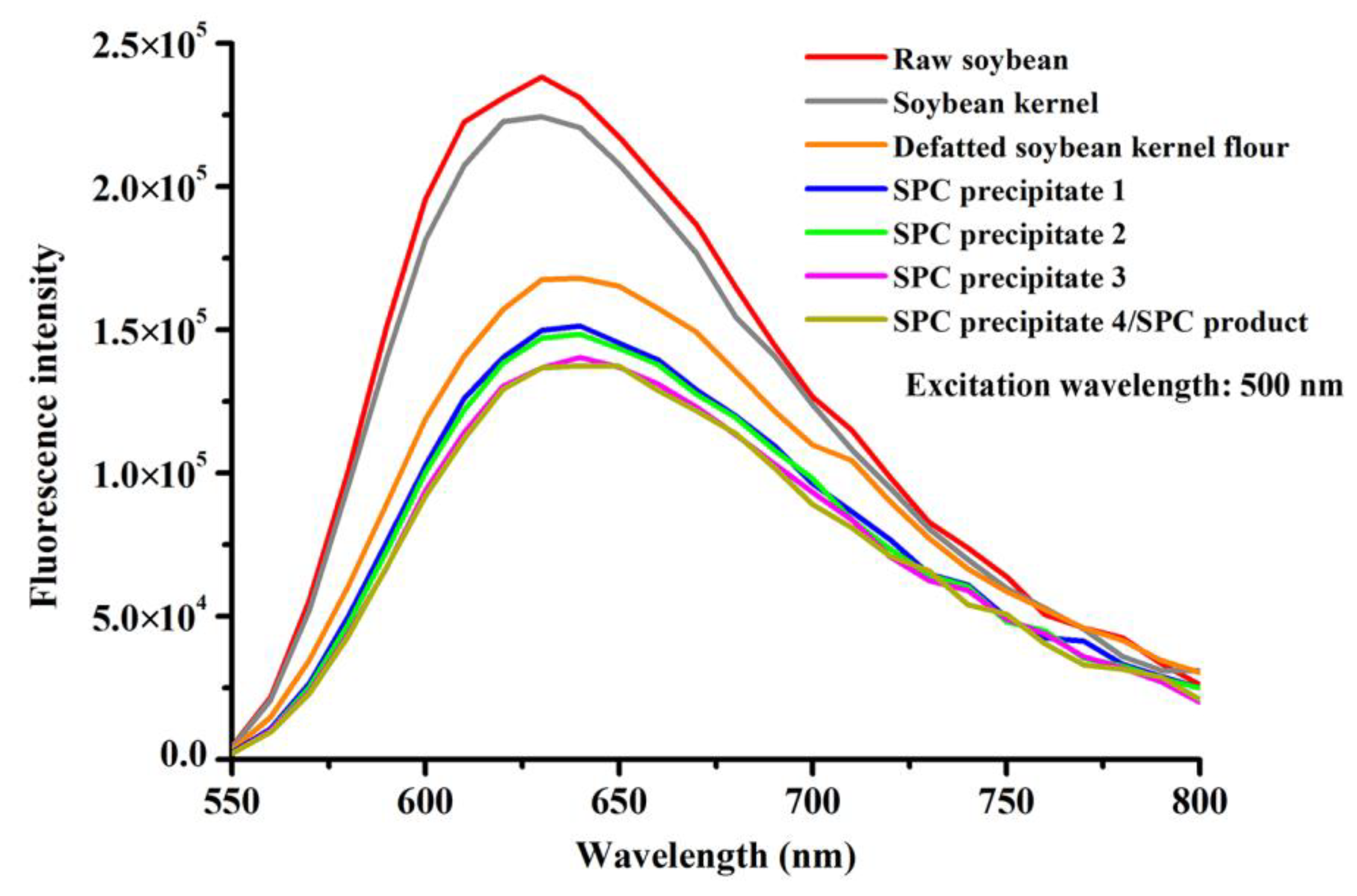
3.4. Variations in the Fluorescence Emission Spectra of DNA during SPC Preparation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change; ISAAA Brief No. 54; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Kyrova, V.; Ostry, V.; Surmanova, P.; Ruprich, J. Monitoring of genetically modified food on the Czech food market and a cross-country comparison. Acta Aliment. 2018, 47, 10–16. [Google Scholar] [CrossRef]
- Torretta, V.; Katsoyiannis, I.A.; Viotti, P.; Rada, E.C. Critical Review of the Effects of Glyphosate Exposure to the Environment and Humans through the Food Supply Chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Mesnage, R.; Tsatsakis, A.M.; Golokhvast, K.S.; Yang, S.H.; Antoniou, M.N.; Chung, G. Addressing concerns over the fate of DNA derived from genetically modified food in the human body: A review. Food Chem. Toxicol. 2018, 124, 423–430. [Google Scholar] [CrossRef]
- Kharazmi, M.; Bauer, T.; Hammes, W.P.; Hertel, C. Effect of Food Processing on the Fate of DNA with Regard to Degradation and Transformation Capability in Bacillus subtilis. Syst. Appl. Microbiol. 2003, 26, 495–501. [Google Scholar] [CrossRef]
- de Vries, J.; Meier, P.; Wackernagel, W. The natural transformation of the soil bacteria Pseudomonas stutzeri and Acinetobacter sp. by transgenic plant DNA strictly depends on homologous sequences in the recipient cells. FEMS Microbiol. Lett. 2001, 195, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Weller, P.; Hammes, W.P.; Hertel, C. The effect of processing parameters on DNA degradation in food. Eur. Food Res. Technol. 2003, 217, 338–343. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, Y.; Wang, Y. Effect of critical processing procedures on transgenic components in quality and quantity level during soymilk processing of Roundup Ready Soybean. Eur. Food Res. Technol. 2007, 225, 119–126. [Google Scholar] [CrossRef]
- Ballari, R.V.; Martin, A. Assessment of DNA degradation induced by thermal and UV radiation processing: Implications for quantification of genetically modified organisms. Food Chem. 2013, 141, 2130–2136. [Google Scholar] [CrossRef]
- Du, Y.; Chen, F.; Chen, C.; Liu, K. Monitoring and traceability of genetically modified soya bean event GTS 40-3-2 during soya bean protein concentrate and isolate preparation. R. Soc. Open Sci. 2020, 7, 201147. [Google Scholar] [CrossRef]
