Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Extraction and Determination of CML and CEL
2.4. Extraction and Determination of Acrylamide
2.5. Determination of Protein, Amino Acids, Sugars and Fatty Acids
2.6. Statistical Analysis
3. Results and Discussion
3.1. CML and CEL Contents of Plant-Based Meat Analogs
3.2. Acrylamide Content of Plant-Based Meat Analogs
3.3. Protein Content of Plant-Based Meat Analogs
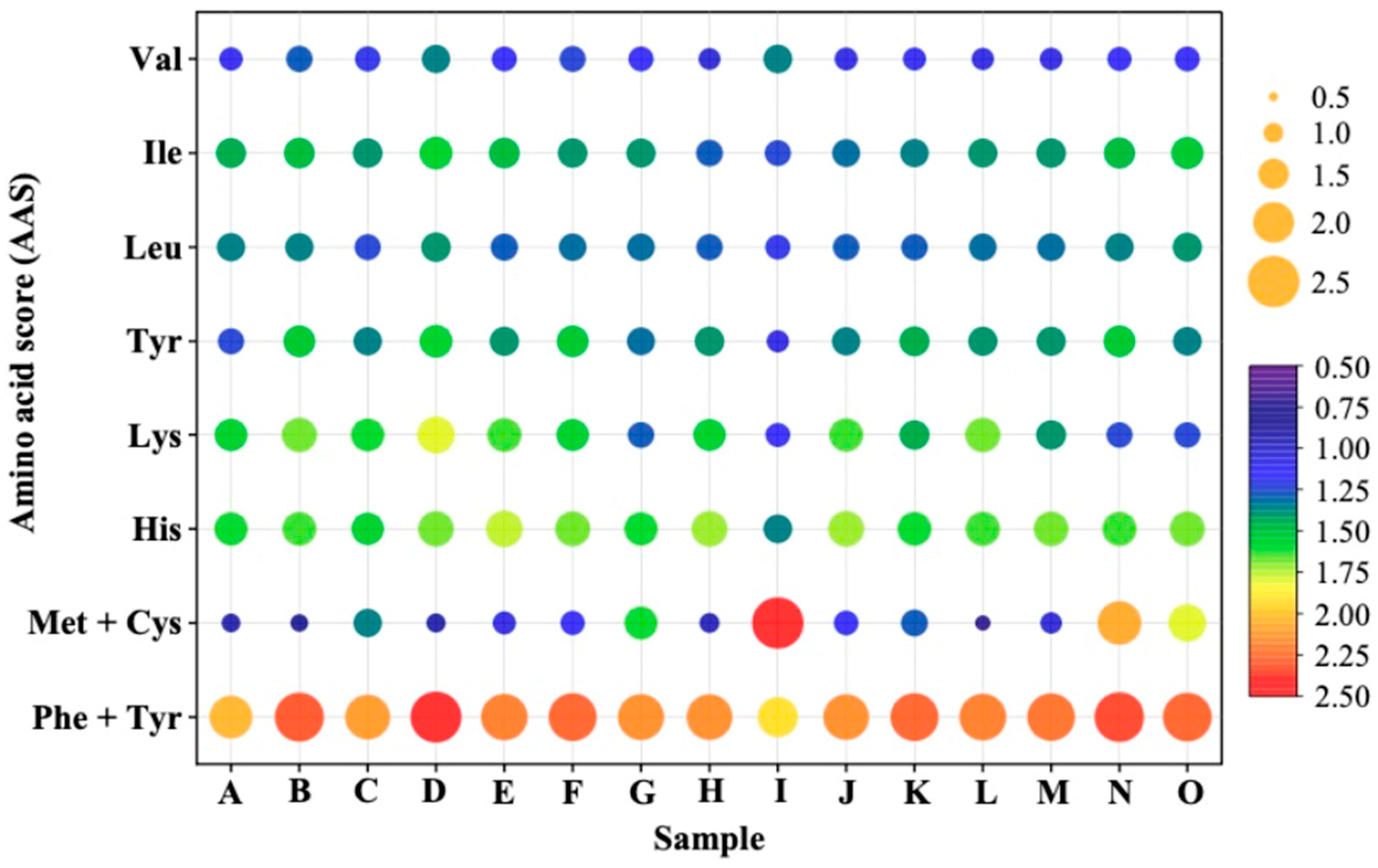
3.4. Amino Acid Profile of Plant-Based Meat Analogs
3.5. Fatty Acid Profile of Plant-Based Meat Analogs
3.6. Sugar Content of Plant-Based Meat Analogs
3.7. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akillioglu, H.G.; Lund, M.N. Quantification of advanced glycation end products and amino acid cross-links in foods by high-resolution mass spectrometry: Applicability of acid hydrolysis. Food Chem. 2022, 366, 130601. [Google Scholar] [CrossRef]
- Ortiz, S.J.; Wrobel, K.; Ojeda, A.G.; Acevedo-Aguilar, F.J.; Escobosa, A.R.C.; Barrientos, E.Y.; Garay-Sevilla, M.E.; Wrobel, K. Nε-(carboxymethyl)-ʟ-lysine content in cheese, meat and fish products is affected by the presence of copper during elaboration process. Eur. Food Res. Technol. 2018, 244, 225–234. [Google Scholar] [CrossRef]
- Yu, L.; Chai, M.; Zeng, M.; He, Z.; Chen, J. Effect of lipid oxidation on the formation of Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine in Chinese-style sausage during storage. Food Chem. 2018, 269, 466–472. [Google Scholar] [CrossRef]
- Koschinsky, T.; He, J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Ueda, S.; Okuda, S. Food-derived advanced glycation end products (AGEs): A novel therapeutic target for various disorders. Curr. Pharm. Des. 2007, 13, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Acrylamide. In IARC monographs on the evaluation of carcinogenic risks to humans. In Some Industrial Chemicals; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Acrylamide in Foods. 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-acrylamide-foods (accessed on 15 March 2023).
- Pointke, M.; Pawelzik, E. Plant-based alternative products: Are they healthy alternatives? Micro- and macronutrients and nutritional scoring. Nutrients 2022, 14, 601. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Shah, F.; Xu, Y.; Wang, Q. High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: Effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocoll. 2020, 99, 105311. [Google Scholar] [CrossRef]
