Microbial Transglutaminase Cross-Linking Enhances the Textural and Rheological Properties of the Surimi-like Gels Made from Alkali-Extracted Protein Isolate from Catfish Byproducts and the Role of Disulfide Bonds in Gelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Protein Isolate
2.2. Preparation of Gels
2.3. Color Analysis of Gels
2.4. Texture Profile Analysis
2.5. Dynamic Rheological Analysis
2.6. Solubility Analysis in Non-Reducing and Reducing Solutions
2.7. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Color Profile of Gels Made with Different Levels of MTGase
3.2. Texture Profile Analysis (TPA) of Gels
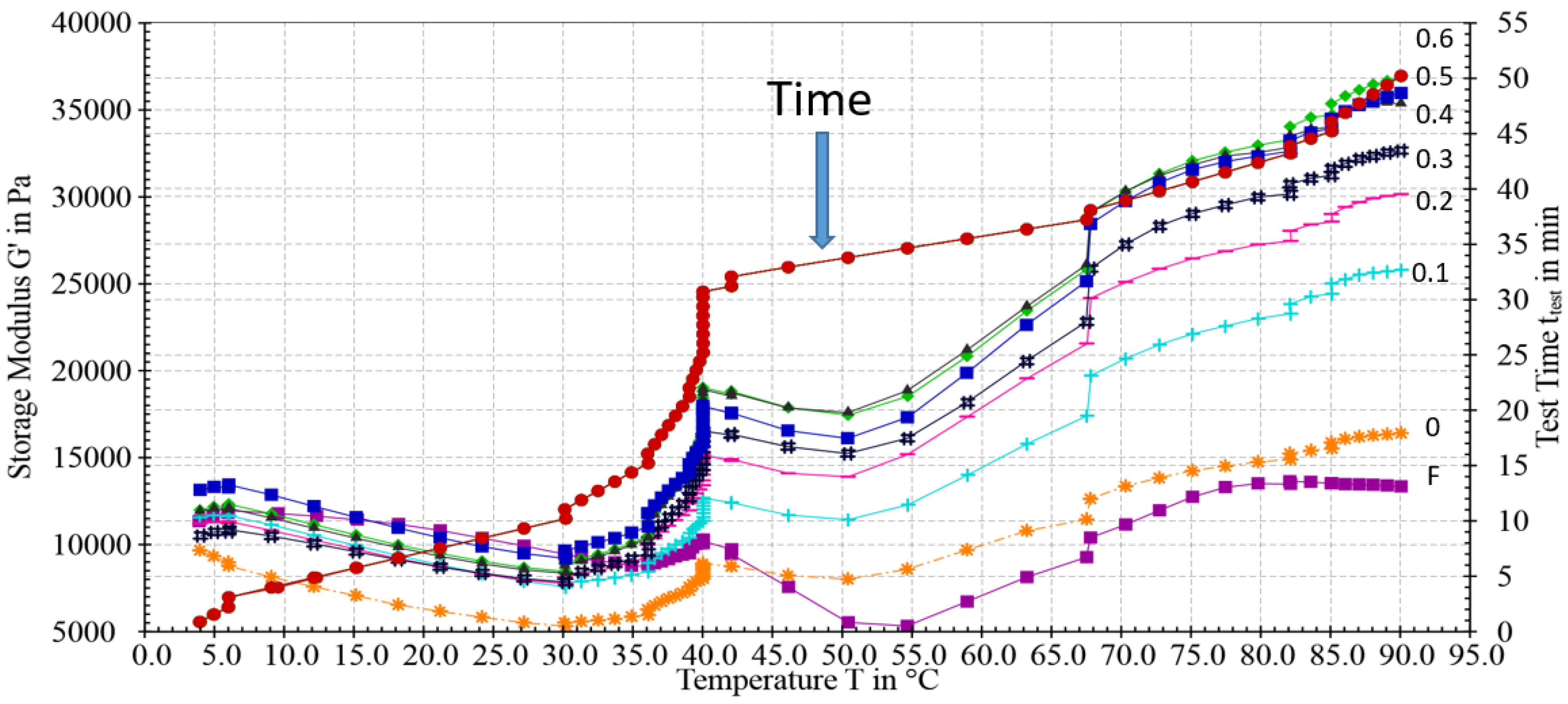
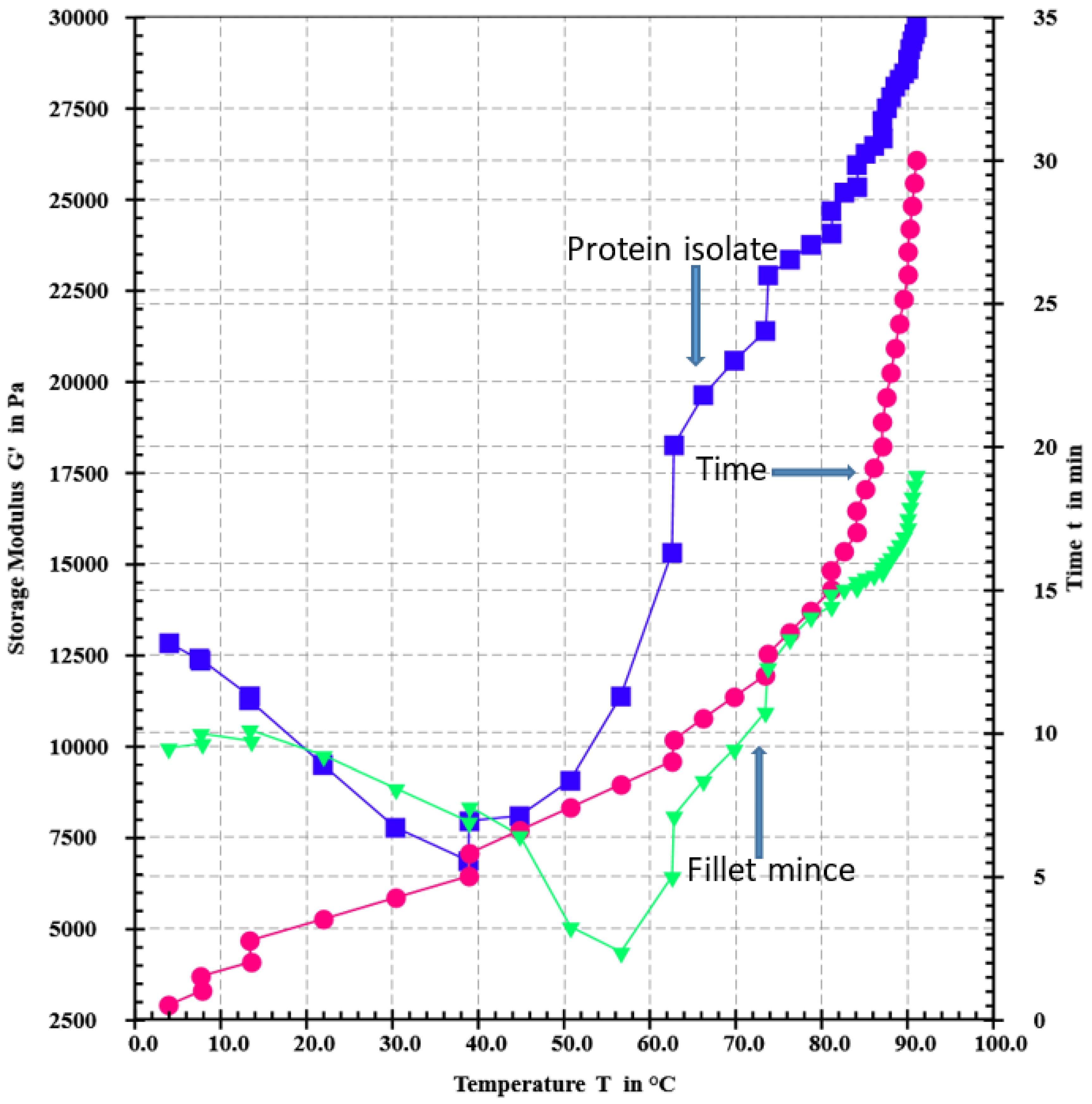
3.3. Dynamic Rheological Analysis
3.4. Solubility in Non-Reducing and Reducing Solutions
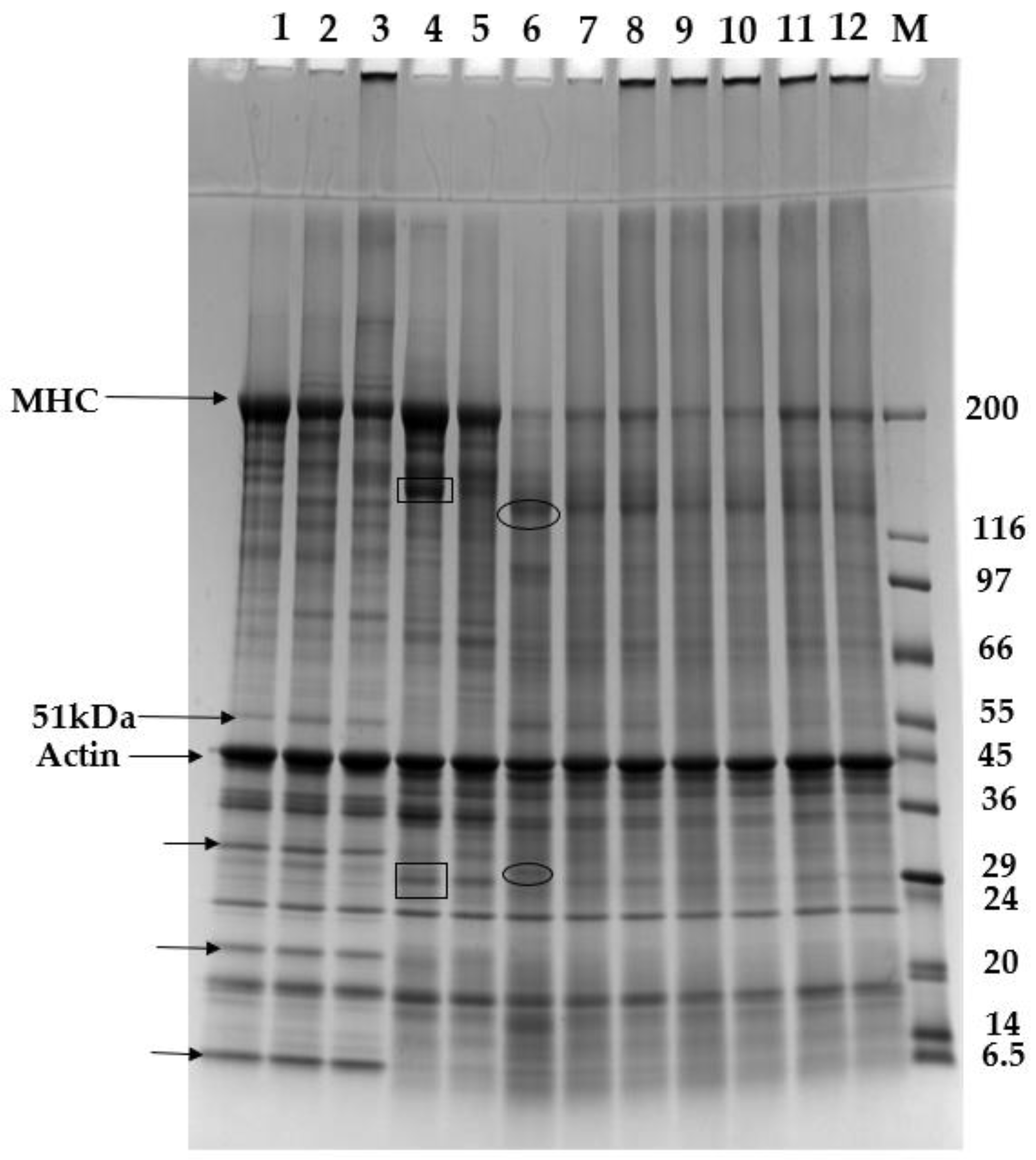
3.5. SDS-PAGE Patterns of Proteins and Gels
3.6. Pearson Correlation Analysis among Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nakahara, C.; Nozawa, H.; Seki, N. A comparison of cross-linking of fish myofibrillar proteins by endogenous and microbial transglutaminases. Fish. Sci. 1999, 65, 138–144. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Kristinsson, H.G. Conformational and rheological changes in catfish myosin during alkali-induced unfolding and refolding. Food Chem. 2008, 107, 385–398. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Gao, H.R.; Chang, S.K.C.; Bechtel, P.J.; Mahmoud, B.S.M. Comparative studies on the yield and characteristics of myofibrillar proteins from catfish heads and frames extracted by two methods for making surimi-like protein gel products. Food Chem. 2019, 272, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chang, S.K.C. Protein extraction pH and cross-linking affect physicochemical and textural properties of protein gels made from channel catfish by-products. J. Sci. Food Agric. 