Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
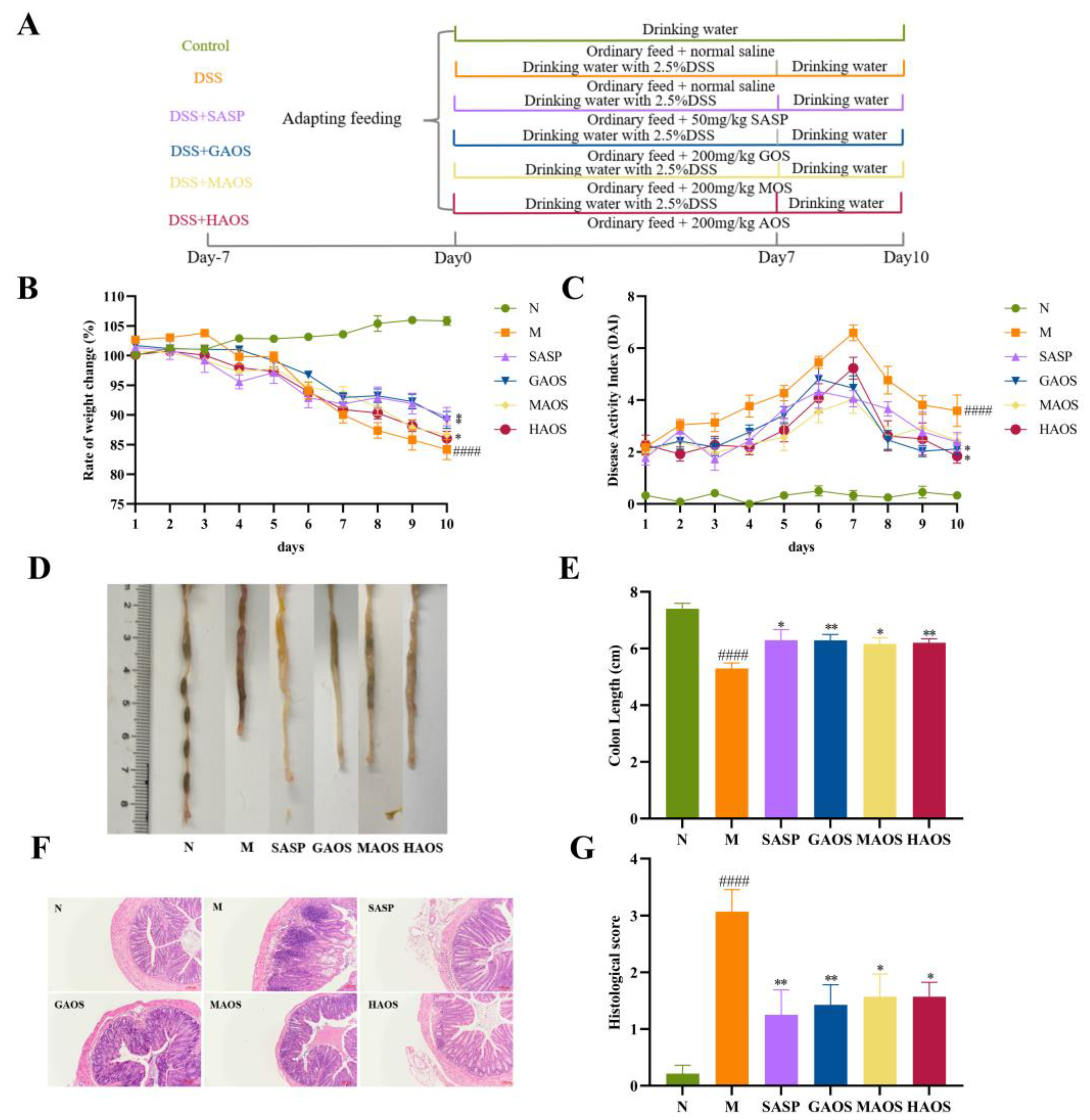
2.2. Animals and Experimental Design
2.3. Assessment of Colitis
2.4. Histological Analysis
2.5. Biochemical Analysis
2.6. Western Blotting Analysis
2.7. Short-Chain Fatty Acids (SCFAs) Detection
2.8. 16S rRNA Gene Sequencing
2.9. Statistical Analysis
3. Results
3.1. Alginate Oligosaccharides Treatment Alleviates the Histopathological Damage of Colitis Mice
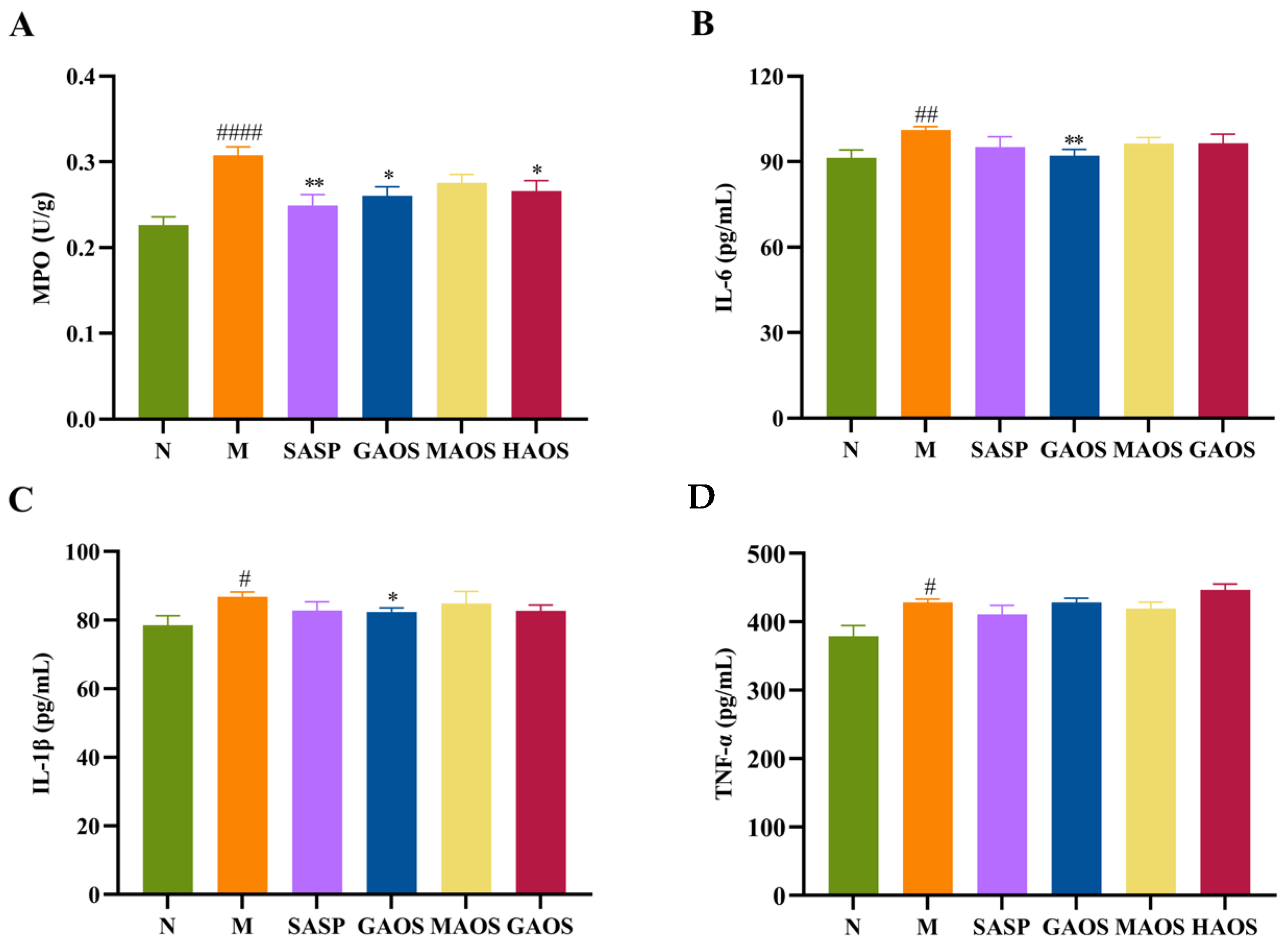
3.2. Alginate Oligosaccharides Treatment Regulated the Inflammatory Response in Colitis Mice
3.3. Alginate Oligosaccharides Treatment Regulates the Intestinal Barrier in Colitis Mice
3.4. Alginate Oligosaccharides Treatment Promoted the Production of SCFAs in Colitis Mice
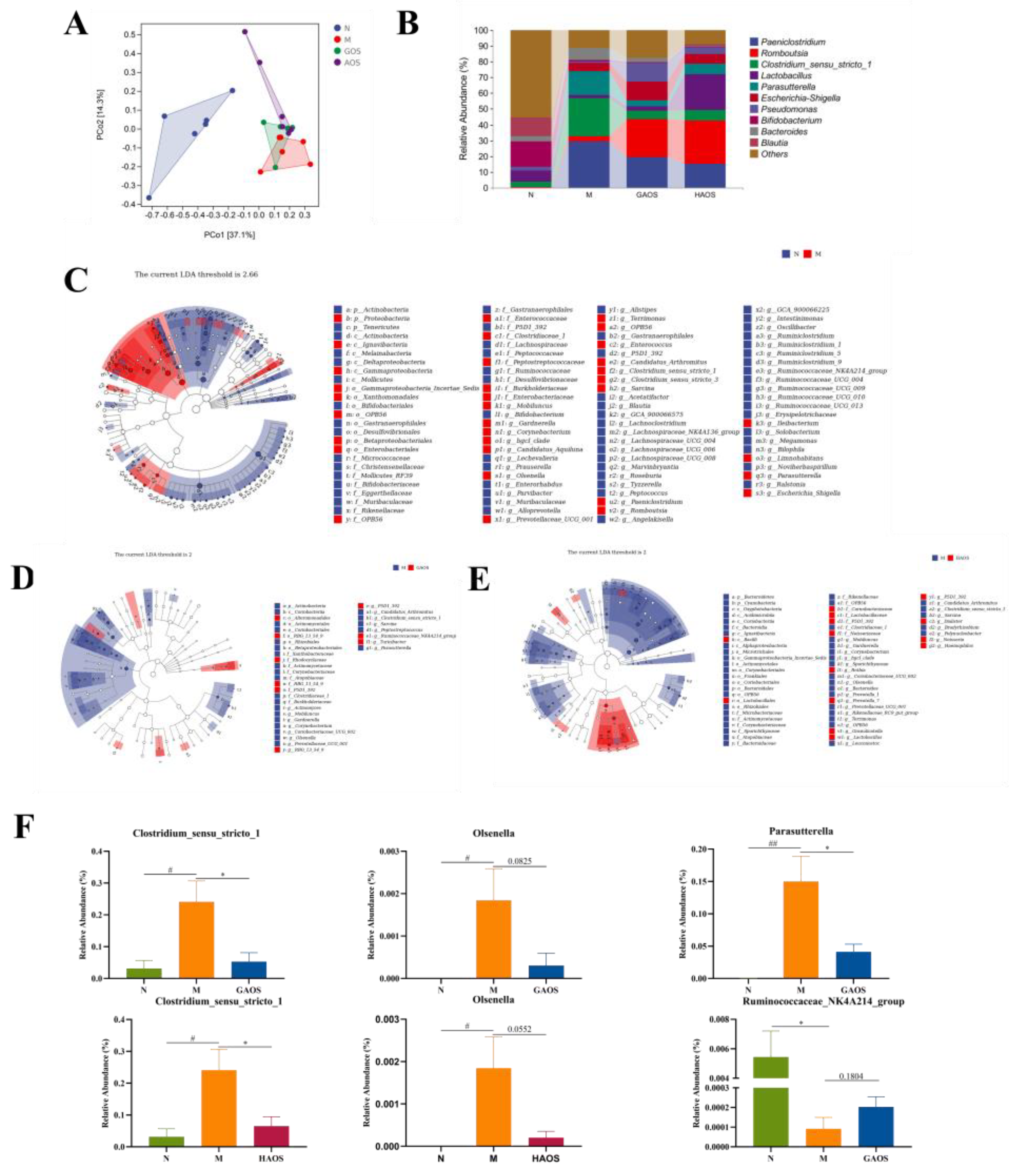
3.5. Alginate Oligosaccharides Treatment Regulates the Gut Microbiota in Colitis Mice
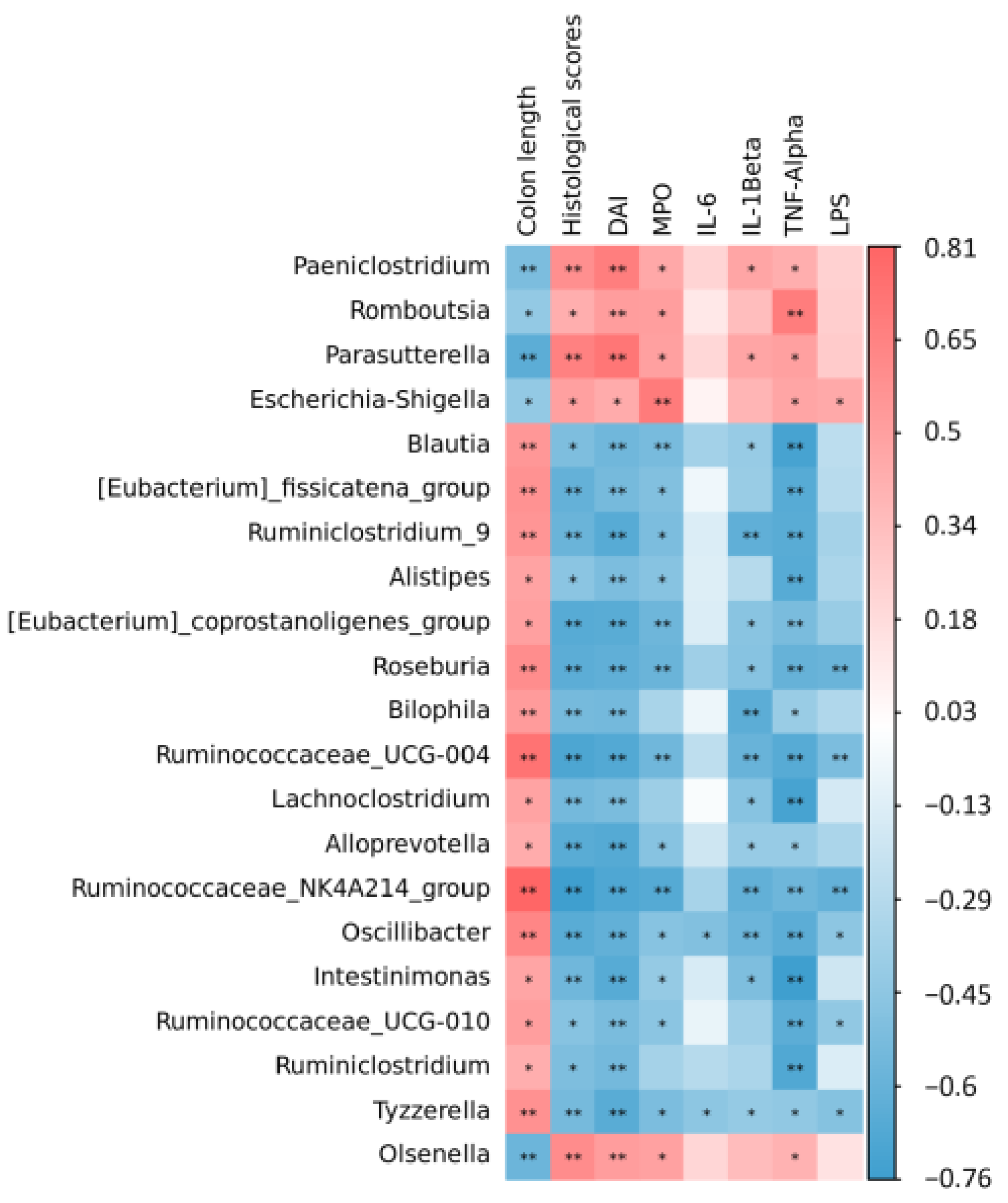
3.6. Spearman Correlation between Colitis Indexes and Gut Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Palence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Kim, H.R.; Jeong, S.J.; Yang, H.J.; Ryu, M.S.; Jeong, D.Y.; Kim, S.Y.; Jung, C.H. Protective Effects of Fermented Soybeans (Cheonggukjang) on Dextran Sodium Sulfate (DSS)-Induced Colitis in a Mouse Model. Foods 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Li, W.; Sun, J.; Yu, P.; Baldwin, R.L.; Urban, J.F. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci. Rep. 2016, 6, 20606. [Google Scholar] [CrossRef]