- Guan, J.J.; Qiu, A.Y.; Liu, X.Y.; Hua, Y.F.; Ma, Y.H. Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Palermo, M.; Fiore, A.; Fogliano, V. Okara promoted acrylamide and carboxymethyl-lysine formation in bakery products. J. Agric. Food Chem. 2012, 60, 10141–10146. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, C.; Bosch, G.; van der Poel, A.F.B.; Wierenga, P.A.; Alexander, L.; Hendriks, W.H. Quantitation of Maillard reaction products in commercially available pet Foods. J. Agric. Food Chem. 2014, 62, 8883–8891. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Chen, Y.; Xie, S.; Xue, C.; He, Z.; Chen, Q.; Wang, Z.; Qin, F.; Chen, J.; Zeng, M. Accumulation of heterocyclic amines and advanced glycation end products in various processing stages of plant-based burgers by UHPLC-MS/MS. J. Agric. Food Chem. 2022, 70, 14771–14783. [Google Scholar] [CrossRef] [PubMed]
- de Marchi, M.; Costa, A.; Pozza, M.; Goi, A.; Manuelian, C.L. Detailed characterization of plant-based burgers. Sci. Rep. 2021, 11, 2049. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M.P.L.V.O. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef]
- Scheijen, J.L.J.M.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of Nɛ-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of free and protein-bound carboxymethyllysine and carboxyethyllysine in meats during commercial sterilization. Meat Sci. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, X.; Zhang, Z.; Ma, L.; Liu, G.; Wang, Q.; Chen, F.; Cheng, K.W. Development of an isotope dilution UHPLC-QqQ-MS/MS-based method for simultaneous determination of typical advanced glycation end products and acrylamide in baked and fried foods. J. Agric. Food Chem. 2021, 69, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, Y.Y.; Xu, X.B.; Wang, X.S.; Liu, H.W.; Zhou, D.Y.; Zhu, B.W.; Thornton, M. Isotope dilution HPLC-MS/MS for simultaneous quantification of acrylamide and 5-hydroxymethylfurfural (HMF) in thermally processed seafood. Food Chem. 2017, 232, 633–638. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.124-2016; National Standards for Food Safety—Determination of Amino Acids in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.8-2016; National Standards for Food Safety—Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.168-2016; National Standards for Food Safety—Determination of Fatty Acids in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Yu, L.; Gao, C.; Zeng, M.; He, Z.; Wang, L.; Zhang, S.; Chen, J. Effects of raw meat and process procedure on Nε-carboxymethyllysine and Nε-carboxyethyl-lysine formation in meat products. Food Sci. Biotechnol. 2016, 25, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 168, 190–195. [Google Scholar] [CrossRef]