- Fernandes, T.J.; Costa, J.; Plácido, A.; Villa, C.; Grazina, L.; Meira, L.; Oliveira, M.B.P.; Mafra, I. Genetically Modified Organism Analysis as Affected by DNA Degradation. In Genetically Modified Organisms in Food-Production, Safety, Regulation, and Public Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 111–118. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, W.; Selvaraj, J.N.; Liu, Y. DNA degradation in genetically modified rice with Cry1Ab by food processing methods: Implications for the quantification of genetically modified organisms. Food Chem. 2015, 174, 132–138. [Google Scholar] [CrossRef]
- Tian, F.; Guan, Q.; Wang, X.; Teng, D.; Wang, J. Influence of Different Processing Treatments on the Detectability of Nucleic Acid and Protein Targets in Transgenic Soybean Meal. Appl. Biochem. Biotechnol. 2014, 172, 3686–3700. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, F.; Bu, G.; Zhang, L. Distribution and degradation of DNA from non-genetically and genetically modified soybean (Roundup Ready): Impact of soybean protein concentrate and soybean protein isolate preparation. Food Chem. 2021, 335, 127582. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Li, X.; Liang, J.; Li, Y.; Zeng, X.; Wu, G. Copy number and zygosity determination of transgenic rapeseed by droplet digital PCR. Oil Crop Sci. 2017, 2, 84–94. [Google Scholar] [CrossRef]
- Demeke, T.; Dobnik, D. Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Anal. Bioanal. Chem. 2018, 410, 4039–4050. [Google Scholar] [CrossRef]
- Scollo, F.; Egea, L.A.; Gentile, A.; La Malfa, S.; Dorado, G.; Hernandez, P. Absolute quantification of olive oil DNA by droplet digital-PCR (ddPCR): Comparison of isolation and amplification methodologies. Food Chem. 2016, 213, 388–394. [Google Scholar] [CrossRef]
- Chammas, O.; Bonass, W.A.; Thomson, N.H. Effect of heparin and heparan sulphate on open promoter complex formation for a simple tandem gene model using ex situ atomic force microscopy. Methods 2017, 120, 91–102. [Google Scholar] [CrossRef]
- Yang, T.; Gao, H. Atomic force microscopy study of EDTA induced desorption of metal ions immobilized DNA from mica surface. Ultramicroscopy 2019, 199, 7–15. [Google Scholar] [CrossRef]