- Eisenstein, M. Biology: A slow-motion epidemic. Nature 2016, 540, S98–S99. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Bouma, G. Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immun. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Lane, E.R.; Zisman, T.L.; Suskind, D.L. The microbiota in inflammatory bowel disease: Current and therapeutic insights. J. Inflamm. Res. 2017, 10, 63–73. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; Dullemen, H.M.V.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv. Drug. Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Zhang, H.; Bao, Y.I.; Everaert, N. Alginate oligosaccharides preparation, biological activities and their application in livestock and poultry. J. Integr. Agric. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Cote, G.L.; Krull, L.H. Characterization of the exocellular polysaccharides from Azotobacter chroococcum. Carbohydr. Res. 1988, 181, 143–152. [Google Scholar] [CrossRef]
- Lu, S.; Na, K.; Wei, J.; Zhang, L.; Guo, X. Alginate oligosaccharides: The structure-function relationships and the directional preparation for application. Carbohydr. Polym. 2022, 284, 119225. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, F.; Li, Y.; Li, J.; Liu, X.; Wang, X.; Hu, L.; An, Y. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis. Drug. Des. Devel. Ther. 2017, 11, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Yin, H.; Chen, D.; Yu, B.; He, J. Ameliorative effects of alginate oligosaccharide on tumour necrosis factor-α-induced intestinal epithelial cell injury. Int. Immunopharmacol. 2020, 89, 107084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiong, B.; Chen, L.; Ge, W.; Yin, S.; Feng, Y.; Sun, Z.; Sun, Q.; Zhao, Y.; Shen, W.; et al. Rescue of male fertility following faecal microbiota transplantation from alginate oligosaccharide-dosed mice. Gut 2021, 70, 2213–2215. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Ma, L.L.; Shi, H.T.; Zhu, J.B.; Wu, J.; Ding, Z.W.; An, Y.; Zou, Y.Z.; Ge, J.B. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Chin, Y.; Li, R.; Xue, C.; Tang, Q. Transcriptomic analysis reveals effects of fucoxanthin on intestinal glucose transport. J. Funct. Foods 2018, 49, 205–213. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.; Carmody, R.; Gootenberg, D.; Button, J.; Wolfe, B.; Ling, A.; Devlin, A.S.; Varma, Y.; Fischbach, M.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, C.; Chin, Y.; Chen, X.; Gao, Y.; Yuan, S.; Xue, C.; Wang, Y.; Tang, Q. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. 2019, 10, 277–288. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, L.; Chin, Y.; Liu, F.; Li, R.W.; Yuan, S.; Xue, C.; Xu, J.; Tang, Q. Astaxanthin n-Octanoic Acid Diester Ameliorates Insulin Resistance and Modulates Gut Microbiota in High-Fat and High-Sucrose Diet-Fed Mice. Int. J. Mol. Sci. 2020, 21, 2149. [Google Scholar] [CrossRef]
- Ogata, H.; Hibi, T. Cytokine and Anti-cytokine Therapies for Inflammatory Bowel Disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Neutrophil-Dependent Oxidative Stress in Inflammatory Gastrointestinal Diseases. Front. Gastrointest. Res. 2011, 29, 612–615. [Google Scholar]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Ng, J.; Hirota, S.A.; Gross, O.; Li, Y.; Ulke-Lemee, A.; Potentier, M.S.; Schenck, L.P.; Vilaysane, A.; Seamone, M.E.; Feng, H.; et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010, 139, 542–552.e3. [Google Scholar] [CrossRef] [PubMed]
- Billmeier, U.; Dieterich, W.; Neurath, M.F.; Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 9300–9313. [Google Scholar]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Smalley-Freed, W.G.; Efimov, A.; Burnett, P.E.; Short, S.P.; Davis, M.A.; Gumucio, D.L.; Washington, M.K.; Coffey, R.J.; Reynolds, A.B. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J. Clin. Investig. 