Phenolic-Degrading Enzymes: Effect on Haze Active Phenols and Chill Haze in India Pale Ale Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes, Chemicals, and Wort Samples
2.2. Bradford Method
2.3. Laccase Activity Assay
2.4. Tannase Activity Assay
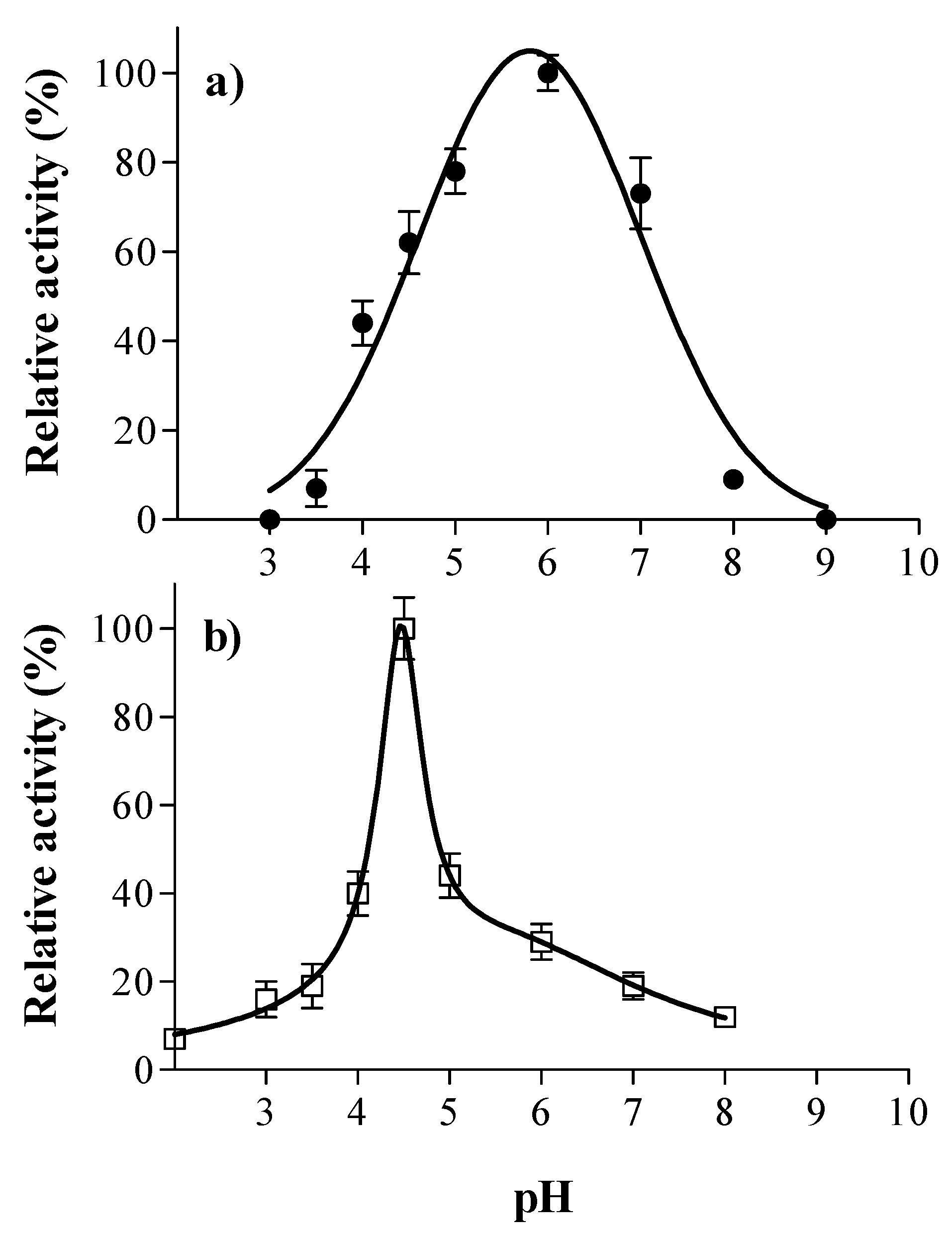
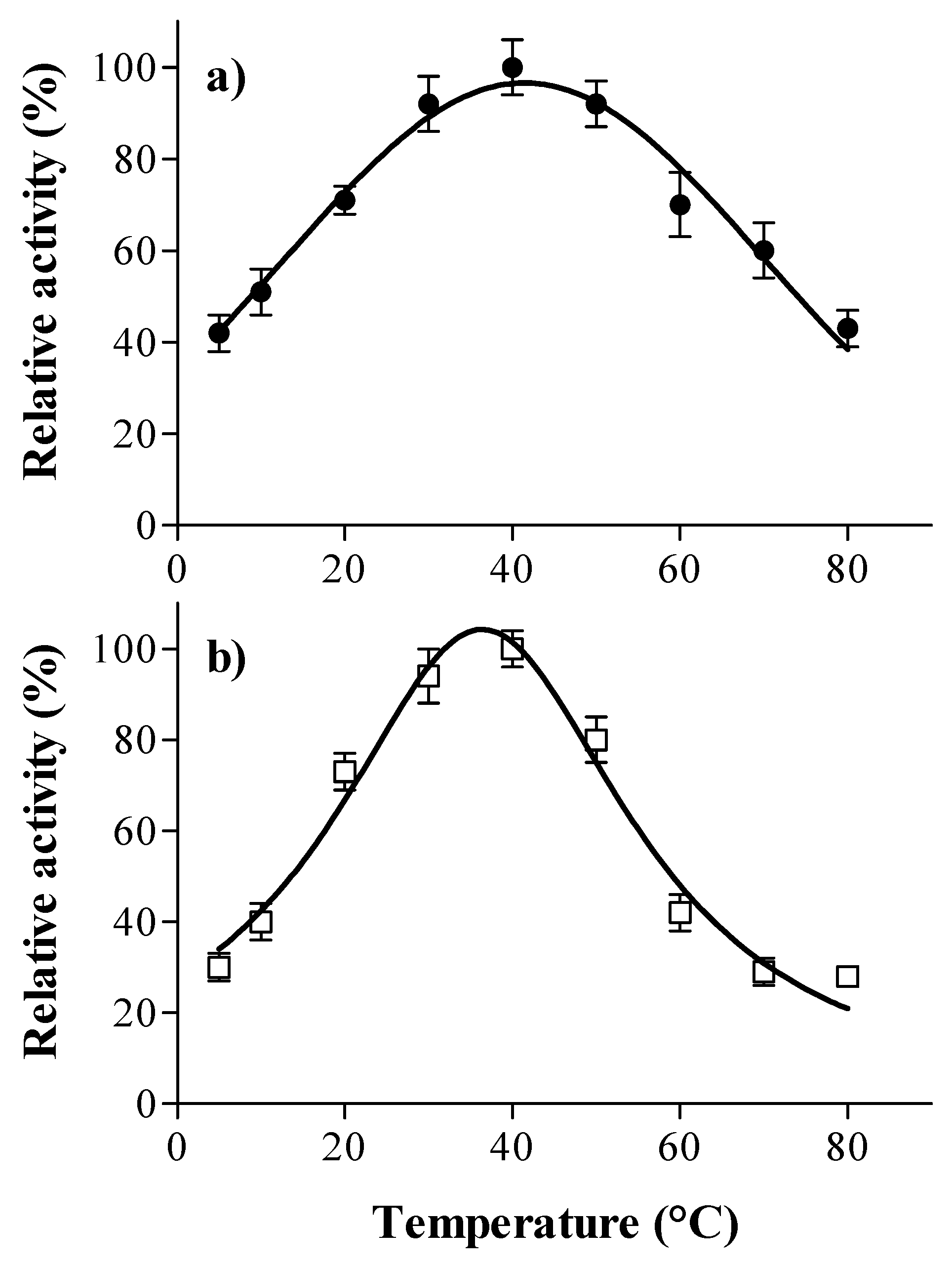
2.5. Optimal pH and Temperature
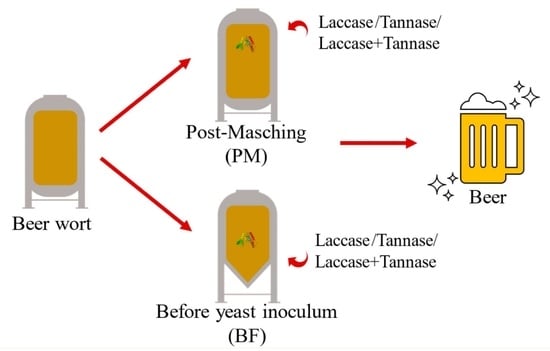
2.6. Enzymatic Treatment of Wort
2.7. Haze Active Phenols
2.8. Chill Haze
2.9. CIELAB Parameters and Browning Index
2.10. Statistical Analysis
