Feature Reduction for the Classification of Bruise Damage to Apple Fruit Using a Contactless FT-NIR Spectroscopy with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Experimental
2.3. Sampling
2.4. Data Pre-Processing
2.5. Analytical Workflow
2.5.1. Introduction
2.5.2. Baseline Method
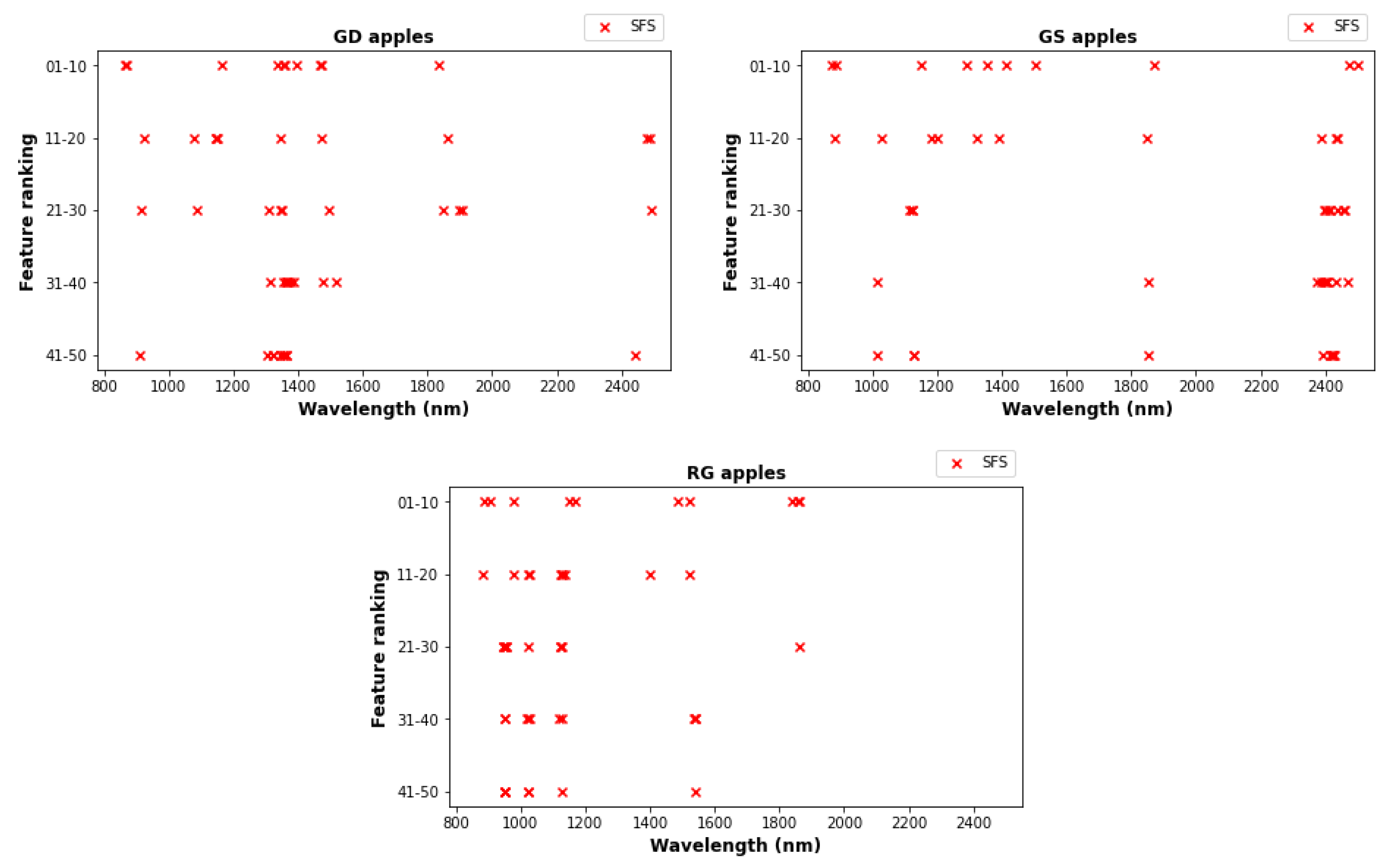
2.5.3. Feature Selection
- In recursive feature elimination (RFE), the model is first trained on the entire set of features, and deletes the least important features. This training-deletion procedure is repeated recursively on the remaining features until the desired number of features remain.
- In sequential feature selection (SFS), initially models based on each individual feature are computed, and the feature with the best cross-validation score is selected. Then all models consisting of the selected features and one additional feature are computed, and the best chosen. This process is repeated recursively, adding one feature each time. The default cross validation score was used for this step. There is a backward selection variant of this method that is similar to RFS but we found that execution is too slow.
