Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Instruments
2.2. Sample Handling
2.2.1. Sample Pretreatment
2.2.2. Sample Digestion
2.3. Determination of Trace Elements
2.4. Data Processing
3. Results
3.1. Determination of Nine Traces Mineral Elements in Buckwheat Flour
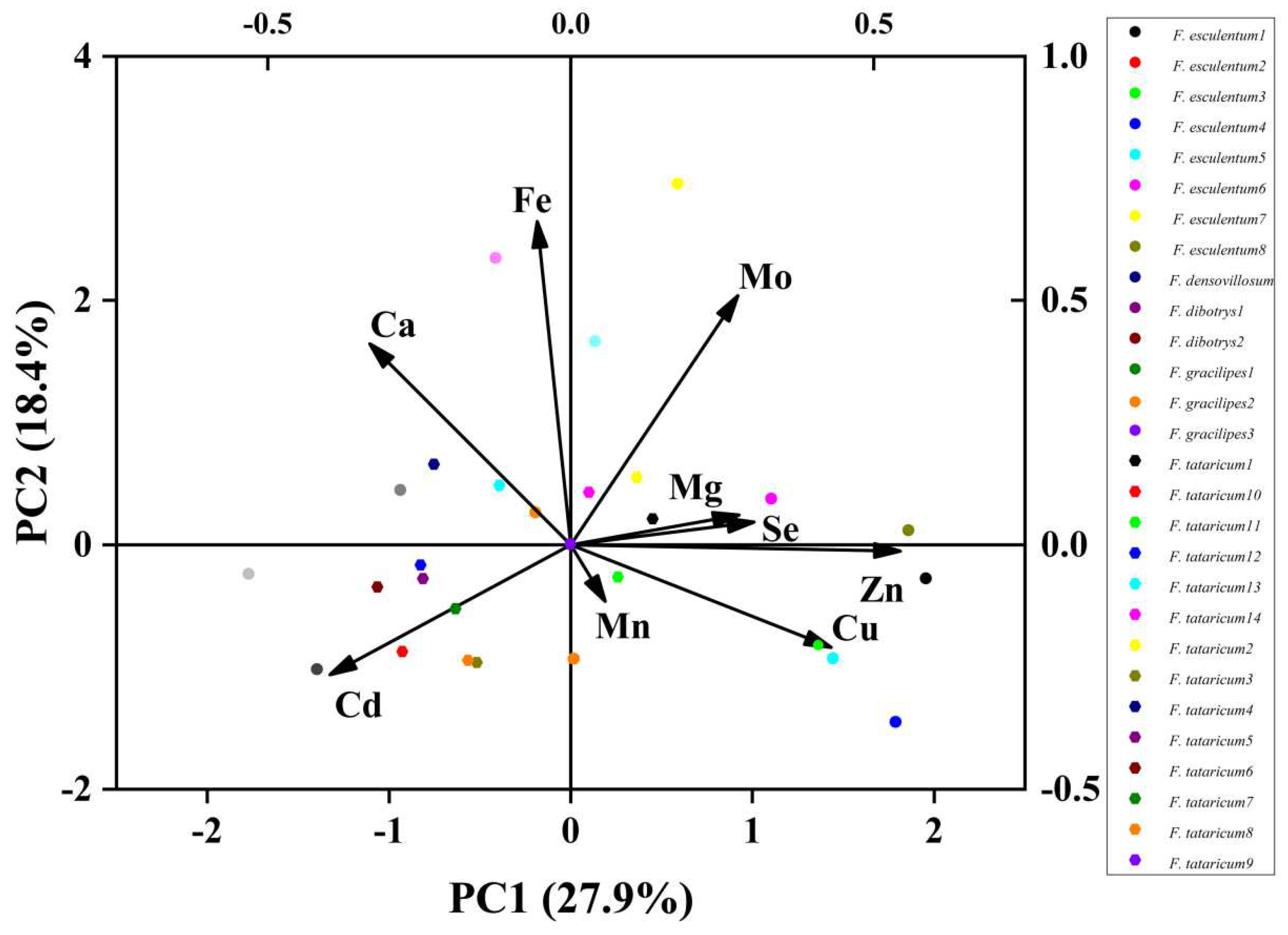
3.2. Principal Component Analysis
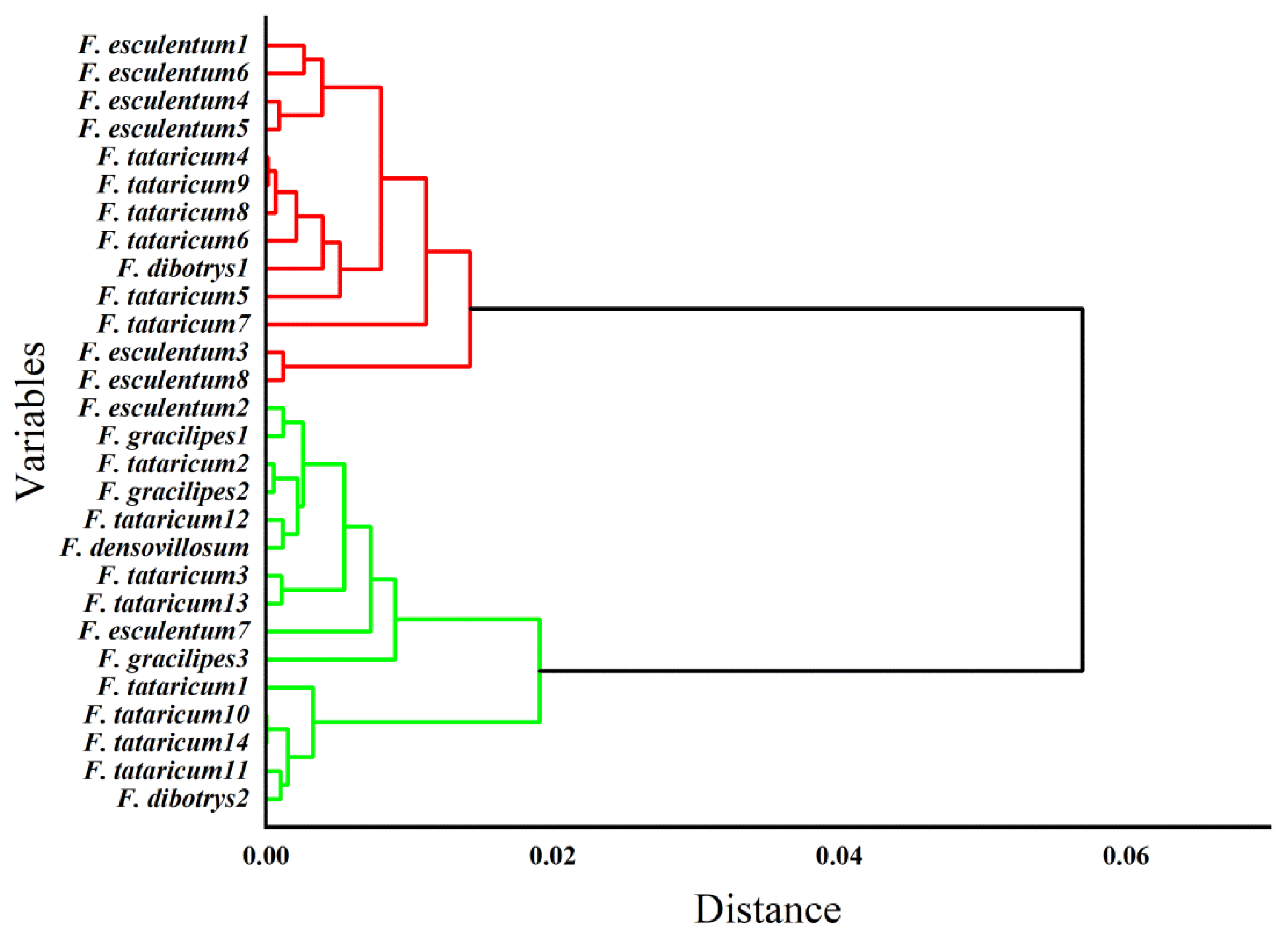
3.3. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary Buckwheat in Human Nutrition. Plants 2021, 10, 700. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Zhang, L.L.; He, Y.; Sheng, F.; Hu, Y.F.; Song, Y.; Li, W.; Chen, J.; Zhang, J.; Zou, L. Towards a better understanding of Fagopyrum dibotrys: A systematic review. Chin. Med. 2021, 16, 89. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, M.; Ruan, J.; Peng, Y.; Ma, C.; Wu, W.; Gao, A.; Weng, W.; Cheng, J. Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Int. J. Mol. Sci. 2022, 23, 10698. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Jeromel, L.; Vavpetič, P.; Pelicon, P.; Kaulich, B.; Gianoncelli, A.; Eichert, D.; Regvar, M.; Kreft, I. Spatially resolved distributions of the mineral elements in the grain of tartary buckwheat (Fagopyrum tataricum). Food Res. Int. 2013, 54, 125–131. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.J.; Huang, C.F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, C.; Wang, K.; Zhao, J.; Shao, J.; Chen, H.; Zhou, M.; Zhu, X. Metal Tolerance Protein Encoding Gene Family in Fagopyrum tartaricum: Genome-Wide Identification, Characterization and Expression under Multiple Metal Stresses. Plants 2022, 11, 850. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Yuan, Y.; Xu, Q.; Chen, P. Transcriptome profiling of Fagopyrum tataricum leaves in response to lead stress. BMC Plant Biol. 2020, 20, 54. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Yan, J.; Zhou, M.; Woo, S.H.; Weng, W.; Cheng, J.; Zhang, K. Tartary buckwheat: An under-utilized edible and medicinal herb for food and nutritional security. Food Rev. Int. 2022, 38, 440–454. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Potisek, M.; Kovačec, E.; Budič, B.; Kump, P.; Regvar, M.; Kreft, I. Mineral and trace element composition and importance for nutritional value of buckwheat grain, groats, and sprouts. In Molecular Breeding and Nutritional Aspects of Buckwheat; Academic Press: Cambridge, MA, USA, 2016; pp. 261–271. [Google Scholar]