3. Results and Discussion
3.1. Laccase and Tannase Activity: Optimal pH and Temperature
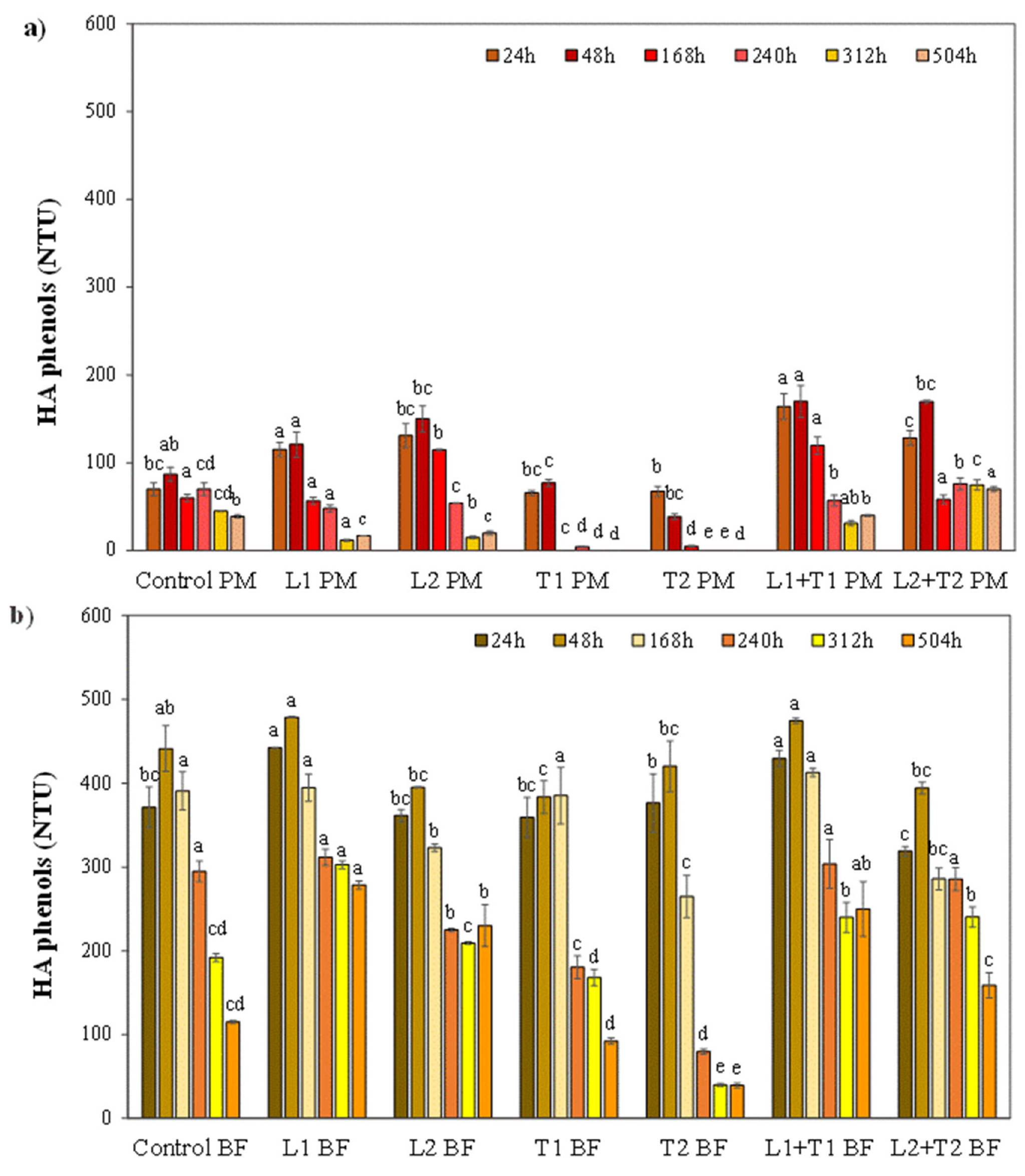
3.2. Effect of Enzymatic Treatment on the Haze Active Phenols and Haze Stability
3.2.1. The Fate of Haze Active Phenols from Malted Barley and Hop
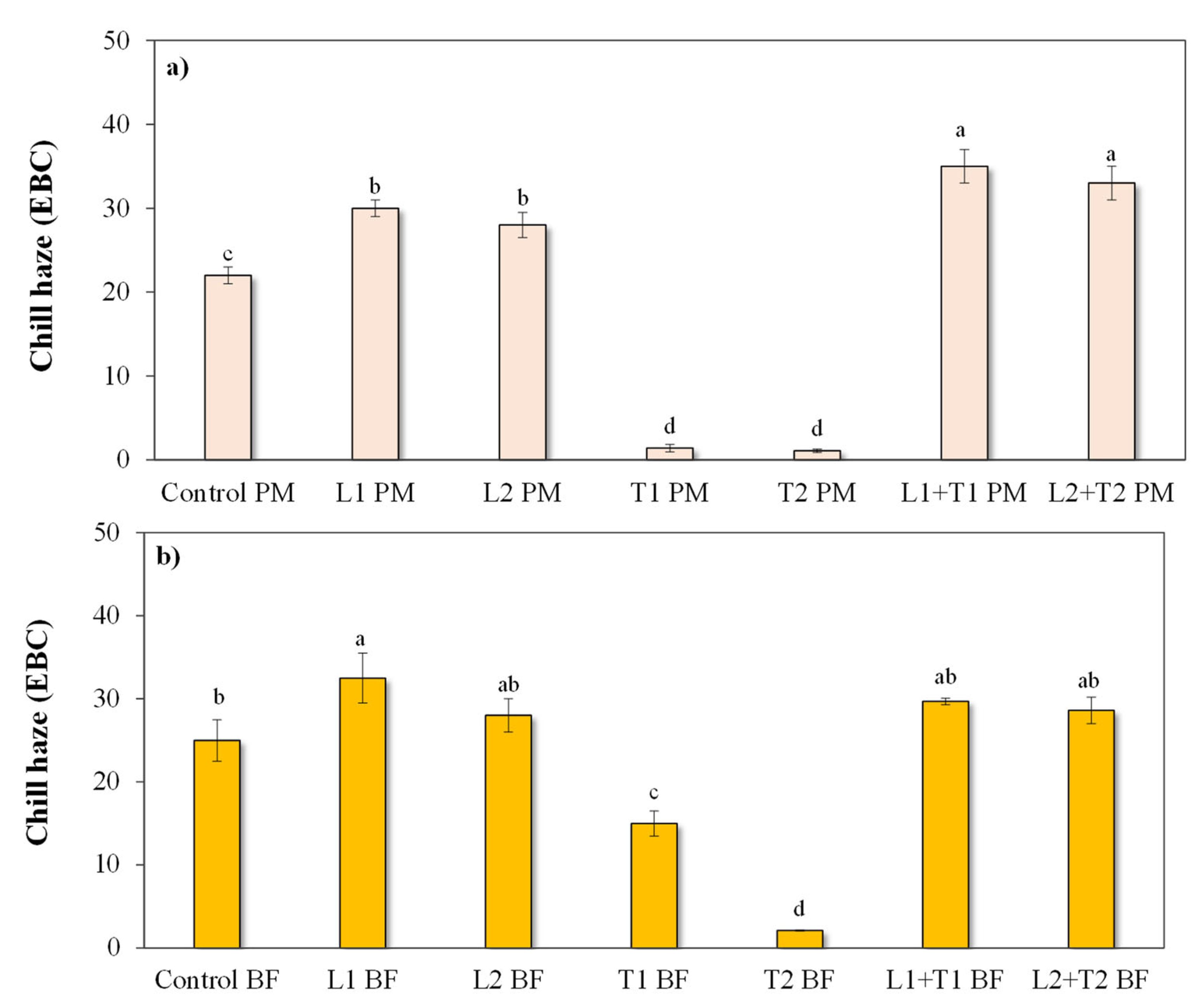
3.2.2. Chill Haze Behavior
3.3. Effect of Enzymatic Treatment on Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer–the importance of colloidal stability (non-biological haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Ciont, C.; Epuran, A.; Kerezsi, A.D.; Coldea, T.E.; Mudura, E.; Pasqualone, A.; Pop, O.L. Beer safety: New challenges and future trends within craft and large-scale production. Foods 2022, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Huang, Y.; Dai, F.; Ning, H.; Li, C.; Zhou, M.; Zhang, G. Identification of two key genes controlling chill haze stability of beer in barley (Hordeum vulgare L). BMC Genom. 2015, 16, 449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Siebert, K.J. Effects of protein− polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef]
- Jongberg, S.; Andersen, M.L.; Lund, M.N. Covalent Protein-Polyphenol Bonding as Initial Steps of Haze Formation in Beer. J. Am. Soc. Brew. Chem. 2020, 78, 153–164. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, L. Haze in Beer: Its Formation and Alleviating Strategies, from a Protein–Polyphenol Complex Angle. Foods 2021, 10, 3114. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A discussion of polyphenols in beer physical and flavour stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Inge, R.; Graham, S. Handbook of Alcoholic Beverages: Beer, a Quality Perspective; Elsevier: Burlington, MA, USA, 2009. [Google Scholar]
- European Parliament and Council. No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Chan, C.H. Food Allergen Labelling and Information Requirements under the EU Food Information for Consumers Regulation No. 1169/2011: Technical Guidance. Food Standards Agency. 2014. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20210108111734/https://www.food.gov.uk/sites/default/files/media/document/food-allergen-labelling-technical-guidance.pdf (accessed on 8 January 2021).