- Potaman, V.; Bannikov, Y.; Shlyachtenko, L. Sedimentation of DNA in ethanol-water solutions within the interval of B→A transition. Nucleic Acids Res. 1980, 8, 635–642. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Kwak, M.-S.; Sung, M.-H. Protection of Radiation-Induced DNA Damage by Functional Cosmeceutical Poly-Gamma-Glutamate. J. Microbiol. Biotechnol. 2018, 28, 527–533. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhao, Q.; Ouyang, J.; Wu, Y. Rapid detection of authenticity and adulteration of walnut oil by FTIR and fluorescence spectroscopy: A comparative study. Food Chem. 2015, 181, 25–30. [Google Scholar] [CrossRef]
- Košir, A.B.; Demšar, T.; Štebih, D.; Žel, J.; Milavec, M. Digital PCR as an effective tool for GMO quantification in complex matrices. Food Chem. 2019, 294, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Košir, A.B.; Spilsberg, B.; Holst-Jensen, A.; Žel, J.; Dobnik, D. Development and inter-laboratory assessment of droplet digital PCR assays for multiplex quantification of 15 genetically modified soybean lines. Sci. Rep. 2017, 7, 8601. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Song, J.-Y.; Hong, Y.; Kim, H.-Y. Monitoring of genetically modified soybean events in sausage products in South Korea. Food Control 2016, 67, 63–67. [Google Scholar] [CrossRef]
- Nikolić, Z.; Petrović, G.; Panković, D.; Ignjatov, M.; Marinković, D.; Stojanović, M.; Đorđević, V. Threshold Level and Traceability of Roundup Ready Soybean in Tofu Production. Food Technol. Biotechnol. 2017, 55, 439–444. [Google Scholar] [CrossRef]
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Bioresour. Technol. 2020, 303, 122899. [Google Scholar] [CrossRef]
- Bui, V.-C.; Nguyen, T.-H. DNA aggregation induced by Mg2+ ions under different conditions. J. Mol. Recognit. 2018, 31, e2721. [Google Scholar] [CrossRef]
- Wang, Y.; Ran, S.; Man, B.; Yang, G. Ethanol induces condensation of single DNA molecules. Soft Matter 2011, 7, 4425–4434. [Google Scholar] [CrossRef]
- Castellanos, A.P. Simulation and experimental verification of DNA damage due to X-rays interaction. arXiv 2018, arXiv:1810.05815. Available online: https://ui.adsabs.harvard.edu/abs/2018arXiv181005815P (accessed on 13 October 2018).