2021, 101, 4799–4807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, S.K.C. Protein extraction from catfish byproducts and physicochemical properties of the protein isolate. J. Food Sci. 2021, 86, 3061–3074. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Dynamic rheological, microstructural and physicochemical properties of blend fish protein recovered from kilka (Clupeonella cultriventris) and silver carp (Hypophthalmichthys molitrix) by the pH-shift or washing-based technology. Food Chem. 2017, 229, 695–709. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S. Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolate. Food Chem. 2013, 136, 929–937. [Google Scholar] [CrossRef]
- An, Y.Q.; You, J.; Xiong, S.B.; Yin, T. Short-term frozen storage enhances cross-linking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 257, 216–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K.C. Color and texture of surimi-like gels made of protein isolate extracted from catfish byproducts are improved by washing and adding soy whey. J. Food Sci. 2022, 87, 3057–3070. [Google Scholar] [CrossRef]
- Park, J.W. Functional protein additives in surimi gel. J. Food Sci. 1994, 59, 525–527. [Google Scholar] [CrossRef]
- Meng, S.; Chang, S.; Gillen, A.M.; Zhang, Y. Protein and quality analyses of accessions from the USDA soybean germplasm collection for tofu production. Food Chem. 2016, 213, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.C.; Zhang, Y. Protein analysis. In Food Analysis; Nielsen, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 315–331. [Google Scholar]
- Laemmli, U.K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Shi, L.; Xiong, S.B.; Hu, Y.; You, J.; Huang, Q.L.; Yin, T. Gelling properties of vacuum-freeze dried surimi powder as influenced by heating method and microbial transglutaminase. LWT-Food Sci. Technol. 2019, 99, 105–111. [Google Scholar] [CrossRef]