2.5.4. Machine Learning Classifiers
2.5.5. Comparison of Classifier Performance
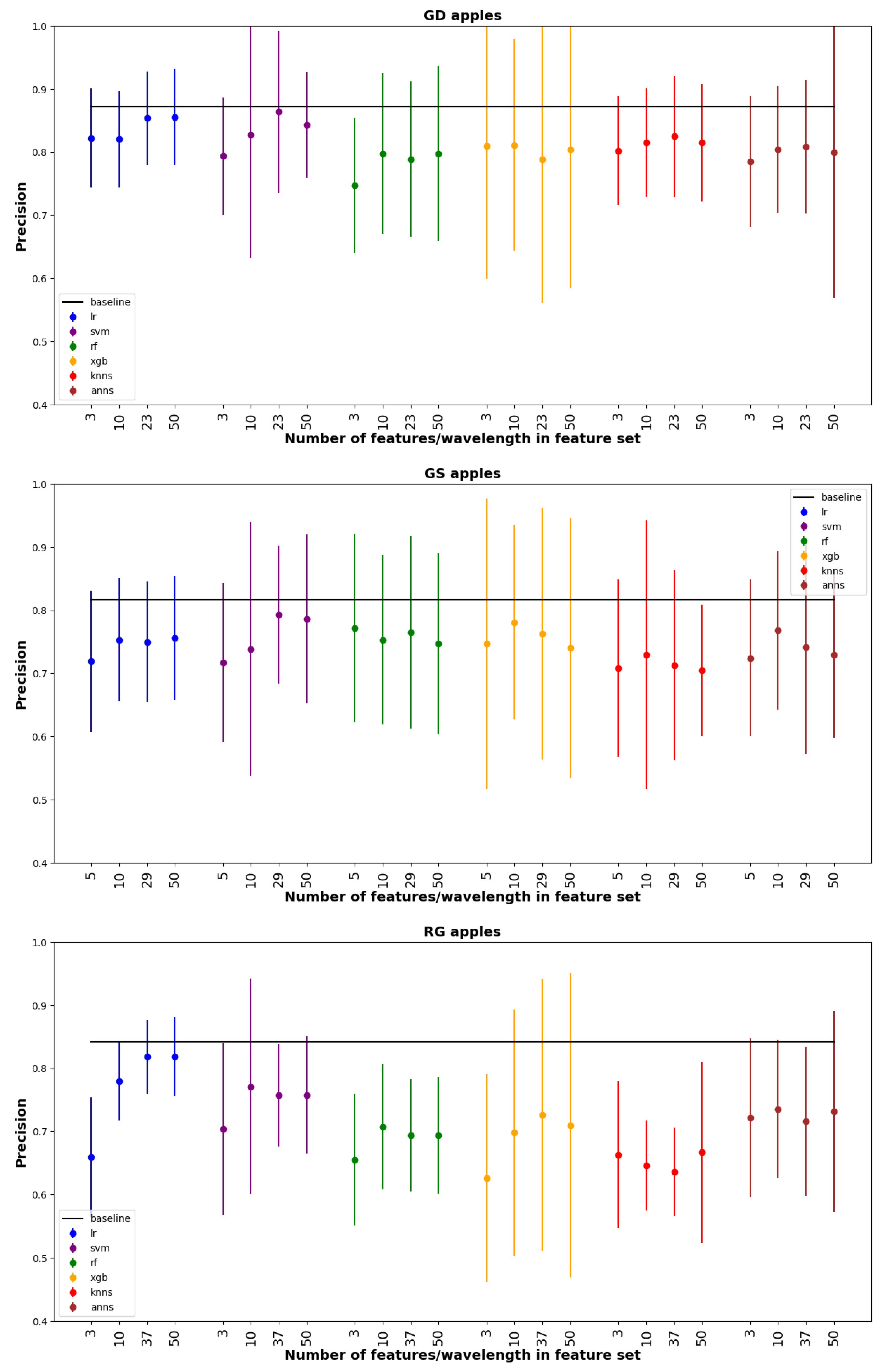
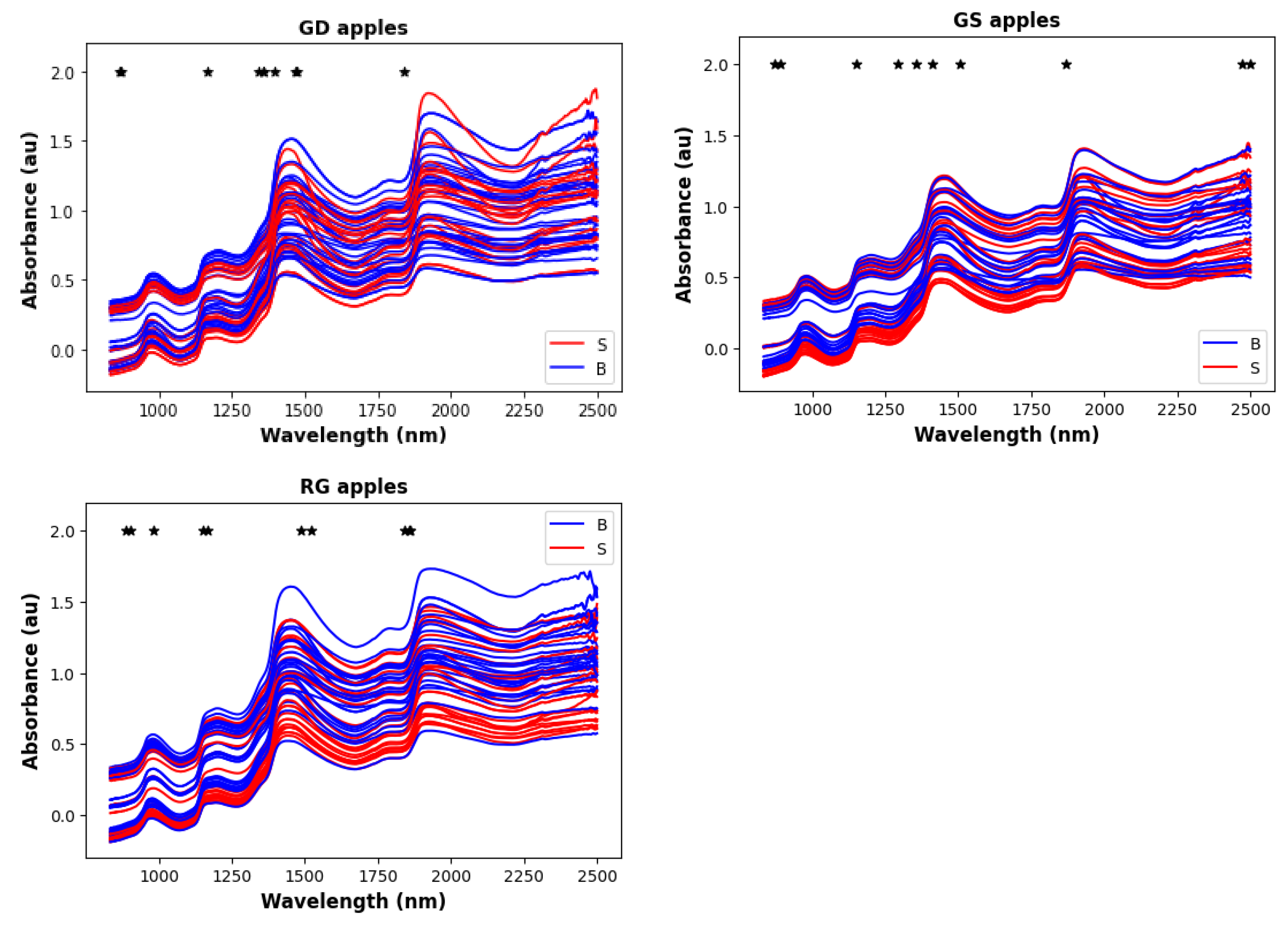
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Priority | GD Wavelength (nm) | GS Wavelength (nm) | RG Wavelength (nm) |
|---|---|---|---|
| 1 | 1393.9 | 2473.9 | 1840.1 |
| 2 | 864.5 | 1356.7 | 1486.6 |
| 3 | 1357.4 | 872.4 | 904.6 |
| 4 | 1468.1 | 1411.3 | 1168.9 |
| 5 | 1836.1 | 1292.4 | 888.5 |
| 6 | 1472.3 | 1150.2 | 980.6 |
| 7 | 868.8 | 1505.6 | 1152.3 |
| 8 | 1163.1 | 889.7 | 1522.4 |
| 9 | 1338.5 | 1870.6 | 1857.2 |
| 10 | 1354.6 | 2500.1 | 1861.2 |
| 11 | 1144.1 | 1180.6 | 976.5 |
| 12 | 1145.2 | 883.1 | 882.5 |
| 13 | 1862.5 | 1201.4 | 1136.1 |
| 14 | 2488.1 | 2432.1 | 1523.3 |
| 15 | 1151.3 | 2436.7 | 1399.2 |
| 16 | 1076.2 | 1391.6 | 1123.8 |
| 17 | 2478.6 | 1029.6 | 1022.3 |
| 18 | 924.0 | 2387.3 | 1028.0 |
| 19 | 1346.1 | 1321.4 | 1126.7 |
| 20 | 1473.9 | 1846.6 | 1127.2 |
| 21 | 1496.9 | 2455.2 | 949.0 |
| 22 | 1343.3 | 2439.0 | 953.9 |
| 23 | 915.2 | 2409.5 | 1862.5 |
| 24 | 2492.9 | 2396.2 | 1024.0 |
| 25 | 1309.4 | 1124.3 | 1125.3 |
| 26 | 1896.6 | 2459.8 | 1122.8 |
| 27 | 1909.2 | 1125.3 | 946.6 |
| 28 | 1850.6 | 2414.0 | 954.6 |
| 29 | 1351.0 | 2400.6 | 951.8 |
| 30 | 1086.6 | 1115.6 | 1127.7 |
| 31 | 1384.2 | 2372.0 | 1125.8 |
| 32 | 1376.9 | 2389.5 | 1537.7 |
| 33 | 1312.7 | 2398.4 | 1026.8 |
| 34 | 1477.3 | 1015.5 | 1541.4 |
| 35 | 1366.7 | 1851.9 | 1120.9 |
| 36 | 1518.8 | 2402.8 | 1019.5 |
| 37 | 1367.4 | 2407.3 | 953.5 |
| 38 | 1361.7 | 2405.0 | 952.8 |
| 39 | 1363.1 | 2434.4 | 1538.7 |
| 40 | 1356.7 | 2469.2 | 1025.6 |
| 41 | 1324.1 | 1128.2 | 949.7 |
| 42 | 1344.7 | 1853.2 | 952.1 |
| 43 | 1363.8 | 1126.7 | 1126.3 |
| 44 | 2441.3 | 1016.3 | 1539.6 |
| 45 | 1352.4 | 2425.3 | 951.4 |
| 46 | 1320.8 | 2416.3 | 950.0 |
| 47 | 1353.2 | 2391.7 | 951.1 |
| 48 | 1364.5 | 2418.5 | 952.5 |
| 49 | 1306.8 | 2429.8 | 1025.2 |
| 50 | 911.3 | 2423.0 | 1023.1 |
References