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, W.; Tang, J.; Wang, E.; Dong, S. Conformational transition of DNA induced by cationic lipid vesicle in acidic solution: Spectroscopy investigation. Biophys. Chem. 2002, 97, 7–16. [Google Scholar] [CrossRef]
- Bokma, J.T.; Curtis, W.J., Jr.; Blok, J. CD of the li-salt of DNA in ethanol/water mixtures: Evidence for the B- to C-form transition in solution. Biopolymers 1987, 26, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fujimoto, S. Molecular dynamics simulation of the A-DNA to B-DNA transition in aqueous RbCl solution. Sci. China Chem. 2013, 56, 524–532. [Google Scholar] [CrossRef]
- Rudd, L.; Lee, D.J.; Kornyshev, A.A. The role of electrostatics in the B to A transition of DNA: From solution to assembly. J. Physics: Condens. Matter 2007, 19, 416103. [Google Scholar] [CrossRef] [PubMed]
- Malenkov, G.; Minchenkova, L.; Minyat, E.; Schyolkina, A.; Ivanov, V. The nature of the B-A transition of DNA in solution. FEBS Lett. 1975, 51, 38–42. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Zheng, X.; Guo, H. Modern Molecular Biology, 4th ed.; Higher Education Press: Beijing, China, 2013; Chapter 2. [Google Scholar]
- Cheatham, T.E.; Crowley, M.F.; Fox, T.; Kollman, P.A. A molecular level picture of the stabilization of A-DNA in mixed ethanol–water solutions. Proc. Natl. Acad. Sci. USA 1997, 94, 9626–9630. [Google Scholar] [CrossRef]
- Oosting, T.; Hilario, E.; Wellenreuther, M.; Ritchie, P.A. DNA degradation in fish: Practical solutions and guidelines to improve DNA preservation for genomic research. Ecol. Evol. 2020, 10, 8643–8651. [Google Scholar] [CrossRef]
- Banavali, N.K.; Roux, B. Free Energy Landscape of A-DNA to B-DNA Conversion in Aqueous Solution. J. Am. Chem. Soc. 2005, 127, 6866–6876. [Google Scholar] [CrossRef]
- Trantírek, L.; Štefl, R.; Vorlíčková, M.; Koča, J.; Sklenářář, V.; Kypr, J. An A-type double helix of DNA having B-type puckering of the deoxyribose rings. J. Mol. Biol. 2000, 297, 907–922. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, X.; Fu, P. Spectroscopic studies on the interaction between carbaryl and calf thymus DNA with the use of ethidium bromide as a fluorescence probe. J. Photochem. Photobiol. B Biol. 2012, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, I.J.; Kearns, D.R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry 1977, 16, 3647–3654. [Google Scholar] [CrossRef]
- Cai, L.; Cherian, M. Zinc-metallothionein protects from DNA damage induced by radiation better than glutathione and copper- or cadmium-metallothioneins. Toxicol. Lett. 2003, 136, 193–198. [Google Scholar] [CrossRef] [PubMed]
| DNA Target | Primer Sequence (5′-3′) | Amplicon Size (bp) | Location of Amplified Fragment in GenBank Sequence | |
|---|---|---|---|---|
| lectin (K00821) | F: | GCTTCGCCGCTTCCTTC | 84 | 1269–1352 |
| R: | TTGGTGCGAGAAAGAAGGC | |||
| Probe: | FAM-TTCACCTTCTATGCCC-BHQ-1 | |||
| cp4 epsps (AB209952) | F: | CCTCCGATGATCGACGAAT | 87 | 1476–1562 |
| R: | GAGTTCTTCCAGACCGTTCATC | |||
| Probe: | FAM-CCGATTCTCGCTGTC-BHQ-1 | |||
| Samples | ms (g) | m0 (g) | V (μL) | lectin Target | cp4 epsps Target | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | c (Copies/μL) | Copy Number * (Copies) | RSD% | Degradation Ratio (%) | D | c (Copies/μL) | Copy Number * (Copies) | RSD% | Degradation Ratio (%) | ||||||