2010, 120, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Dahlhoff, M.; Horst, D.; Hirschi, B.; Trülzsch, K.; Müller-Höcker, J.; Vogelmann, R.; Allgäuer, M.; Gerhard, M.; Steininger, S.; et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS ONE 2010, 5, e14325. [Google Scholar] [CrossRef]
- Grill, J.I.; Neumann, J.; Hiltwein, F.; Kolligs, F.T.; Schneider, M.R. Intestinal E-cadherin Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis. Dig. Dis. Sci. 2015, 60, 895–902. [Google Scholar] [CrossRef]
- Jankowski, J.A.; Bedford, F.K.; Boulton, R.A.; Cruickshank, N.; Hall, C.; Elder, J.; Allan, R.; Forbes, A.; Kim, Y.S.; Wright, N.A.; et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn’s colitis. Lab. Investig. 1998, 78, 1155–1167. [Google Scholar]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Chen, E.S.; Xiao, S.; Snapper, S.B.; Bao, B.; An, D.; Blumberg, R.S.; et al. Foxo1 controls gut homeostasis and commensalism by regulating mucus secretion. J. Exp. Med. 2021, 218, e20210324. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immun. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Duszka, K.; Oresic, M.; May, C.L.; König, J.; Wahli, W. PPARγ Modulates Long Chain Fatty Acid Processing in the Intestinal Epithelium. Int. J. Mol. Sci. 2017, 18, 2559. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, W.; Deng, Q.; Huang, Q.; Wang, X.; Yang, C.; Huang, F. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct. 2020, 11, 8077–8088. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Matsui, H.; Matsuda, Y.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Fukunaga, K.; Yoshida, M. Oyster (Crassostrea gigas) extract attenuates dextran sulfate sodium-induced acute experimental colitis by improving gut microbiota and short-chain fatty acids compositions in mice. Foods 2022, 11, 373. [Google Scholar] [CrossRef]
