Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of GSH-CdTe QDs
2.3. Fluorescence Responses to H2O2 in Milk
2.4. Fluorescence Responses to MnO2 NSs
2.5. Preparation of Fluorescent Signal Probe (QDs-OVA)
2.6. Preparation of Fluorescent Quenching Probe (MnO2 NSs-Ab)
2.7. Preparation of Enrofloxacin Coating Antigen (ENR-OVA)
2.8. Detection Procedure
3. Results and Discussion
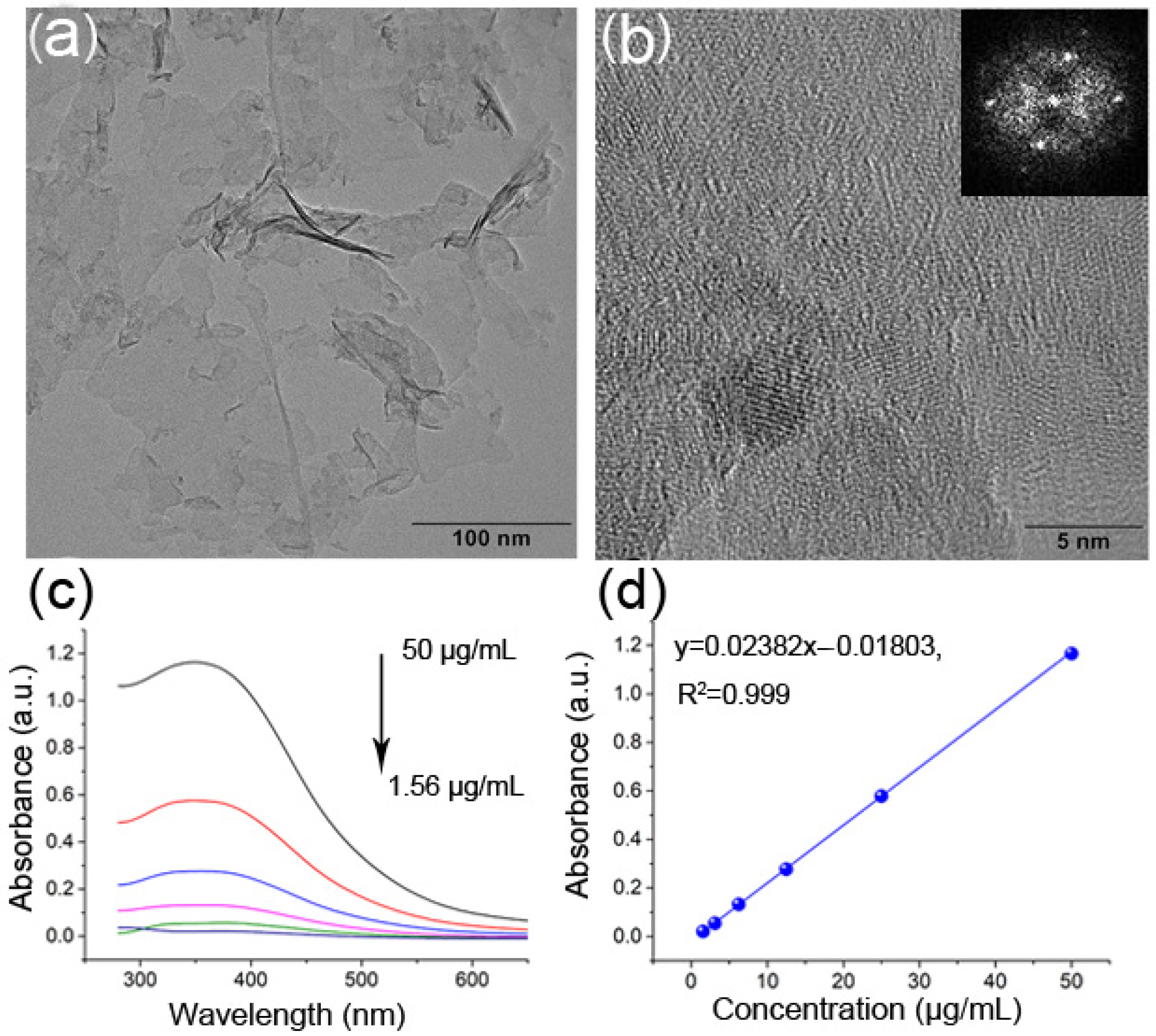
3.1. Characterization of GSH-CdTe QDs
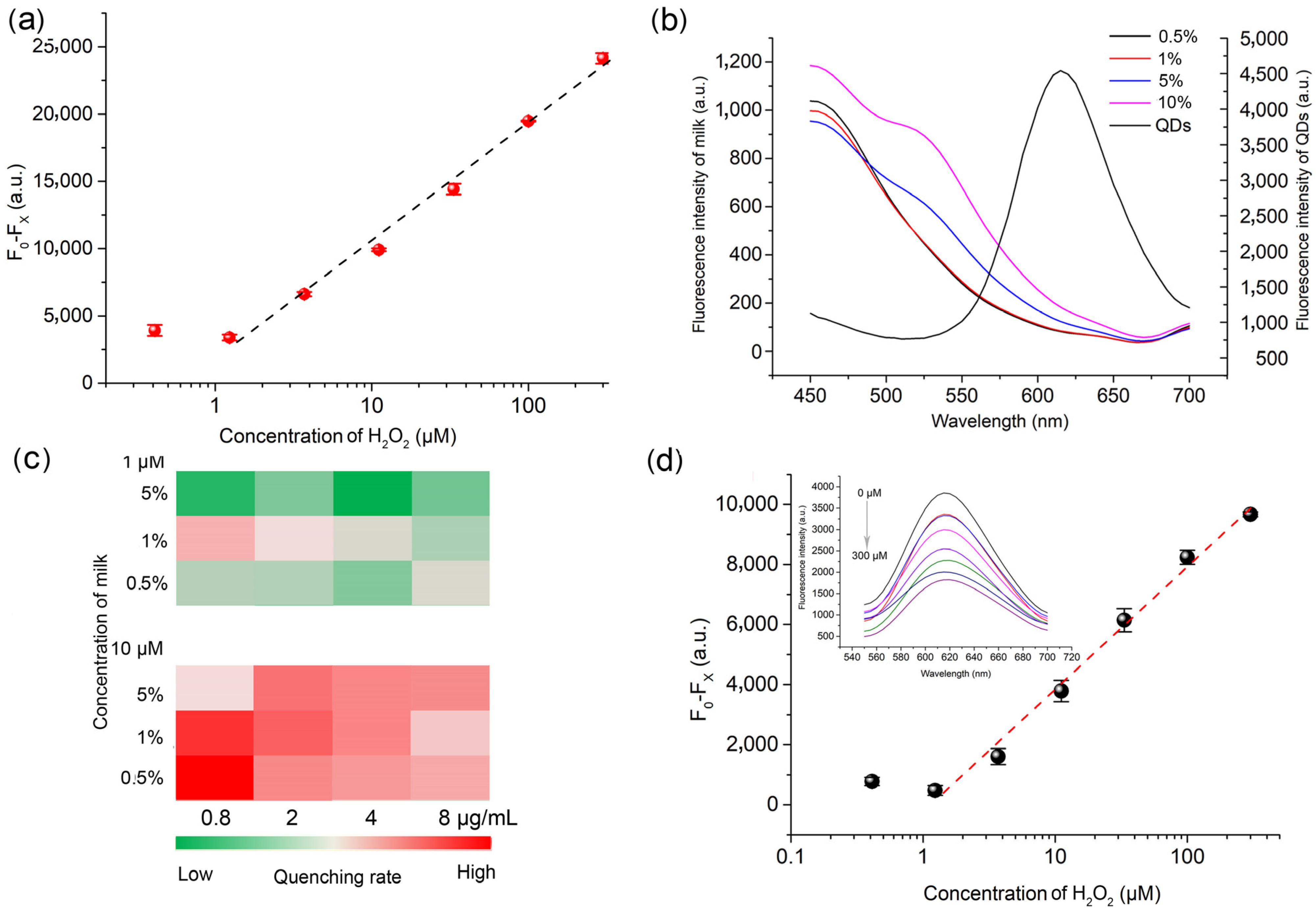
3.2. H2O2 Detection in Milk Powder
3.3. Characterization of MnO2 NSs
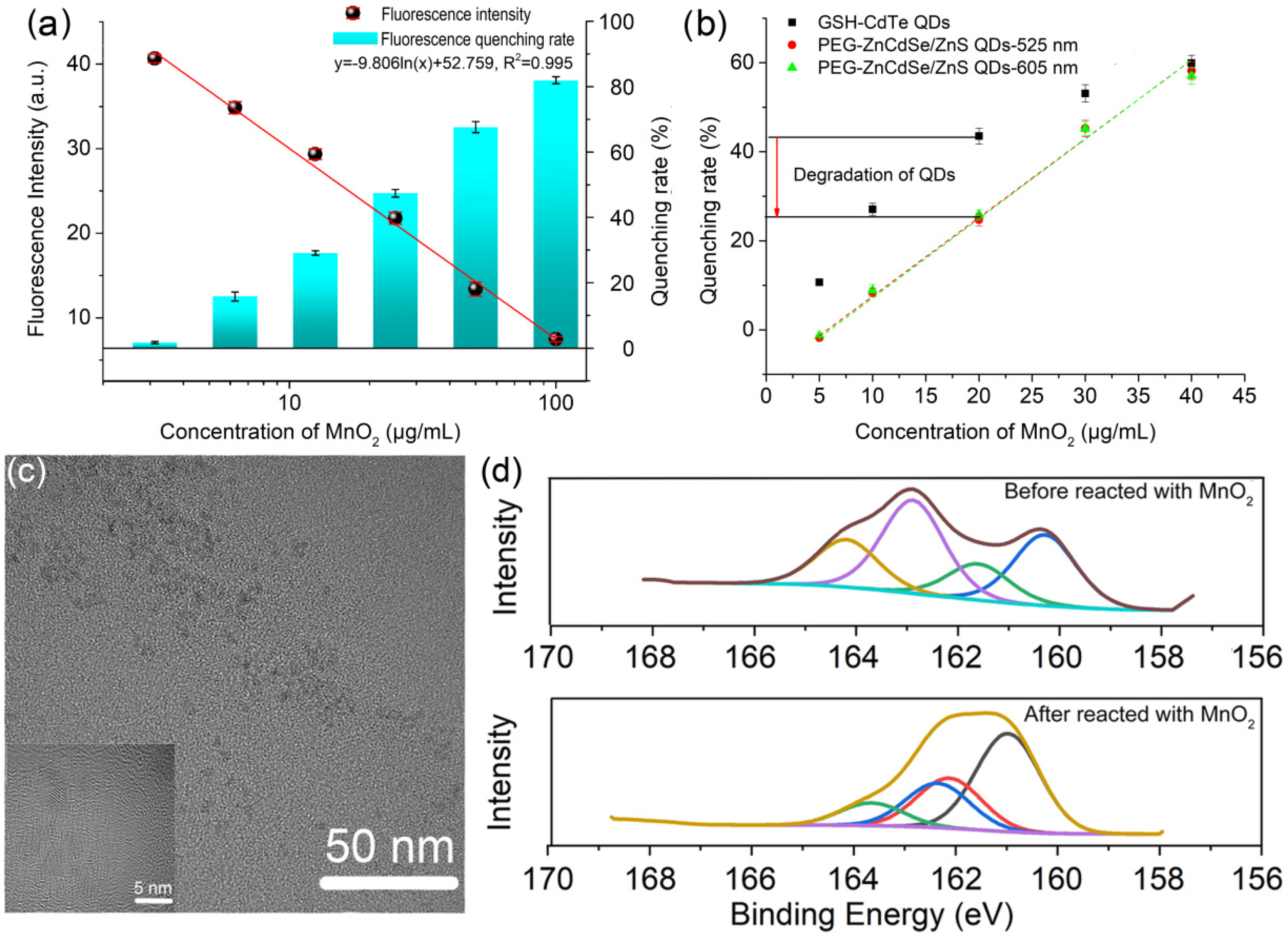
3.4. Feasibility Analysis of MnO2/GSH-CdTe QDs Fluorescent Sensing System
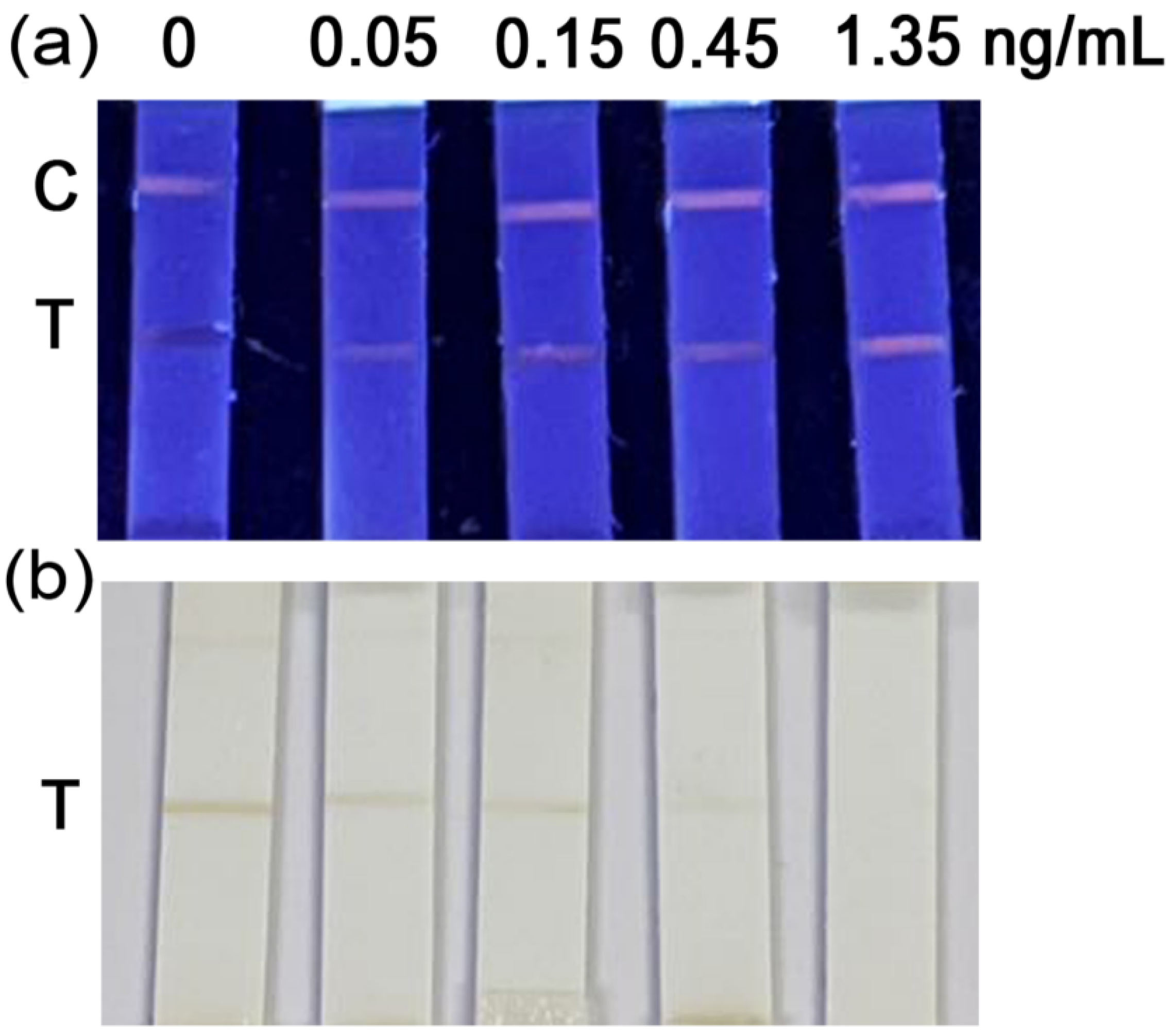
3.5. Establishment of Fluorescence Quenching Immunosensors (FQIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Li, H.; Shen, J.; Wu, Y. Nontargeted Detection Methods for Food Safety and Integrity. Annu. Rev. Food Sci. Technol. 2019, 10, 429–455. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, S.; Fang, B.; Zhang, G.; Lai, X.; Su, L.; He, W.; Lai, W. Lateral flow immunoassay based on polydopa-mine-coated metal–organic framework for the visual detection of enrofloxacin in milk. Anal. Bioanal. Chem. 2022, 414, 7315–7323. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Zhang, M.; You, F.; Hao, N.; Ding, C.; Wang, K. Simultaneous detection of enrofloxacin and ciprofloxacin in milk using a bias potentials controlling-based photoelectrochemical aptasensor. J. Hazard. Mater. 2021, 416, 125988. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.J.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a new dual electrochemical immunosensor for a rapid and sensitive detection of enrofloxacin in meat samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef]