- Siebert, K.J.; Lynn, P.Y. Mechanisms of beer colloidal stabilization. J. Am. Soc. Brew. Chem. 1997, 55, 73–78. [Google Scholar] [CrossRef]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cerreti, M.; Liburdi, K.; Benucci, I.; Spinelli, S.E.; Lombardelli, C.; Esti, M. Optimization of pectinase and protease clarification treatment of pomegranate juice. LWT 2017, 82, 58–65. [Google Scholar] [CrossRef]
- Nunes, C.S.; Kunamneni, A. Laccases—Properties and applications. In Enzymes in Human and Animal Nutrition; Academic Press: Cambridge, MA, USA, 2018; pp. 133–161. [Google Scholar]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Uses of laccases in the food industry. Enzym. Res. 2010, 2010, 918761. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Rodríguez, R.; Gutiérrez-Sánchez, G.; Augur, C.; Favela-Torres, E.; Prado-Barragan, L.A.; Contreras-Esquivel, J.C. Microbial tannases: Advances and perspectives. Appl. Microbiol. Biotechnol. 2007, 76, 47–59. [Google Scholar] [CrossRef]
- Yao, J.; Guo, G.S.; Ren, G.H.; Liu, Y.H. Production, characterization and applications of tannase. J. Mol. Catal. 2014, 101, 137–147. [Google Scholar] [CrossRef]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Gomaa, A.M. Application of enzymes in brewing. J. Nutr. Food Sci. Forecast 2018, 1, 1–5. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grassin, C.; Dubourdieu, D. Optimisation de la méthode de dosage de l’activité laccase de Botrytis cinerea par la syringaldazine. OENO One 1986, 20, 125–130. [Google Scholar] [CrossRef]
- Manole, A.; Herea, D.; Chiriac, H.; Melnig, V. Laccase activity determination. Cuza Univ. Sci. Ann. Lasi 2008, 4, 17–24. [Google Scholar]
- Otto, B.; Schlosser, D. First laccase in green algae: Purification and characterization of an extracellular phenol oxidase from Tetracystis aeria. Planta 2014, 240, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Ire, F.S.; Nwanguma, A.C. Comparative evaluation on tannase production by Lasiodiplodia plurivora ACN-10 under submerged fermentation (SmF) and solid state fermentation (SSF). Asian J. Biotechnol. Bioresour. Technol. 2020, 6, 39–49. [Google Scholar] [CrossRef]
- Sharma, S.; Bhat, T.K.; Dawra, R.K. A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 2000, 279, 85–89. [Google Scholar] [CrossRef]
- Siebert, K.J.; Lynn, P.Y. Comparison of methods for measuring polyphenols in beer. J. Am. Soc. Brew. Chem. 2006, 64, 127–134. [Google Scholar] [CrossRef]
- Chapon, L. Nephelometry as a method for studying the relations between polyphenols and proteins. J. Inst. Brew. 1993, 99, 49–56. [Google Scholar] [CrossRef]
- Koren, D.; Hegyesné Vecseri, B.; Kun-Farkas, G.; Urbin, Á.; Nyitrai, Á.; Sipos, L. How to objectively determine the color of beer? J. Food Sci. Technol. 2020, 57, 1183–1189. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of browning in apples using colour and textural features by image analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Skoronski, E.; Souza, D.H.; Ely, C.; Broilo, F.; Fernandes, M.; Júnior, A.F.; Ghislandi, M.G. Immobilization of laccase from Aspergillus oryzae on graphene nanosheets. Int. J. Biol. Macromol. 2017, 99, 121–127. [Google Scholar] [CrossRef]