| Main products | a | Raw soybean | 100.00 | 0.1 | 50 | 40 | 528 ± 39 | (1.06 ± 0.08) × 109 A | 7% | / | 10 | 2570 ± 167 | (1.29 ± 0.08) × 109 A | 6% | |
| b | Soybean kernel | 86.36 | 0.1 | 50 | 40 | 534 ± 20 | (9.22 ± 0.35) × 108 B | 4% | 13.02 ± 3.31 | 10 | 2740 ± 53 | (1.18 ± 0.02) × 109 B | 2% | 8.53 ± 1.78 | |
| c | Defatted soybean kernel flour | 60.20 | 0.1 | 50 | 40 | 414 ± 15 | (4.98 ± 0.18) × 108 C | 4% | 53.02 ± 1.72 | 10 | 1806 ± 60 | (5.44 ± 0.18) × 108 C | 3% | 57.83 ± 1.40 | |
| d | SPC precipitate 1 | 45.23 | 0.1 | 50 | 10 | 264 ± 12 | (5.97 ± 0.27) × 107 D | 5% | 94.37 ± 0.26 | 10 | 302 ± 15 | (6.83 ± 0.34) × 107 D | 5% | 94.71 ± 0.27 | |
| e | SPC precipitate 2 | 41.46 | 0.1 | 50 | 10 | 196 ± 12 | (4.06 ± 0.25) × 107 DE | 6% | 96.17 ± 0.24 | 10 | 280 ± 16 | (5.80 ± 0.32) × 107 D | 6% | 95.50 ± 0.25 | |
| f | SPC precipitate 3 | 38.89 | 0.1 | 50 | 5 | 158 ± 4 | (1.54 ± 0.04) × 107 E | 3% | 98.55 ± 0.04 | 5 | 274 ± 18 | (2.66 ± 0.18) × 107 DE | 7% | 97.94 ± 0.14 | |
| g | SPC precipitate 4/SPC product | 35.28 | 0.1 | 50 | 5 | 118 ± 9 | (1.04 ± 0.08) × 107 E | 7% | 99.02 ± 0.07 | 5 | 134 ± 19 | (1.18 ± 0.17) × 107 E | 14% | 99.09 ± 0.13 | |
| By-products | h | Soybean hull | 12.52 | 0.1 | 50 | 10 | 380 ± 18 | (2.38 ± 0.11) × 107 DE | 5% | / | 10 | 382 ± 28 | (2.39 ± 0.18) × 107 DE | 7% | / |
| i | Soybean oil | 25.58 | 9.3 | 30 | 1 | 46 ± 11 | (3.80 ± 0.92) × 103 E | 24% | / | 1 | 15.4 ± 3.1 | (1.27 ± 0.25) × 103 E | 20% | / | |
| j | SPC supernatant 1 | 7.80 | 0.1 | 20 | 1 | 32 ± 7 | (4.99 ± 1.12) × 104 E | 22% | / | 1 | 7.2 ± 1.8 | (1.12 ± 0.28) × 104 E | 25% | / | |
| k | SPC supernatant 2 | 3.17 | 0.1 | 20 | 1 | 58 ± 9 | (3.68 ± 0.58) × 104 E | 16% | / | 1 | 6.6 ± 1.4 | (4.18 ± 0.89) × 103 E | 21% | / | |
| l | SPC supernatant 3 | 1.66 | 0.1 | 20 | 1 | 42 ± 8 | (1.39 ± 0.27) × 104 E | 19% | / | 1 | 15.0 ± 2.6 | (4.98 ± 0.86) × 103 E | 17% | / | |
| m | SPC supernatant 4 | 0.86 | 0.1 | 20 | 1 | 42 ± 5 | (7.22 ± 0.91) × 103 E | 13% | / | 1 | 9 ± 1 | (1.55 ± 0.17) × 103 E | 11% | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Chen, F.; Liu, K.; Chen, C. Effect of Soybean Protein Concentrate Preparation on Copy Numbers and Structural Characteristics of DNA from Genetically Modified Soybean. Foods 2023, 12, 2031. https://doi.org/10.3390/foods12102031
Du Y, Chen F, Liu K, Chen C. Effect of Soybean Protein Concentrate Preparation on Copy Numbers and Structural Characteristics of DNA from Genetically Modified Soybean. Foods. 2023; 12(10):2031. https://doi.org/10.3390/foods12102031
Chicago/Turabian StyleDu, Yan, Fusheng Chen, Kunlun Liu, and Chen Chen. 2023. "Effect of Soybean Protein Concentrate Preparation on Copy Numbers and Structural Characteristics of DNA from Genetically Modified Soybean" Foods 12, no. 10: 2031. https://doi.org/10.3390/foods12102031
APA StyleDu, Y., Chen, F., Liu, K., & Chen, C. (2023). Effect of Soybean Protein Concentrate Preparation on Copy Numbers and Structural Characteristics of DNA from Genetically Modified Soybean. Foods, 12(10), 2031. https://doi.org/10.3390/foods12102031