- Gill, R.; Bahshi, L.; Freeman, R.; Willner, I. Optical Detection of Glucose and Acetylcholine Esterase Inhibitors by H2O2-Sensitive CdSe/ZnS Quantum Dots. Angew. Chem. 2008, 120, 1700–1703. [Google Scholar] [CrossRef]
- Lima, L.S.; Rossini, E.L.; Pezza, L.; Pezza, H.R. Bioactive paper platform for detection of hydrogen peroxide in milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117774. [Google Scholar] [CrossRef]
- Ren, H.-X.; Qian, Z.-J.; Li, M.; Peng, C.-F.; Wang, Z.-P.; Wei, X.-L.; Xu, J.-G. Mesoporous silica-loaded gold nanocluster with enhanced fluorescence and ratiometric fluorescent detection of thiram in foods. Mikrochim. Acta 2021, 188, 363. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Hassan, M.; Li, H.; Ouyang, Q.; Chen, Q. Fluorescence resonance energy transfer-based aptasensor for sensitive detection of kanamycin in food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120147. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-B.; Rau, J.-Y.; Chiou, H.-C.; Tseng, W.-L. Synthesis of gold nanoclusters-loaded lysozyme nanoparticles for rati-ometric fluorescent detection of cyanide in tap water, cyanogenic glycoside-containing plants, and soils. Environ. Res. 2022, 207, 112144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhong, H.; Zhao, P.; Shen, A.; Li, H.; Liu, X. Carbon quantum dot fluorescent probes for food safety detection: Progress, opportunities and challenges. Food Control. 2022, 133, 108591. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Lin, X.; Pan, X.; Wu, Z.; Gai, H. Digital Duplex Homogeneous Immunoassay by Counting Immunocomplex Labeled with Quantum Dots. Anal. Chem. 2021, 93, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.O.; Mosquera, M.A.; Jiang, Y.; Weiss, E.A.; Schatz, G.C.; Ratner, M.A. Thermodynamics and Mechanism of a Photocatalyzed Stereoselective [2 + 2] Cycloaddition on a CdSe Quantum Dot. J. Am. Chem. Soc. 2020, 142, 15488–15495. [Google Scholar] [CrossRef]
- Zhou, W.; Coleman, J.J. Semiconductor quantum dots. Curr. Opin. Solid State Mater. Sci. 2016, 20, 352–360. [Google Scholar] [CrossRef]
- Jiao, X.; Zhou, Y.; Zhao, D.; Pang, D.; Wang, C.; Du, H.; Wen, Y.; Zhang, X. An indirect ELISA-inspired dual-channel fluorescent immunoassay based on MPA-capped CdTe/ZnS QDs. Anal. Bioanal. Chem. 2019, 411, 5437–5444. [Google Scholar] [CrossRef]
- Zhu, Y.; Chao, J.; Zhu, F.; Zhu, N.; Zhang, Q.; Gyimah, E.; Yakubu, S.; Zou, Y.; Zhang, Z. Ratiometric fluorescence immunoassay based on FAM-DNA–functionalized CdSe/ZnS QDs for the sensitive detection of tetrabromobisphenol A in foodstuff and the environment. Anal. Bioanal. Chem. 2020, 412, 3605–3613. [Google Scholar] [CrossRef]
- Dou, X.; Dai, H.; Twardowska, I.; Wei, S. Hyperaccumulation of Cd by Rorippa globosa (Turcz.) Thell. from soil enriched with different Cd compounds, and impact of soil amendment with glutathione (GSH) on the hyperaccumulation efficiency. Environ. Pollut. 2019, 255, 113270. [Google Scholar] [CrossRef]
- Qian, H.; Dong, C.; Weng, J.; Ren, J. Facile One-Pot Synthesis of Luminescent, Water-Soluble, and Biocompatible Glutathione-Coated CdTe Nanocrystals. Small 2006, 2, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Yu, J.