- Yin, Q.; Zhou, G.; Peng, C.; Zhang, Y.; Kües, U.; Liu, J.; Fang, Z. The first fungal laccase with an alkaline pH optimum obtained by directed evolution and its application in indigo dye decolorization. AMB Express 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases–occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Slomczynski, D.; Nakas, J.; Tanenbaum, S. Production and characterization of laccase from Botrytis cinerea 61-34. Appl. Environ. Microbiol. 1995, 61, 907–912. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Renuka, P.S.; Malini, S.; Veena, S.M. Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 248735. [Google Scholar] [CrossRef]
- Abdel-Naby, M.A.; Sherif, A.A.; El-Tanash, A.B.; Mankarios, A.T. Immobilization of Aspergillus oryzae tannase and properties of the immobilized enzyme. J. Appl. Microbiol. 1999, 87, 108–114. [Google Scholar] [CrossRef]
- Mizuno, T.; Shiono, Y.; Koseki, T. Biochemical characterization of Aspergillus oryzae native tannase and the recombinant enzyme expressed in Pichia pastoris. J. Biosci. Bioeng. 2014, 118, 392–395. [Google Scholar] [CrossRef]
- Belmares, R.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Coronel, A.R.; Aguilar, C.N. Microbial production of tannase: An enzyme with potential use in food industry. LWT 2004, 37, 857–864. [Google Scholar] [CrossRef]
- Rajakumar, S.; Nandy, S.C. Isolation, purification and some properties of Penicillium chrysogenum tannase. Appl. Environ. Microbiol. 1983, 46, 525–527. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Regerat, F.; Pourrat, H. Production, purification and characterization of a tannase from Aspergillus niger LCF 8. J. Ferment. Bioeng. 1994, 77, 320–323. [Google Scholar] [CrossRef]
- Ordoñez, R.M.; Colombo, I.; Alberto, M.R.; Isla, M.I. Production of tannase from wood-degrading fungus using as substrate plant residues: Purification and characterization. World J. Microbiol. Biotechnol. 2011, 27, 2325–2333. [Google Scholar] [CrossRef]
- Fortina, M.G.; Acquati, A.; Rossi, P.; Manachini, P.L.; Di Gennaro, C. Production of laccase by Botrytis cinerea and fermentation studies with strain F226. J. Ind. Microbiol. 1996, 17, 69–72. [Google Scholar] [CrossRef]
- Mukherjee, G.; Banerjee, R. Effects of temperature, pH and additives on the activity of tannase produced by a co-culture of Rhizopus oryzae and Aspergillus foetidus. World J. Microbiol. Biotechnol. 2006, 22, 207–212. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Guido, L.F. A review on the fate of phenolic compounds during malting and brewing: Technological strategies and beer styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef] [PubMed]
- Langos, D.; Granvogl, M. Studies on the simultaneous formation of aroma-active and toxicologically relevant vinyl aromatics from free phenolic acids during wheat beer brewing. J. Agric. Food Chem. 2016, 64, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review. Food Rev. Int. 2009, 26, 1–84. [Google Scholar] [CrossRef]
- Jurková, M.; Horák, T.; Hašková, D.; Čulík, J.; Čejka, P.; Kellner, V. Control of antioxidant beer activity by the mashing process. J. Inst. Brew. 2012, 118, 230–235. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzlé, M.; Zhao, M.; Didierjean, L.; Taidi, B.; Ennahar, S. Effects of processing steps on the phenolic content and antioxidant activity of beer. J. Agric. Food Chem. 2011, 59, 1249–1255. [Google Scholar] [CrossRef]