- Huang, X.Y.; Zeller, F.J.; Huang, K.F.; Shi, T.X.; Chen, Q.F. Variation of major minerals and trace elements in seeds of tartary buckwheat (Fagopyrum tataricum Gaertn.). Genet. Res. Crop Evo. 2014, 61, 567–577. [Google Scholar] [CrossRef]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. Int. J. Mol. Sci. 2022, 23, 3923. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.; Pestorić, M.; Mišan, A.; Nedeljković, N.; Jambrec, D.; Jovanov, P.; Banjac, V.; Torbica, A.; Hadnađev, M.; Mandić, A. Antioxidant Capacity, Mineral Content and Sensory Properties of Gluten-Free Rice and Buckwheat Cookies. Food Technol. Biotechnol. 2015, 53, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Krejpcio, Z. Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J. Food Sci. Technol. 2014, 51, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Fan, X.; Teng, C.; Li, Y.; Everaert, N.; Blecker, C. The Beneficial Effect of Coarse Cereals on Chronic Diseases through Regulating Gut Microbiota. Foods 2021, 10, 2891. [Google Scholar] [CrossRef] [PubMed]
- Collumb, C.J.; Delelegn, A.A.; Fernandez, G.M.; Hudson, A.C.; Kimberley, K.W.; Sims, D.B.; Walton, D.J. Trace Elements in Gluten-free Pastas and Flours from Markets Located in the Las Vegas, Nevada Area. J. Food Res. 2019, 8, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, E.; Kaminski, W. Dynamics modeling of multicomponent metal ions’ removal onto low-cost buckwheat hulls. Environ. Sci. Pollut. Res. Int. 2021, 28, 46504–46513. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J.; Grygier, A.; Muzsik-Kazimierska, A.; Rudzińska, M.; Gramza- Michałowska, A. Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats. Molecules 2022, 27, 4394. [Google Scholar] [CrossRef]
- Duliński, R.; Zdaniewicz, M.; Pater, A.; Poniewska, D.; Żyła, K. The Impact of Phytases on the Release of Bioactive Inositols, the Profile of Inositol Phosphates, and the Release of Selected Minerals in the Technology of Buckwheat Beer Production. Biomolecules 2020, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Kasar, C.; Thanushree, M.P.; Gupta, S.; Inamdar, A.A. Milled fractions of common buckwheat (Fagopyrum esculentum) from the Himalayan regions: Grain characteristics, functional properties and nutrient composition. J. Food Sci. Technol. 2021, 58, 3871–3881. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Huang, Y.; Jiang, Y.; Liu, Y.; Wen, W.; Li, H.; Shao, J.; Wang, C.; Zhu, X. Antioxidant capacity, metal contents, and their health risk assessment of tartary buckwheat teas. Acs Omega 2020, 5, 9724–9732. [Google Scholar] [CrossRef]