-S. Selective synthesis of CdTe and high luminescence CdTe/CdS quantum dots: The effect of ligands. J. Colloid Interface Sci. 2009, 333, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, S.G.; Liang, J.Y.; Li, N.B.; Luo, H.Q. Free-label dual-signal responsive optical sensor by combining resonance Rayleigh scattering and colorimetry for sensitive detection of glutathione based on ultrathin MnO2 nanoflakes. Sensors Actuators B Chem. 2019, 288, 195–201. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Pan, S.; Liu, H.; Hu, X. A novel “off-on-off” fluorescent-nanoprobe based on B, N co-doped carbon dots and MnO2 nanosheets for sensitive detection of GSH and Ag+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 244, 118831. [Google Scholar] [CrossRef]
- Fan, D.; Shang, C.; Gu, W.; Wang, E.; Dong, S. Introducing Ratiometric Fluorescence to MnO2 Nanosheet-Based Biosensing: A Simple, Label-Free Ratiometric Fluorescent Sensor Programmed by Cascade Logic Circuit for Ultrasensitive GSH Detection. ACS Appl. Mater. Interfaces 2017, 9, 25870–25877. [Google Scholar] [CrossRef]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO 2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef]
- Zhang, S.; Sunami, Y.; Hashimoto, H. Mini Review: Nanosheet Technology towards Biomedical Application. Nanomaterials 2017, 7, 246. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horizons 2018, 4, 321–338. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, J.; Zhang, K.; Zhu, H.; Yu, H.; Sun, M.; Huang, D.; Wang, S. Visualizing Gaseous Nitrogen Dioxide by Ratiometric Fluorescence of Carbon Nanodots–Quantum Dots Hybrid. Anal. Chem. 2015, 87, 2087–2093. [Google Scholar] [CrossRef]
- Gendloff, E.; Casale, W.; Ram, B.; Tai, J.; Pestka, J.; Hart, L. Hapten-protein conjugates prepared by the mixed anhydride method: Cross-reactive antibodies in heterologous antisera. J. Immunol. Methods 1986, 92, 15–20. [Google Scholar] [CrossRef]