- Li, P.; Xiao, N.; Zeng, L.; Xiao, J.; Huang, J.; Xu, Y.; Chen, Y.; Ren, Y.; Du, B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020, 250, 116958. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Caruso, R.; Lo, B.C.; Núñez, G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immun. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, C.; Lou, Y.; Xu, H. Whole and polysaccharide powdered Sporisorium reliance improves DSS-induced colitis in BALB/c mice by modulating gut microbiota. J. Funct. Foods 2021, 79, 104409. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Cammarotaa, G.; Ianiroa, G.; Ciancia, R.; Bibbòa, S.; Gasbarrinia, A.; Curròb, D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol. Ther. 2015, 149, 191–212. [Google Scholar] [CrossRef]
- Liu, R.X.; Li, Y.C.; Zhang, B. The effects of konjac oligosaccharide on TNBS-induced colitis in rats. Int. Immunopharmacol. 2016, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial Population Differentials between Mucosal and Submucosal Intestinal Tissues in Advanced Crohn’s Disease of the Ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lee, Y.; Lu, H.; Chou, C.; Wang, C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 2019, 14, e0205784. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef]
- Geng, Y.; Yue, Y.; Guan, Q.; Ren, Y.; Guo, L.; Fan, Y.; Lu, Z.; Shi, J.; Xu, Z. Cereal Vinegar Sediment Alleviates Spontaneous Ulcerative Colitis in Il-10 Deficient Mice. Mol. Nutr. Food Res. 2021, 65, e2001227. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Zhao, Q. Polyphenol-Rich Loquat Fruit Extract Prevents Fructose-Induced Nonalcoholic Fatty Liver Disease by Modulating Glycometabolism, Lipometabolism, Oxidative Stress, Inflammation, the Intestinal Barrier and the Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 7726–7737. [Google Scholar] [CrossRef]