- Benjakul, S.; Phatcharat, S.; Tammatinna, A.; Visessanguan, W.; Kishimura, H. Improvement of gelling properties of lizardfish mince as influenced by microbial transglutaminase and fish freshness. J. Food Sci. 2008, 73, 239–246. [Google Scholar] [CrossRef]
- Zhang, N.N.; Yang, N.; Yu, W.Y.; Jin, Z.; Jiang, P.F.; Yu, C.X.; Dong, X.P. Effects of microbial transglutaminase on textural, water distribution, and microstructure of frozen-stored longtail southern cod (Patagonotothen ramsayi) fish mince gel. J. Texture Stud. 2021, 53, 844–853. [Google Scholar] [CrossRef]
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Biosci. 2013, 1, 39–47. [Google Scholar] [CrossRef]
- Perez-Mateos, M.; Lanier, T.C. Comparison of Atlantic menhaden gels from surimi processed by acid or alkaline solubilization. Food Chem. 2006, 101, 1223–1229. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Liu, W.J.; Yuan, C.H.; Morioka, K.; Chen, S.G.; Liu, D.H.; Ye, X.Q. Enhancement of the gelation properties of hairtail (Trichiurus haumela) muscle protein with curdlan and transglutaminase. Food Chem. 2015, 176, 115–122. [Google Scholar] [CrossRef]
- Gilleland, G.M.; Lanier, T.C.; Hamann, D.D. Covalent bonding in pressure-induced fish protein gels. J. Food Sci. 1997, 62, 713–716. [Google Scholar] [CrossRef]
- Asagami, T.; Ogiwara, M.; Wakameda, A.; Noguchi, S.F. Effect of microbial transglutaminase on the quality of frozen surimi made from various kinds of fish species. Fish. Sci. 1995, 61, 267–272. [Google Scholar] [CrossRef]
- Seguro, K.; Kumazawa, Y.; Ohtsuka, T.; Toiguchi, S.; Motoki, M. Microbial transglutaminase and ε-(Υ-glutamyl) lysine crosslink effects on elastic properties of kamaboko gels. J. Food Sci. 1995, 60, 305–311. [Google Scholar] [CrossRef]
- Wongwichian, C.; Chaijan, M.; Panpipat, W.; Klomklao, S.; Benjakul, S. Autolysis and characterization of sarcoplasmic and myofibril associated proteinase of oxeye scad (Selar boops) muscle. J. Aquat. Food Prod. Technol. 2016, 25, 1132–1143. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Mendes, R.O.; Saraiva, J.A.; Vaz-Pires, P.R.; Nunes, M.L. Quality Characteristics of high pressure-induced hake (Merluccius capensis) protein gels with and without MTGase. J. Aquat. Food Prod. Technol. 2010, 19, 193–213. [Google Scholar] [CrossRef]