- Li, H.; Lu, W.; Song, B.; Zhou, J.; Zhao, G.; Han, G. The design of Mn2+&Co2+ co-doped CdTe quantum dot sensitized solar cells with much higher efficiency. RSC Adv. 2020, 10, 35701–35708. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Sharmoukh, W. Intrinsic catalase-mimicking MOFzyme for sensitive detection of hydrogen peroxide and ferricions. Microchem. J. 2021, 163, 105873. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Chen, Z.; Wang, C.; Liu, C.; Yang, Y.; Wu, Z.; Song, Y. CeO2 quantum dots modified electrode for detecting hydrogen peroxide. Inorg. Chem. Commun. 2019, 101, 62–68. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Liu, Y.; Wang, Y.; Tang, J. Catalase-inorganic hybrid microflowers modified glassy carbon electrode for amperometric detection of hydrogen peroxide. Mater. Lett. 2019, 243, 9–12. [Google Scholar] [CrossRef]
- Ragavan, K.; Ahmed, S.R.; Weng, X.; Neethirajan, S. Chitosan as a peroxidase mimic: Paper based sensor for the detection of hydrogen peroxide. Sensors Actuators B Chem. 2018, 272, 8–13. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Heterostructural Graphene Quantum Dot/MnO2 Nanosheets toward High-Potential Window Electrodes for High-Performance Supercapacitors. Adv. Sci. 2018, 5, 1700887. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. The Copper(II)-Catalyzed Oxidation of Glutathione. Chem. A Eur. J. 2016, 22, 15937–15944. [Google Scholar] [CrossRef]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical Instability of CdSe Nanocrystals Coated by Hydrophilic Thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef]