| Score | Weight Loss | Fecal State | Bloody Situation |
|---|---|---|---|
| 0 | <1% | Normal | Negative |
| 1 | 1–5% | Soft but well-formed | Weak negative |
| 2 | 5–10% | Soft and shapeless | Positive |
| 3 | 10–15% | Very loose and moist | Stool bleeding |
| 4 | >15% | Diarrhea | Rectal bleeding |
| Score | Scoring Criteria |
|---|---|
| 0 | Normal |
| 1 | Mild inflammation and edema of the mucosal layer; loss of the basal 1/3 of the crypt. |
| 2 | Moderate inflammation of the mucosal layer; loss of the basal 2/3 of the crypt. |
| 3 | Moderate inflammation of the mucosal layer with complete disappearance of the crypt, but the epithelial layer is still intact. |
| 4 | Heavy inflammation of the mucosal, submucosal, and muscular layers; total disappearance of the crypt and epithelium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Gao, Y.; Kan, R.; Ren, P.; Xue, C.; Kong, B.; Tang, Q. Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota. Foods 2023, 12, 220. https://doi.org/10.3390/foods12010220
Wu A, Gao Y, Kan R, Ren P, Xue C, Kong B, Tang Q. Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota. Foods. 2023; 12(1):220. https://doi.org/10.3390/foods12010220
Chicago/Turabian StyleWu, Axue, Yuan Gao, Ruotong Kan, Pengfei Ren, Changhu Xue, Biao Kong, and Qingjuan Tang. 2023. "Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota" Foods 12, no. 1: 220. https://doi.org/10.3390/foods12010220
APA StyleWu, A., Gao, Y., Kan, R., Ren, P., Xue, C., Kong, B., & Tang, Q. (2023). Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota. Foods, 12(1), 220. https://doi.org/10.3390/foods12010220