- Žilić, S.; Mogol, B.A.; Akıllıoğlu, G.; Serpen, A.; Delić, N.; Gökmen, V. Effects of extrusion, infrared and microwave processing on Maillard reaction products and phenolic compounds in soybean. J. Sci. Food Agric. 2014, 94, 45–51. [Google Scholar] [CrossRef]
- Fanelli, N.S.; Bailey, H.M.; Thompson, T.W.; Delmore, R.; Nair, M.N.; Stein, H.H. Digestible indispensable amino acid score (DIAAS) is greater in animal-based burgers than in plant-based burgers if determined in pigs. Eur. J. Nutr. 2022, 61, 461–475. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- and animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- França, F.; Harada-Padermo, S.D.S.; Frasceto, R.A.; Saldaña, E.; Lorenzo, J.M.; Vieira, T.M.F.D.S.; Selani, M.M. Umami ingredient from shiitake (Lentinula edodes) by-products as a flavor enhancer in low-salt beef burgers: Effects on physicochemical and technological properties. LWT-Food Sci. Technol 2022, 154, 112724. [Google Scholar] [CrossRef]
- WHO/FAO/UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Chen, Q.; Zhang, J.; Zhang, Y.; Wang, Q. Effect of fatty acid saturation degree on the rheological properties of pea protein and its high-moisture extruded product quality. Food Chem. 2022, 390, 133139. [Google Scholar] [CrossRef]
- Koutsidis, G.; Simons, S.P.J.; Thong, Y.H.; Haldoupis, Y.; Mojica-Lazaro, J.; Wedzicha, B.L.; Mottram, D.S. Investigations on the effect of amino acids on acrylamide, pyrazines, and michael addition products in model systems. J. Agric. Food Chem. 2009, 57, 9011–9015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Ye, B.; Yan, H.; Zhao, Y.; Liu, L. Effect of unsaturated fatty acids on glycation product formation pathways. Food Res. Int. 2021, 143, 110288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hu, H.; Xie, J.; Shen, M. Investigation into the contents of nutrients, Nε-carboxymethyllysine and Nε-carboxyethyllysine in various commercially canned fishes to find the correlation between them. J. Food Compost. 2021, 96, 103737. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tang, J.; Lai, K.; Rasco, B.A.; Huang, Y. Formation of protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in ground pork during commercial sterilization as affected by the type and concentration of sugars. Food Chem. 2021, 336, 127706. [Google Scholar] [CrossRef]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nɛ-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, beta-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; McClements, D.J.; Nie, S.; Shen, M.; Li, C.; Huang, Y.; Zhong, Y.; Chen, J.; Zeng, M.; et al. pH and lipid unsaturation impact the formation of acrylamide and 5-hydroxymethylfurfural in model system at frying temperature. Food Res. Int. 2019, 123, 403–413. [Google Scholar] [CrossRef]
| Sample Code | CML (mg/kg) | CEL (mg/kg) | Acrylamide (μg/kg) |
|---|---|---|---|
| A | 22.21 ± 1.12 ef | 39.97 ± 1.71 efg | 31.81 ± 0.26 f |
| B | 43.03 ± 2.87 ab | 35.45 ± 1.93 fgh | 43.07 ± 0.53 e |
| C | 22.47 ± 2.18 ef | 31.39 ± 2.20 hi | 33.59 ± 2.38 f |
| D | 40.59 ± 1.01 bc | 29.47 ± 0.41 hi | 64.00 ± 0.33 d |
| E | 34.05 ± 2.03 d | 51.97 ± 1.92 c | 63.07 ± 6.07 d |
| F | 47.61 ± 3.82 a | 46.25 ± 4.78 cde | 63.00 ± 1.47 d |
| G | 46.05 ± 1.46 ab | 86.23 ± 2.11 a | 69.00 ± 0.62 d |
| H | 22.79 ± 0.46 e | 42.32 ± 0.80 def | 49.07 ± 2.25 e |
| I | 41.67 ± 0.87 ab | 29.02 ± 1.70 hi | 66.00 ± 0.29 d |
| J | 43.42 ± 2.54 ab | 65.61 ± 4.13 b | 43.41 ± 0.50 e |
| K | 22.69 ± 0.48 e | 48.63 ± 4.43 cd | 78.63 ± 5.58 c |
| L | 25.92 ± 1.78 e | 25.21 ± 3.12 i | 48.96 ± 1.40 e |
| M | 34.53 ± 0.79 cd | 30.40 ± 0.83 hi | 120.78 ± 5.40 b |
| N | 26.16 ± 3.97 e | 71.56 ± 4.96 b | 67.22 ± 2.99 d |
| O | 16.46 ± 0.67 f | 31.94 ± 1.50 ghi | 186.70 ± 1.26 a |
| Sample Code | Total Amino Acids | Protein |
|---|---|---|
| A | 37.18 ± 0.98 e | 39.05 ± 0.32 gh |
| B | 39.87 ± 1.06 cd | 43.56 ± 0.36 de |
| C | 38.83 ± 0.54 cde | 40.76 ± 0.08 f |
| D | 38.7 ± 0.74 de | 40.33 ± 0.12 fg |
| E | 40.55 ± 0.82 cd | 42.81 ± 0.69 e |
| F | 41.21 ± 1.10 c | 45.15 ± 0.45 d |
| G | 34.28 ± 0.94 f | 37.62 ± 0.33 h |
| H | 34.35 ± 0.71 f | 37.81 ± 0.67 h |
| I | 21.89 ± 0.4 h | 24.03 ± 0.21 k |
| J | 47.68 ± 2.19 a | 53.18 ± 0.39 a |
| K | 46.33 ± 1.14 ab | 49.38 ± 0.73 b |
| L | 30.77 ± 0.64 g | 33.7 ± 0.29 i |
| M | 30.34 ± 0.68 g | 31.88 ± 0.34 j |
| N | 45.09 ± 0.49 b | 47.21 ± 1.30 c |
| O | 23.63 ± 0.21 h | 24.18 ± 0.83 k |
| Sample Code | SFA (g/100 g) | MUFA (g/100 g) | PUFA (g/100 g) | TFA (g/100 g) | n-3 (g/100 g) | n-6 (g/100 g) | n-6/n-3 |
|---|---|---|---|---|---|---|---|
| A | 10.36 ± 0.08 f | 15.41 ± 0.04 bc | 6.91 ± 0.03 f | 32.68 ± 0.15 de | 1.89 ± 0.01 b | 5.00 ± 0.02 ef | 2.65 ± 0 |