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and Postharvest Factors Affecting Bruise Damage of Fresh Fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Opara, U.L. Mechanical damage of fresh produce in postharvest transportation: Current status and future prospects. Trends Food Sci. Technol. 2022, 124, 195–207. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest factors influencing bruise damage of fresh fruits—A review. Sci. Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, C. Quantitative evaluation of mechanical damage to fresh fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.; Nieuwoudt, H.H.; Perold, W.J.; Opara, U.L. Detecting bruise damage and level of severity in apples using a contactless nir spectrometer. Appl. Eng. Agric. 2020, 36, 257–270. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Perold, W.J.; Opara, U.L. Classification learning of latent bruise damage to apples using shortwave infrared hyperspectral imaging. Sensors 2021, 21, 4990. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, D.; Hui, G.; Mao, J.; Liu, T.; Zhou, W.; Zhao, Y.; Chen, Z.; Chen, F. Loquat Bruise Detection Using Optical Coherence Tomography Based on Microstructural Parameters. Food Anal. Methods 2018, 11, 2692–2698. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Non-destructive defect detection of apples by spectroscopic and imaging technologies: A review. Trans. ASABE 2017, 60, 1765–1790. [Google Scholar] [CrossRef]
- Baranowski, P.; Mazurek, W.; Wozniak, J.; Majewska, U. Detection of early bruises in apples using hyperspectral data and thermal imaging. J. Food Eng. 2012, 110, 345–355. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Wang, Q.; Chen, L. Development of a multispectral imaging system for online detection of bruises on apples. J. Food Eng. 2015, 146, 62–71. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, B.; Tian, G.; Zhou, J.; Huang, J.; Xiong, Y. Challenges and solutions of optical-based nondestructive quality inspection for robotic fruit and vegetable grading systems: A technical review. Trends Food Sci. Technol. 2018, 81, 213–231. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.; Holmes, M.; Hanpin, M. Variables selection methods in near-infrared spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Opara, U.L. Machine learning applications to non-destructive defect detection in horticultural products. Biosyst. Eng. 2020, 189, 60–83. [Google Scholar] [CrossRef]