- Herranz, B.; Tovar, C.A.; Borderias, A.J.; Moreno, H.M. Effect of high-pressure and/or microbial transglutaminase on physicochemical, rheological and microstructural properties of flying fish surimi. Innov. Food Sci. Emerg. Technol. 2013, 20, 24–33. [Google Scholar] [CrossRef]
- Marmon, S.K.; Undeland, I. Protein isolation from gutted herring (Clupea harengus) using pH-shift processes. J. Agric. Food Chem. 2013, 58, 10480–10486. [Google Scholar] [CrossRef]
- Yang, N.; Fan, X.R.; Yu, W.Y.; Huang, Y.Z.; Yu, C.X.; Konno, K.; Dong, X.P. Effects of microbial transglutaminase on gel formation of frozen-stored longtail southern cod (Patagonotothen ramsayi) mince. LWT-Food Sci. Technol. 2020, 128, 109444. [Google Scholar] [CrossRef]
- Ramirez-Suarez, J.C.; Addo, K.; Xiong, Y.L. Gelation of mixed myofibrillar/wheat gluten proteins treated with microbial transglutaminase. Food Res. Int. 2005, 38, 1143–1149. [Google Scholar] [CrossRef]
- Huang, Y.P.; Seguro, K.; Motoki, M.; Tawada, K. Cross-linking of contractile proteins from skeletal muscle by treatment with microbial transglutaminase. J. Biochem. 1992, 112, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.X.; Xiong, S.B.; Hu, Y.; Yin, T.; You, J. In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by microbial transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160. [Google Scholar] [CrossRef]
- Yongsawatigul, J.; Piyadhammaviboon, P. Effect of microbial transglutaminase on autolysis and gelation of lizardfish surimi. J. Sci. Food Agric. 2005, 85, 1453–1460. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Yatmaz, H.A.; Gokoglu, N.; Ucak, I. The quality alternations of rainbow trout mince treated with transglutaminase. LWT-Food Sci. Technol. 2017, 84, 815–820. [Google Scholar] [CrossRef]