- Dogra, D.; Awasthi, C.P. Variation of major minerals and trace elements in grains of some tatary buckwheat (Fagopyrum tataricum gaertn.) grown in Himachal Pradesh. Prog. Agric. 2018, 18, 219–223. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.G. Determining the geographical origin of common buckwheat from China by multivariate analysis based on mineral elements, amino acids and vitamins. Sci. Rep. 2017, 7, 9696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowicz, R.; Biel, W. A novel method for analyzing mineral ratio profiles of treated buckwheat sprouts (Fagopyrum esculentum Moench). J. Food Compos. Anal. 2022, 114, 104800. [Google Scholar] [CrossRef]
- Golijan, J.M.; Lekić, S.S.; Dojčinović, B.P.; Dramićanin, A.M.; Milinčić, D.D.; Pešić, M.B.; Barać, M.B.; Kostić, A.Ž. Mineral and nutritional assessments of soybean, buckwheat, spelt, and maize grains grown conventionally and organically. Int. Food Res. J. 2022, 29, 646–658. [Google Scholar] [CrossRef]
- Çelik, S.A.; Ayran, İ.; Asuman, K.A.N. Elemental characterization of buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Int. J. Agric. Environ. Food Sci. 2018, 2, 190–192. [Google Scholar] [CrossRef]
- Jiang, Y.; El Mehdawi, A.F.; Tripti Lima, L.W.; Stonehouse, G.; Fakra, S.C.; Hu, Y.; Qi, H.; Pilon-Smits, E. Characterization of Selenium Accumulation, Localization and Speciation in Buckwheat-Implications for Biofortification. Front. Plant Sci. 2018, 9, 1583. [Google Scholar] [CrossRef]
- Aubert, L.; Decamps, C.; Jacquemin, G.; Quinet, M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants 2021, 10, 258. [Google Scholar] [CrossRef]
- Kara, D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Wang, H.W.; Liu, Y.Q. Evaluation of trace and toxic element concentrations in Paris polyphylla from China with empirical and chemometric approaches. Food Chem. 2010, 121, 887–892. [Google Scholar] [CrossRef]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borovaya, S.; Klykov, A.; Barsukova, E.; Chaikina, E. Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro. Plants 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Ozyazici, G.; Turan, N. Effect of Vermicompost Application on Mineral Nutrient Composition of Grains of Buckwheat (Fagopyrum esculentum M.). Sustainability 2021, 13, 6004. [Google Scholar] [CrossRef]