- Luo, X.; Takahashi, T.; Kyo, K.; Zhang, S. Wavelength selection in vis/NIR spectra for detection of bruises on apples by ROC analysis. J. Food Eng. 2012, 109, 457–466. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Nieuwoudt, H.H.; Opara, U.L.; Perold, W.J. Performance of genetic algorithm in optimization of NIRS PLS models to predict apple fruit quality. Acta Hortic. 2018, 1201, 355–362. [Google Scholar] [CrossRef]
- Li, L.; Jang, X.; Li, B.; Liu, Y. Wavelength selection method for near-infrared spectroscopy based on standard-sample calibration transfer of mango and apple. Comput. Electron. Agric. 2021, 190, 106448. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Dedicated non-destructive devices for food quality measurement: A review. Trends Food Sci. Technol. 2018, 78, 197–205. [Google Scholar] [CrossRef]
- Amuah, C.L.; Teye, E.; Lamptey, F.P.; Nyandey, K.; Opoku-Ansah, J.; Adueming, P.O.W. Feasibility Study of the Use of Handheld NIR Spectrometer for Simultaneous Authentication and Quantification of Quality Parameters in Intact Pineapple Fruits. J. Spectrosc. 2019, 2019, 5975461. [Google Scholar] [CrossRef]
- Srivastava, S.; Shashikant, B.V. Handheld, smartphone based spectrometer for rapid and nondestructive testing of citrus cultivars. J. Food Meas. Charact. 2021, 15, 892–904. [Google Scholar] [CrossRef]
- Opara, L.U.; AlGhafri, A.; Agzoun, H.; AlIssai, J.; AlJabri, F. Design and development of a new device for measuring susceptibility to impact damage of fresh produce. New Zealand J. Crop. Hortic. Sci. 2007, 35, 245–251. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Terry, L.A.; Landahl, S.; Cronje, P.J.; Nieuwoudt, H.; Mouazen, A.M.; Saeys, W.; Nicolaï, B.M. Prediction of ’Nules Clementine’ mandarin susceptibility to rind breakdown disorder using Vis/NIR spectroscopy. Postharvest Biol. Technol. 2012, 74, 1–10. [Google Scholar] [CrossRef][Green Version]
- Bruker. Matrix-F FT-NIR Spectrometer. Available online: https://www.bruker.com/en/products-and-solutions/infrared-and-raman/ft-nir-spectrometers/matrix-f-ft-nir-spectrometer.html (accessed on 15 December 2022).