| Samples | L* | a* | b* | Whiteness |
|---|---|---|---|---|
| Protein isolate without setting | 74.17 ± 0.74 a | −1.04 ± 0.13 a | 12.26 ± 0.27 a | 71.39 ± 0.74 a |
| Protein isolate with setting | 74.77 ± 1.10 a | −1.07 ± 0.09 a | 11.47 ± 0.31 b | 72.26 ± 0.81 a |
| 0.1 units/g with setting | 74.72 ± 0.36 a | −0.97 ± 0.10 a | 12.17 ± 0.24 a | 71.93 ± 0.44 a |
| 0.2 units/g with setting | 74.20 ± 0.54 a | −1.01 ± 0.11 a | 12.17 ± 0.30 a | 71.46 ± 0.49 a |
| 0.3 units/g with setting | 74.40 ± 0.49 a | −0.95 ± 0.02 a | 12.32 ± 0.17 a | 71.57 ± 0.27 a |
| 0.4 units/g with setting | 74.11 ± 0.82 a | −0.99 ± 0.06 a | 12.18 ± 0.21 a | 71.37 ± 0.78 a |
| 0.5 units/g with setting | 74.76 ± 1.33 a | −1.02 ± 0.05 a | 12.31 ± 0.29 a | 71.90 ± 0.91 a |
| 0.6 units/g with setting | 74.99 ± 1.47 a | −1.07 ± 0.08 a | 12.40 ± 0.25 a | 72.06 ± 0.40 a |
| Samples | Hardness at Maximum Force (g) | Cohesiveness | Springiness (%) | Chewiness (g) | Resilience (%) | Fracturability (g) | Deformation (mm) |
|---|---|---|---|---|---|---|---|
| Fillet mince without setting | 22,504 ± 1328 c | 0.626 ± 0.005 a | 80.11 ± 1.04 b | 11,278 ± 502 c | 26.08 ± 0.41 b | ND | ND |
| Fillet mince with setting | 27,096 ± 762 b | 0.648 ± 0.014 a | 85.77 ± 1.43 a | 15,058 ± 181 a | 29.93 ± 0.57 a | ND | ND |
| Protein isolate without setting | 10,288 ± 1032 f | 0.303 ± 0.011 de | 79.98 ± 2.08 b | 2484 ± 31 g | 8.09 ± 0.11 f | 8963 ± 609 e | 7.88 ± 0.27 e |
| Protein isolate with setting | 9975 ± 366 f | 0.280 ± 0.005 e | 79.03 ± 0.52 b | 2203 ± 27 g | 7.27 ± 0.38 f | 5823 ± 343 f | 6.51 ± 0.19 f |
| 0.1 units/g with setting | 11,335 ± 577 f | 0.318 ± 0.012 d | 80.34 ± 2.88 b | 2890 ± 144 g | 8.19 ± 0.06 f | 9613 ± 267 e | 8.21 ± 0.16 d |
| 0.2 unit/g with setting | 15,243 ± 619 e | 0.355 ± 0.019 c | 85.80 ± 0.17 a | 4633 ± 139 f | 9.65 ± 0.29 e | 14,258 ± 332 d | 9.83 ± 0.15 c |