- Sheng, W.; Li, S.; Liu, Y.; Wang, J.; Zhang, Y.; Wang, S. Visual and rapid lateral flow immunochromatographic assay for enrofloxacin using dyed polymer microspheres and quantum dots. Microchim. Acta 2017, 184, 4313–4321. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Mu, X.; Sheng, W.; Wang, J.; Wang, S. Two fluorescence quenching immunochromatographic assays based on carbon dots and quantum dots as donor probes for the determination of enrofloxacin. Anal. Methods 2019, 11, 2378–2384. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wen, W.; Sheng, W.; Wang, J.; Wang, S.; Wang, J. A high-sensitivity thermal analysis immunochromatographic sensor based on au nanoparticle-enhanced two-dimensional black phosphorus photothermal-sensing materials. Biosens. Bioelectron. 2019, 133, 223–229. [Google Scholar] [CrossRef] [PubMed]


| Materials | Synthetic Conditions | Methods | Wavelength | LOD | Detection Range | Linear/Logarithmic Relationship | Ref. |
|---|---|---|---|---|---|---|---|
| Ce-MOF | Solvothermal, 85 °C for 16 h | Fluorescence | λex at 356 nm, λem at 540 nm | 10 µM | 200–1500 µM | Linear | [32] |
| CeO-QDs | Calcined at 900 °C for 3 h, stirring for 24 h in water and reacted at 120 °C for a further 6 h. | GCE | —— | 26.5 µM | 294 μM–1.47 mM | Linear | [33] |
| Cat-HMFs | ultrasonication at 25 °C for 30 min | GCE | —— | 50 µM | 100 μM–3 mM | Linear | [34] |
| Chitosan | Waterthermal, 60 °C for 30 min | Colorimetric | λAb = 652 nm | 1.55 µM | 10 μM–10 mM | Logarithmic | [35] |
| QDs | Waterthermal, refluxing for 2 h | Fluorescence | λex at 365 nm, λem at 610 nm | 0.61 µM | 1.23–300 µM | Logarithmic | This work |
| Sample | Conc. (ng/g(mL)) | Detection Results (n = 3) | Observed Results | ELISA Kits (n = 3) (ng/g(mL)) |
|---|---|---|---|---|