- Ning, J.; Ye, H.; Sun, Y.; Zhang, J.; Mei, Z.; Xiong, S.; Zhang, S.; Li, Y.; Hui, G.; Yi, X.; et al. Study on apple damage detecting method based on relaxation single-wavelength laser and convolutional neural network. J. Food Meas. Charact. 2022, 16, 3321–3330. [Google Scholar] [CrossRef]
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Mueller, A.; Grisel, O.; Niculae, V.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; et al. API design for machine learning software: Experiences from the scikit-learn project. arXiv 2013, arXiv:1309.0238. [Google Scholar] [CrossRef]
- Hussein, E. Apple Classification, first release. Zenodo v1.0 2022. Available online: https://zenodo.org/record/7007001/export/dcite4#.Y7PBHRVBxPY (accessed on 15 March 2012). [CrossRef]
- Altmann, A.; Toloşi, L.; Sander, O.; Lengauer, T. Permutation importance: A corrected feature importance measure. Bioinformatics 2010, 26, 1340–1347. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Nieuwoudt, H.H.; Perold, W.J.; Opara, U.L. Non-destructive measurement of internal quality of apple fruit by a contactless NIR spectrometer with genetic algorithm model optimization. Sci. Afr. 2019, 3, e00051. [Google Scholar] [CrossRef]
- Mendez, K.M.; Reinke, S.N.; Broadhurst, D.I. A comparative evaluation of the generalised predictive ability of eight machine learning algorithms across ten clinical metabolomics data sets for binary classification. Metabolomics 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Aguasca-Colomo, R.; Castellanos-Nieves, D.; Méndez, M. Comparative analysis of rainfall prediction models using machine learning in islands with complex orography: Tenerife Island. Appl. Sci. 2019, 9, 4931. [Google Scholar] [CrossRef]
- Hussein, E.A.; Thron, C.; Ghaziasgar, M.; Vaccari, M.; Marnewick, J.L.; Hussein, A.A. Comparison of Phenolic Content and Antioxidant Activity for Fermented and Unfermented Rooibos Samples Extracted with Water and Methanol. Plants 2022, 11, 16. [Google Scholar] [CrossRef]
- Hussein, E.A.; Ghaziasgar, M.; Thron, C.; Vaccari, M.; Bagula, A. Basic Statistical Estimation Outperforms Machine Learning in Monthly Prediction of Seasonal Climatic Parameters. Atmosphere 2021, 12, 539. [Google Scholar] [CrossRef]