- Klepacka, J.; Najda, A.; Klimek, K. Effect of Buckwheat Groats Processing on the Content and Bioaccessibility of Selected Minerals. Foods 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed]
| Instrument Index | Working Conditions |
|---|---|
| Incident power/W | 1300 |
| Auxiliary air flow/(L·min−1) | 1.3 |
| Aperture of sampling cone/mm 1.0 | 1.0 |
| Plasma gas flow/(L/min) | 15.0 |
| Vacuum degree of analysis chamber/Pa | 4.54 × 10−4 |
| Sample lifting rate/ (r ·s−1) | 0.3 |
| Dwell time/ms | 10 |
| Cooling gas flow/(L min−1) | 13.0 |
| Atomizing gas flow/(L min−1) | 0.9 |
| Aperture of intercepted cone/mm | 0.6 |
| Atomizing chamber temperature/°C | 2 |
| Measurement method | Jump peak |
| Analysis time/s | 45 |
| Resolution ratio/amu | 0.7 |
| Carrier gas flow (L/min) | 1.0 |
| Integration time of each element/ms | 300 |
| Trace Element Name | Principal Components | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Mg | 0.752 | 0.023 | 0.191 | 0.168 |
| Ca | −0.257 | −0.257 | 0.796 | −0.028 |
| Fe | 0.145 | 0.252 | 0.873 | 0.028 |
| Zn | 0.647 | 0.136 | −0.064 | 0.600 |
| Mn | 0.177 | −0.171 | 0.081 | 0.881 |
| Cu | 0.838 | 0.082 | −0.306 | −0.046 |
| Se | −0.211 | 0.536 | −0.205 | 0.472 |
| Mo | 0.097 | 0.811 | 0.147 | −0.136 |
| Cd | −0.106 | −0.775 | 0.059 | 0.001 |
| Eigenvalue | 2.39 | 1.60 | 1.57 | 1.04 |
| Variance contribution | 26.60 | 17.81 | 17.47 | 11.58 |
| Cumulative variance contribution rate | 26.60 | 44.41 | 61.88 | 73.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Gou, J.; Zhang, K.; Ruan, J. Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour. Foods 2023, 12, 225. https://doi.org/10.3390/foods12010225
Zhao M, Gou J, Zhang K, Ruan J. Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour. Foods. 2023; 12(1):225. https://doi.org/10.3390/foods12010225
Chicago/Turabian StyleZhao, Mengyu, Junbo Gou, Kaixuan Zhang, and Jingjun Ruan. 2023. "Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour" Foods 12, no. 1: 225. https://doi.org/10.3390/foods12010225
APA StyleZhao, M., Gou, J., Zhang, K., & Ruan, J. (2023). Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour. Foods, 12(1), 225. https://doi.org/10.3390/foods12010225