| Tap water | 0 | −; −; − a | 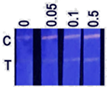 | ND b |
| 0.05 | +; + c; ± d | ND | ||
| 0.1 | +; +; + | ND | ||
| 0.5 | +; +; + | 0.47 ± 0.04 | ||
| Milk | 0 | −; −; − | 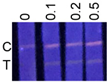 | ND |
| 0.1 | +; +; ± | ND | ||
| 0.2 | +; +; + | ND | ||
| 0.5 | +; +; + | 0.42 ± 0.07 | ||
| Crucian carp | 0 | −; −; ± | 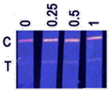 | ND |
| 0.25 | +; +; + | ND | ||
| 0.5 | +; +; + | 0.39 ± 0.08 | ||
| 1 | +; +; + | 0.86 ± 0.11 |
| Signal Type | Signal Element | Detection Limit (ng/mL) | Ref. |
|---|---|---|---|
| Colorimetric | AuNPs | 5 | [39] |
| Black phosphorus-Au nanocomposite | 0.5 | [41] | |
| Fluorescence | Dyed polymer microsphere | 1 | [39] |
| PEG-QDs | 1 | [39] | |
| Fluorescence quenching | AuNPs/PEG-QDs | 0.25 | [40] |
| AgNPs/carbon dots(CDs) | 0.1 | [40] | |
| Chemical degradation + fluorescence quenching | MnO2 NSs/GSH-QDs | 0.05 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Nie, L.; Han, L.; Wen, W.; Wang, J.; Wang, S. Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples. Foods 2023, 12, 62. https://doi.org/10.3390/foods12010062
Li S, Nie L, Han L, Wen W, Wang J, Wang S. Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples. Foods. 2023; 12(1):62. https://doi.org/10.3390/foods12010062
Chicago/Turabian StyleLi, Shijie, Linqing Nie, Lin Han, Wenjun Wen, Junping Wang, and Shuo Wang. 2023. "Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples" Foods 12, no. 1: 62. https://doi.org/10.3390/foods12010062
APA StyleLi, S., Nie, L., Han, L., Wen, W., Wang, J., & Wang, S. (2023). Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples. Foods, 12(1), 62. https://doi.org/10.3390/foods12010062