- Nadler, B.; Coifman, R.R. Partial least squares, Beer’s law and the net analyte signal: Statistical modeling and analysis. J. Chemom. 2005, 19, 45–54. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Mazurek, W. Classification models of bruise and cultivar detection on the basis of hyperspectral imaging data. Comput. Electron. Agric. 2014, 106, 66–74. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Lin, T.; Ying, Y. Food and agro-product quality evaluation based on spectroscopy and deep learning: A review. Trends Food Sci. Technol. 2021, 112, 431–441. [Google Scholar] [CrossRef]
- Purushotham, S.; Meng, C.; Che, Z.; Liu, Y. Benchmarking deep learning models on large healthcare datasets. J. Biomed. Inform. 2018, 83, 112–134. [Google Scholar] [CrossRef]
- Hussein, E.A.; Ghaziasgar, M.; Thron, C.; Vaccari, M.; Jafta, Y. Rainfall Prediction Using Machine Learning Models: Literature Survey. In Artificial Intelligence for Data Science in Theory and Practice; Alloghani, M., Thron, C., Subair, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 75–108. [Google Scholar] [CrossRef]
- Geolla, F.; Geolla, F.; Peiper, U.M. A spectrophotometric method for detecting surface bruises on “Golden Delicious” apples. J. Agric. Eng. Res. 1993, 58, 47–51. [Google Scholar] [CrossRef]
- Upchurch, B.L.; Affeldt, H.A.; Hruschka, W.R.; Norris, K.H.; Throop, J.A. Spectrophotometric study of bruises on whole, ‘Red Delicious’ apples. Trans. Am. Soc. Agric. Eng. 1990, 33, 585–589. [Google Scholar] [CrossRef]
- Miller, W.M.; Throop, J.A.; Upchurch, B.L. Pattern recognition models for spectral reflectance evaluation of apple blemishes. Postharvest Biol. Technol. 1998, 14, 11–20. [Google Scholar] [CrossRef]
- Wen, Z.; Tao, Y. Building a rule-based machine-vision system for defect inspection on apple sorting and packing lines. Expert Syst. Appl. 1999, 16, 307–313. [Google Scholar] [CrossRef]