| 0.3 units/g with setting | 16,993 ± 82 d | 0.362 ± 0.004 c | 87.62 ± 0.32 a | 5382 ± 59 e | 10.30 ± 0.23 e | 16,993 ± 82 c | 9.80 ± 0.06 c |
| 0.4 units/g with setting | 23,199 ± 856 c | 0.416 ± 0.026 b | 86.17 ± 1.47 a | 8304 ± 314 d | 12.95 ± 0.70 d | 23,199 ± 856 b | 10.75 ± 0.13 b |
| 0.5 units/g with setting | 31,768 ± 520 a | 0.434 ± 0.025 b | 88.17 ± 1.45 a | 12,145 ± 700 b | 15.61 ± 0.92 c | 31,768 ± 520 a | 11.13 ± 0.06 a |
| 0.6 units/g with setting | 30,638 ± 615 a | 0.433 ± 0.022 b | 87.81 ± 1.39 a | 11,635 ± 639 bc | 15.23 ± 1.79 c | 30,938 ± 191 a | 11.02 ± 0.15 ab |
| Protein Solubility (%) | ||
|---|---|---|
| Sample | Non-Reducing (5% SDS) | Reducing (5% SDS + 50 mM 2-ME) |
| Fillet mince | 90.63 ± 0.50 aA | 92.60 ± 1.53 cdeA |
| Gel made from fillet mince without setting | 88.71 ± 0.37 abB | 94.68 ± 0.39 abcdA |
| Gel made from fillet mince with setting | 96.36 ± 0.12 aA | 96.66 ± 1.29 abcA |
| Protein isolate | 93.48 ± 0.47 aB | 96.95 ± 0.1 abA |
| Gel made from protein isolate without setting | 79.66 ± 7.61 bcB | 97.91 ± 2.95 aA |
| Gel made from protein isolate with setting | 77.28 ± 4.90 cB | 95.36 ± 1.68 abcdA |
| Gel made from protein isolate with 0.1 unit MTGase/g | 65.64 ± 1.08 dB | 93.41 ± 1.96 bcdA |
| Gel made from protein isolate with 0.2 unit MTGase/g | 66.16 ± 8.76 dB | 95.25 ± 1.21 abcdA |
| Gel made from protein isolate with 0.3 unit MTGase/g | 63.08 ± 9.04 dB | 95.35 ± 1.68 abcdA |
| Gel made from protein isolate with 0.4 unit MTGase/g | 60.79 ± 1.20 dB | 91.73 ± 2.76 deA |
| Gel made from protein isolate with 0.5 unit MTGase/g | 57.46 ± 1.89 dB | 89.07 ± 0.67 efA |
| Gel made from protein isolate with 0.6 unit MTGase/g | 57.58 ± 1.37 dB | 87.49 ± 1.61 fA |
| Reducing Solubility | Hardness | G’ | Cohesiveness | Springiness | Chewiness | Resilience | Fracturability | Deformation | |
|---|---|---|---|---|---|---|---|---|---|
| Non-reducing solubility | 0.759 * | −0.858 * | −0.971 *** | −0.935 ** | −0.849 * | −0.860 * | −0.882 ** | −0.902 ** | −0.949 ** |