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of full-scale brewing process on polyphenols in Italian all-malt and maize adjunct lager beers. J. Food Compos. Anal. 2011, 24, 568–573. [Google Scholar] [CrossRef]
- Eissa, H.A.; Dahdooh, H.; Ibrahim, W.A.; El-Refai, H.A. Kinetic studies of Aspergillus ficuum tannase enzyme and its application in detannification of grape juice. Curr. Trends Biotechnol. Pharm. 2021, 15, 220–232. [Google Scholar]
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
| Sample Code | Enzyme Dosage (VE/L) |
|---|---|
| Control PM | - |
| L1 PM | 100 μL Laccase/L |
| L2 PM | 1 mL Laccase/L |
| T1 PM | 100 μL Tannase/L |
| T2 PM | 1 mL Tannase/L |
| L1 + T1 PM | 100 μL Laccase + 100 μL Tannase/L |
| L2 + T2 PM | 1 mL Laccase + 1 mL Tannase/L |
| Control BF | - |
| L1 BF | 100 μL Laccase/L |
| L2 BF | 1 mL Laccase/L |
| T1 BF | 100 μL Tannase/L |
| T2 BF | 1 mL Tannase/L |
| L1 + T1 BF | 100 μL Laccase + 100 μL Tannase/L |
| L2 + T2 BF | 1 mL Laccase + 1 mL Tannase/L |
| Sample | L* | a* | b* | ΔE | BI |
|---|---|---|---|---|---|
| Control PM | 83.5 ± 0.1 a | 4.2 ± 0.1 b | 18.7 ± 0.1 c | 0.00 | 28.7 ± 0.2 |
| L1 PM | 80.2 ± 0.1 ab | 4.3 ± 0.1 b | 22.0 ± 0.1 b | 6.7 ± 0.1 | 35.4 ± 0.1 |
| L2 PM | 77.4 ± 0.1 b | 7.6 ± 0.1 a | 31.0 ± 0.1 a | 14.2 ± 0.1 | 57.4 ± 0.2 |
| T1 PM | 87.2 ± 0.1 a | 3.2 ± 0.1 b | 14.7 ± 0.1 c | 5.6 ± 0.1 | 20.8 ± 0.1 |
| T2 PM | 85.4 ± 0.1 a | 2.0 ± 0.1 b | 19.1 ± 0.01 c | 2.9 ± 0.1 | 26.6 ± 0.1 |
| L1 + T1 PM | 80.6 ± 0.1 a | 6.3 ± 0.1 a | 20.6 ± 0.4 c | 6.1 ± 0.2 | 34.9 ± 0.1 |
| L2 + T2 PM | 81.7 ± 0.1 a | 6.2 ± 0.1 a | 27.3 ± 0.1 a | 8.9 ± 0.1 | 45.4 ± 0.1 |
| Control BF | 93.5 ± 0.1 a | −1.2 ± 0.1 c | 29.6 ± 0.1 b | 0.00 | 36.1 ± 0.1 |
| L1 BF | 86.4 ± 0.1 b | 4.5 ± 0.1 b | 37.7 ± 0.1 a | 12.2 ± 0.1 | 59.5 ± 0.1 |
| L2 BF | 83.2 ± 0.1 b | 6.4 ± 0.1 a | 39.6 ± 0.1 a | 16.3 ± 0.1 | 68.3 ± 0.1 |
| T1 BF | 93.3 ± 0.1 a | −1.3 ± 0.1 c | 29.8 ± 0.1 b | 0.3 ± 0.1 | 36.4 ± 0.2 |
| T2 BF | 93.7 ± 0.1 a | −1.1 ± 0.1 c | 30.0 ± 0.1 b | 0.4 ± 0.1 | 36.6 ± 0.1 |
| L1 + T1 BF | 87.9 ± 0.1 b | 3.4 ± 0.1 b | 35.4 ± 0.1 a | 9.3 ± 0.1 | 53.2 ± 0.7 |
| L2 + T2 BF | 84.0 ± 0.1 b | 6.4 ± 0.1 a | 40.3 ± 0.1 a | 16.1 ± 0.1 | 68.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Esti, M. Phenolic-Degrading Enzymes: Effect on Haze Active Phenols and Chill Haze in India Pale Ale Beer. Foods 2023, 12, 77. https://doi.org/10.3390/foods12010077
Benucci I, Mazzocchi C, Lombardelli C, Esti M. Phenolic-Degrading Enzymes: Effect on Haze Active Phenols and Chill Haze in India Pale Ale Beer. Foods. 2023; 12(1):77. https://doi.org/10.3390/foods12010077
Chicago/Turabian StyleBenucci, Ilaria, Caterina Mazzocchi, Claudio Lombardelli, and Marco Esti. 2023. "Phenolic-Degrading Enzymes: Effect on Haze Active Phenols and Chill Haze in India Pale Ale Beer" Foods 12, no. 1: 77. https://doi.org/10.3390/foods12010077
APA StyleBenucci, I., Mazzocchi, C., Lombardelli, C., & Esti, M. (2023). Phenolic-Degrading Enzymes: Effect on Haze Active Phenols and Chill Haze in India Pale Ale Beer. Foods, 12(1), 77. https://doi.org/10.3390/foods12010077