- Kleynen, O. Selection of the most efficient wavelength bands for ’Jonagold’ apple sorting. Postharvest Biol. Technol. 2003, 30, 221–232. [Google Scholar] [CrossRef][Green Version]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolaï, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Lu, P.L.; Lin, C.H. Physiology of fruit cracking in wax apple (Syzygium samarangense). Bot. Orient. J. Plant Sci. 2011, 8, 70–76. [Google Scholar] [CrossRef]
- Wang, B.H.; Cao, J.J.; Zhang, B.; Chen, H.Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Xie, Z.; Guan, R.Q.; Du, M.R.; Qiao, Z.H.; Suo, X.Y.; Liu, Z.Y.; Bian, Y.; Qiang, L.; Fu, Y.H. Syzysamalactone, an Unusual 11-Carbon δ-Lactone Derivative from the Fresh Ripe Fruits of Syzygium samarangense (Wax Apple). J. Nat. Prod. 2022, 85, 2100–2103. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, A.; Dekker, M.; Jongen, W. Flavonoids as bioactive components in apple products. Cancer Lett. 1997, 114, 107–108. [Google Scholar] [CrossRef]
- Hagen, S.F.; Solhaug, K.A.; Bengtsson, G.B.; Borge, G.I.A.; Bilger, W. Chlorophyll fluorescence as a tool for non-destructive estimation of anthocyanins and total flavonoids in apples. Postharvest Biol. Technol. 2006, 41, 156–163. [Google Scholar] [CrossRef]
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Nising, A.B.; Hurrell, R.F. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. J. Food Compos. Anal. 2012, 26, 128–135. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Reay, P.F.; Norris, J.; Butler, R.C. Induction of flavonoids and phenolic acids in apple by UV-B and temperature. J. Hortic. Sci. Biotechnol. 2000, 75, 142–148. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential partitioning of triterpenes and triterpene esters in apple peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Lancaster, J.; Dougall, D.K. Regulation of skin color in apples. Crit. Rev. Plant Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Lorenzo, T.V. Chlorophylls in foods. Crit. Rev. Food Sci. Nutr. 1990, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Vargas, E.; Conrad, J.; Hempel, J.; Gras, C.C.; Ziegler, J.U.; Mayer, A.; Jiménez, V.; Esquivel, P.; Carle, R. Carotenoids, carotenoid esters, and anthocyanins of yellow-, orange-, and red-peeled cashew apples (Anacardium occidentale L.). Food Chem. 2016, 200, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Marais, E.; Jacobs, G.; Holcroft, D.M. Postharvest irradiation enhances anthocyanin synthesis in apples but not in pears. HortScience 2001, 36, 738–740. [Google Scholar] [CrossRef]