| Reducing solubility | −0.904 ** | −0.666 | −0.817 * | −0.518 | −0.905 ** | −0.895 ** | −0.881 ** | −0.676 | |
| Hardness | 0.839 * | 0.956 *** | 0.803 * | 0.999 *** | 0.998 *** | 0.995 *** | 0.868 * | ||
| G’ | 0.950 ** | 0.929 ** | 0.841 * | 0.869 * | 0.890 ** | 0.989 *** | |||
| Cohesiveness | 0.882 ** | 0.957 *** | 0.973 *** | 0.977 *** | 0.967 *** | ||||
| Springiness | 0.801 * | 0.819 * | 0.848 * | 0.940 ** | |||||
| Chewiness | 0.998 *** | 0.995 *** | 0.870 * | ||||||
| Resilience | 0.998 *** | 0.894 ** | |||||||
| Fracturability | 0.911 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chang, S.K.C. Microbial Transglutaminase Cross-Linking Enhances the Textural and Rheological Properties of the Surimi-like Gels Made from Alkali-Extracted Protein Isolate from Catfish Byproducts and the Role of Disulfide Bonds in Gelling. Foods 2023, 12, 2029. https://doi.org/10.3390/foods12102029
Zhang Y, Chang SKC. Microbial Transglutaminase Cross-Linking Enhances the Textural and Rheological Properties of the Surimi-like Gels Made from Alkali-Extracted Protein Isolate from Catfish Byproducts and the Role of Disulfide Bonds in Gelling. Foods. 2023; 12(10):2029. https://doi.org/10.3390/foods12102029
Chicago/Turabian StyleZhang, Yan, and Sam K.C. Chang. 2023. "Microbial Transglutaminase Cross-Linking Enhances the Textural and Rheological Properties of the Surimi-like Gels Made from Alkali-Extracted Protein Isolate from Catfish Byproducts and the Role of Disulfide Bonds in Gelling" Foods 12, no. 10: 2029. https://doi.org/10.3390/foods12102029
APA StyleZhang, Y., & Chang, S. K. C. (2023). Microbial Transglutaminase Cross-Linking Enhances the Textural and Rheological Properties of the Surimi-like Gels Made from Alkali-Extracted Protein Isolate from Catfish Byproducts and the Role of Disulfide Bonds in Gelling. Foods, 12(10), 2029. https://doi.org/10.3390/foods12102029