| B | 13.17 ± 0.22 c | 15.87 ± 0.25 ab | 6.73 ± 0.16 fg | 35.77 ± 0.61 c | 1.41 ± 0.03 ef | 5.29 ± 0.12 ef | 3.76 ± 0.01 |
| C | 20.47 ± 0.27 b | 11.75 ± 0.14 e | 1.61 ± 0.02 i | 33.83 ± 0.42 d | 0.04 ± 0 k | 1.57 ± 0.02 h | 41.12 ± 0.23 |
| D | 12.62 ± 0.1 d | 16.03 ± 0.15 a | 7.02 ± 0.07 f | 35.67 ± 0.32 c | 1.50 ± 0.02 d | 5.49 ± 0.06 e | 3.65 ± 0 |
| E | 6.94 ± 0.11 g | 13.96 ± 0.21 d | 6.05 ± 0.10 g | 26.95 ± 0.41 g | 1.34 ± 0.02 f | 4.70 ± 0.08 fg | 3.52 ± 0.01 |
| F | 11.17 ± 0.11 e | 13.66 ± 0.15 d | 6.54 ± 0.06 fg | 31.37 ± 0.31 e | 1.44 ± 0.01 de | 5.07 ± 0.05 ef | 3.51 ± 0.01 |
| G | 5.21 ± 0.13 i | 8.31 ± 0.18 g | 20.51 ± 0.43 c | 34.02 ± 0.74 d | 1.68 ± 0.05 c | 18.81 ± 0.38 c | 11.19 ± 0.08 |
| H | 10.11 ± 0.23 f | 14.95 ± 0.39 c | 6.96 ± 0.18 f | 32.02 ± 0.81 e | 1.50 ± 0.04 d | 5.44 ± 0.15 e | 3.64 ± 0 |
| I | 4.18 ± 0.07 k | 7.31 ± 0.13 h | 16.08 ± 0.28 e | 27.57 ± 0.47 g | 1.16 ± 0.02 g | 14.91 ± 0.25 d | 12.87 ± 0.04 |
| J | 0.26 ± 0 m | 1.15 ± 0.01 k | 0.5 ± 0.01 j | 1.91 ± 0.02 i | 0.05 ± 0 k | 0.44 ± 0.01 i | 8.23 ± 0.08 |
| K | 12.70 ± 0.02 d | 2.83 ± 0 j | 6.06 ± 0.01 g | 21.59 ± 0.03 h | 0.78 ± 0 h | 5.27 ± 0.01 ef | 6.79 ± 0.01 |
| L | 21.93 ± 0.16 a | 6.17 ± 0.04 i | 4.44 ± 0.04 h | 32.54 ± 0.25 de | 0.32 ± 0 i | 4.11 ± 0.04 g | 12.75 ± 0.06 |
| M | 6.36 ± 0.15 h | 9.59 ± 0.23 f | 23.93 ± 0.57 b | 39.88 ± 0.94 b | 2.05 ± 0.05 a | 21.86 ± 0.52 b | 10.64 ± 0.05 |
| N | 3.57 ± 0.05 l | 7.18 ± 0.12 h | 19.10 ± 0.26 d | 29.85 ± 0.43 f | 0.09 ± 0 k | 19.00 ± 0.25 c | 220.83 ± 0.77 |
| O | 4.78 ± 0.06 j | 11.56 ± 0.15 e | 26.01 ± 0.36 a | 42.36 ± 0.57 a | 0.18 ± 0 j | 25.82 ± 0.36 a | 141.57 ± 0.56 |
| Sample Code | Fructose | Glucose | Sucrose | Maltose | Total Sugars |
|---|---|---|---|---|---|
| A | ND | 1.44 ± 0.04 a | 0.80 ± 0.04 e | ND | 2.24 ± 0.03 g |
| B | 0.33 ± 0.01 e | ND | ND | ND | 0.33 ± 0.01 k |
| C | ND | ND | 0.53 ± 0.01 f | ND | 0.53 ± 0.01 j |
| D | 0.45 ± 0.02 bc | ND | ND | 0.53 ± 0.01 d | 0.98 ± 0.02 i |
| E | 0.50 ± 0.03 a | 0.47 ± 0.01 f | 0.58 ± 0.01 f | 1.00 ± 0.01 c | 2.55 ± 0.04 f |
| F | 0.42 ± 0.01 cd | 0.51 ± 0.02 f | ND | 0.51 ± 0.01 d | 1.44 ± 0.03 h |
| G | 0.52 ± 0.02 a | 1.28 ± 0.01 b | ND | 1.47 ± 0.03 b | 3.28 ± 0.05 d |
| H | ND | ND | 0.52 ± 0.01 fg | ND | 0.52 ± 0.01 j |
| I | 0.50 ± 0 a | 1.15 ± 0.01 c | 7.66 ± 0.01 a | ND | 9.31 ± 0.02 a |
| J | ND | 0.47 ± 0.01 f | 2.63 ± 0.02 b | ND | 3.10 ± 0.01 e |
| K | ND | 0.48 ± 0.01 f | 0.46 ± 0.02 g | ND | 0.94 ± 0.03 i |