- Jamrógiewicz, M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J. Pharm. Biomed. Anal. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Pandiselvam, R.; Kothakota, A.; Ishwarya S., P.; Chakraborty, S.K.; Kumar, M.; Cozzolino, D. Emerging non-destructive imaging techniques for fruit damage detection: Image processing and analysis. Trends Food Sci. Technol. 2022, 120, 418–438. [Google Scholar] [CrossRef]
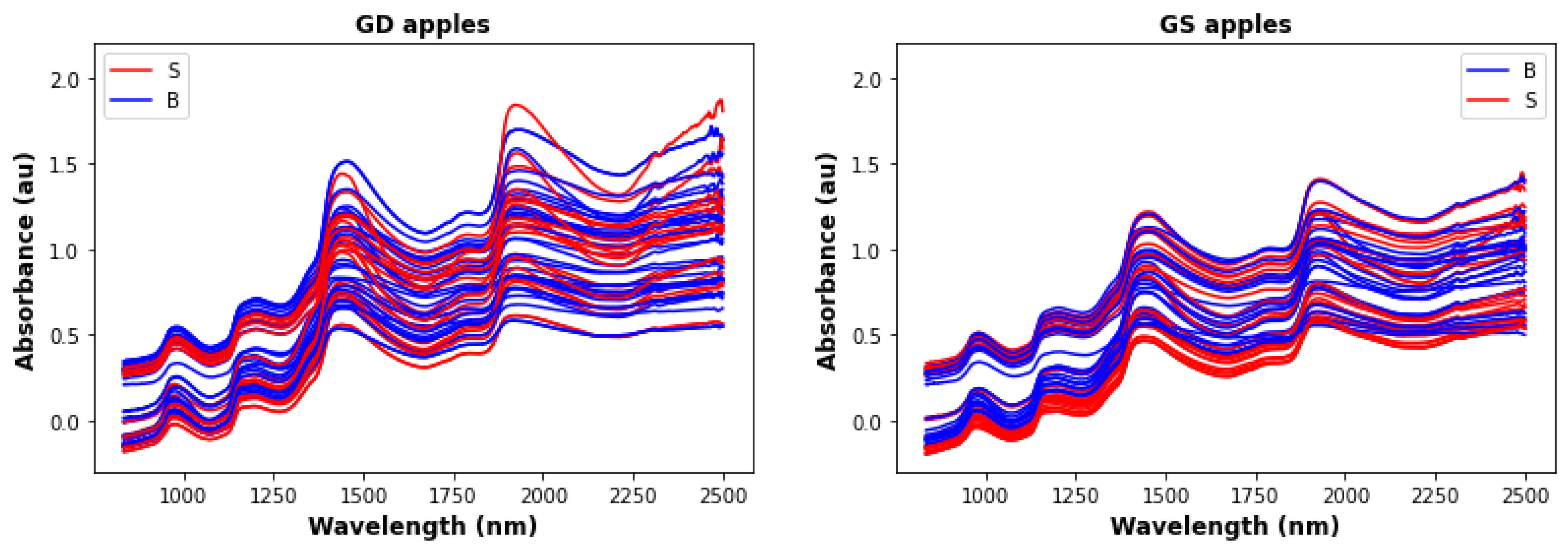
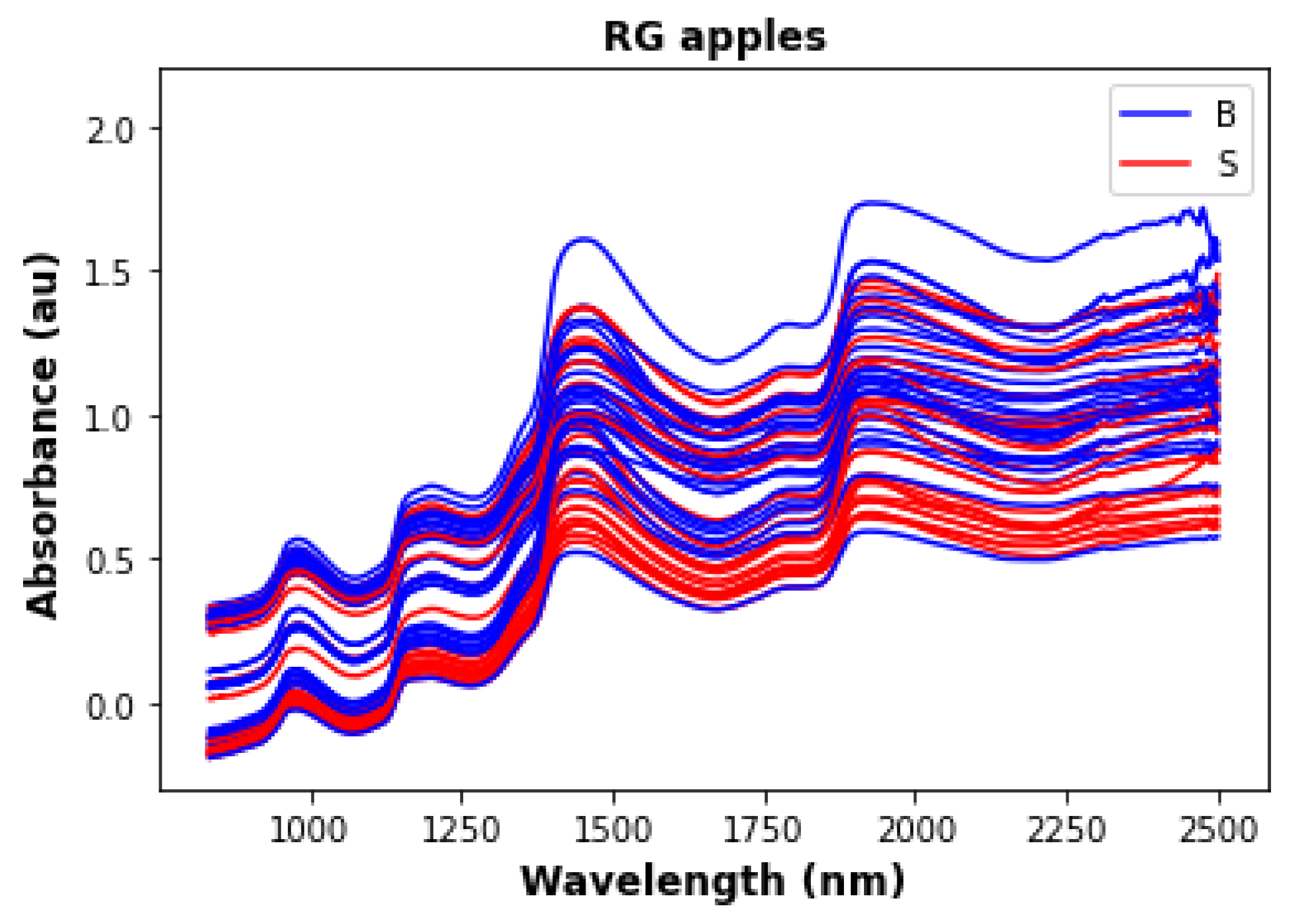
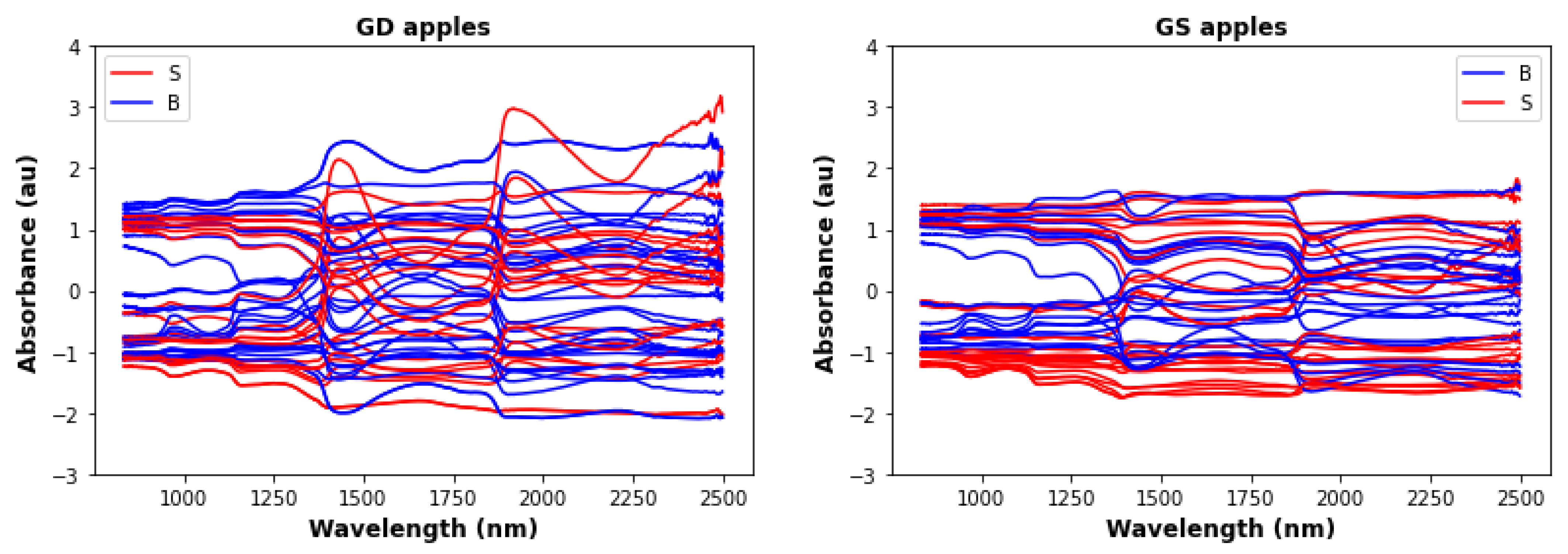




| Type | Non-Bruised Samples | Bruised Samples | Total |
|---|---|---|---|
| GD | 274 | 273 | 547 |
| GS | 252 | 251 | 503 |
| RG | 278 | 284 | 562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nturambirwe, J.F.I.; Hussein, E.A.; Vaccari, M.; Thron, C.; Perold, W.J.; Opara, U.L. Feature Reduction for the Classification of Bruise Damage to Apple Fruit Using a Contactless FT-NIR Spectroscopy with Machine Learning. Foods 2023, 12, 210. https://doi.org/10.3390/foods12010210
Nturambirwe JFI, Hussein EA, Vaccari M, Thron C, Perold WJ, Opara UL. Feature Reduction for the Classification of Bruise Damage to Apple Fruit Using a Contactless FT-NIR Spectroscopy with Machine Learning. Foods. 2023; 12(1):210. https://doi.org/10.3390/foods12010210
Chicago/Turabian StyleNturambirwe, Jean Frederic Isingizwe, Eslam A. Hussein, Mattia Vaccari, Christopher Thron, Willem Jacobus Perold, and Umezuruike Linus Opara. 2023. "Feature Reduction for the Classification of Bruise Damage to Apple Fruit Using a Contactless FT-NIR Spectroscopy with Machine Learning" Foods 12, no. 1: 210. https://doi.org/10.3390/foods12010210
APA StyleNturambirwe, J. F. I., Hussein, E. A., Vaccari, M., Thron, C., Perold, W. J., & Opara, U. L. (2023). Feature Reduction for the Classification of Bruise Damage to Apple Fruit Using a Contactless FT-NIR Spectroscopy with Machine Learning. Foods, 12(1), 210. https://doi.org/10.3390/foods12010210