| L | 0.38 ± 0.02 de | 0.51 ± 0.02 f | 2.57 ± 0.01 b | ND | 3.46 ± 0.04 c |
| M | 0.48 ± 0.01 ab | 1.03 ± 0.03 d | 2.26 ± 0.01 c | 2.74 ± 0.15 a | 6.52 ± 0.19 b |
| N | 0.33 ± 0.01 e | 0.74 ± 0.01 e | 0.46 ± 0.01 g | ND | 1.53 ± 0.03 h |
| O | ND | ND | 1.57 ± 0.04 d | ND | 1.57 ± 0.04 h |
| Amino Acids/Protein | Correlation Coefficient (r) | ||
|---|---|---|---|
| CML | CEL | Acrylamide | |
| Asp | 0.204 | 0.349 | −0.608 * |
| Thr | 0.216 | 0.468 | −0.556 * |
| Ser | 0.158 | 0.535 * | −0.492 |
| Glu | −0.110 | 0.550 * | −0.391 |
| Gly | 0.066 | 0.432 | −0.326 |
| Ala | −0.118 | 0.393 | −0.233 |
| Cys | 0.096 | 0.520 * | −0.286 |
| Val | 0.390 | 0.440 | −0.542 * |
| Met | −0.006 | 0.509 | 0.285 |
| Ile | 0.201 | 0.468 | −0.459 |
| Leu | 0.220 | 0.501 | −0.489 |
| Tyr | 0.215 | 0.444 | −0.426 |
| Phe | 0.203 | 0.517 * | −0.430 |
| Lys | 0.240 | 0.223 | −0.614 * |
| His | 0.201 | 0.513 | −0.474 |
| Arg | 0.291 | 0.353 | −0.550 * |
| TAA | 0.143 | 0.497 | −0.500 |
| Protein | 0.207 | 0.519 * | −0.537 * |
| Sugars | Correlation Coefficient ® | ||
|---|---|---|---|
| CML | CEL | Acrylamide | |
| fructose | 0.652 ** | 0.075 | 0.007 |
| glucose | 0.204 | 0.339 | −0.091 |
| sucrose | 0.115 | −0.312 | 0.102 |
| maltose | 0.332 | 0.123 | 0.309 |
| total sugar | 0.300 | −0.137 | 0.163 |
| Fatty Acids | Correlation Coefficient (r) | ||
|---|---|---|---|
| CML | CEL | Acrylamide | |
| SFA | −0.285 | −0.539 * | −0.363 |
| TUFA | −0.070 | −0.074 | 0.649 ** |
| MUFA | −0.009 | −0.357 | −0.068 |
| PUFA | −0.074 | 0.123 | 0.777 ** |
| n-3 | 0.374 | −0.065 | −0.079 |
| n-6 | −0.108 | 0.130 | 0.792 ** |
| TFA | −0.254 | −0.423 | 0.411 |
| n-6/n-3 | −0.418 | 0.237 | 0.429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Ma, Y.; Wang, Y.; Sun, C.; Chen, F.; Cheng, K.-W.; Liu, B. Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs. Foods 2023, 12, 1967. https://doi.org/10.3390/foods12101967
Fu S, Ma Y, Wang Y, Sun C, Chen F, Cheng K-W, Liu B. Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs. Foods. 2023; 12(10):1967. https://doi.org/10.3390/foods12101967
Chicago/Turabian StyleFu, Shuang, Yurong Ma, Yinan Wang, Chongzhen Sun, Feng Chen, Ka-Wing Cheng, and Bin Liu. 2023. "Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs" Foods 12, no. 10: 1967. https://doi.org/10.3390/foods12101967
APA StyleFu, S., Ma, Y., Wang, Y., Sun, C., Chen, F., Cheng, K.-W., & Liu, B. (2023). Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs. Foods, 12(10), 1967. https://doi.org/10.3390/foods12101967