Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of SM
2.3. Preparation of SM-EGCG-SeNPs
2.4. DPPH Radical Scavenging Assay
2.5. Hydroxyl Radical Scavenging Assay
2.6. Cell Viability Assay
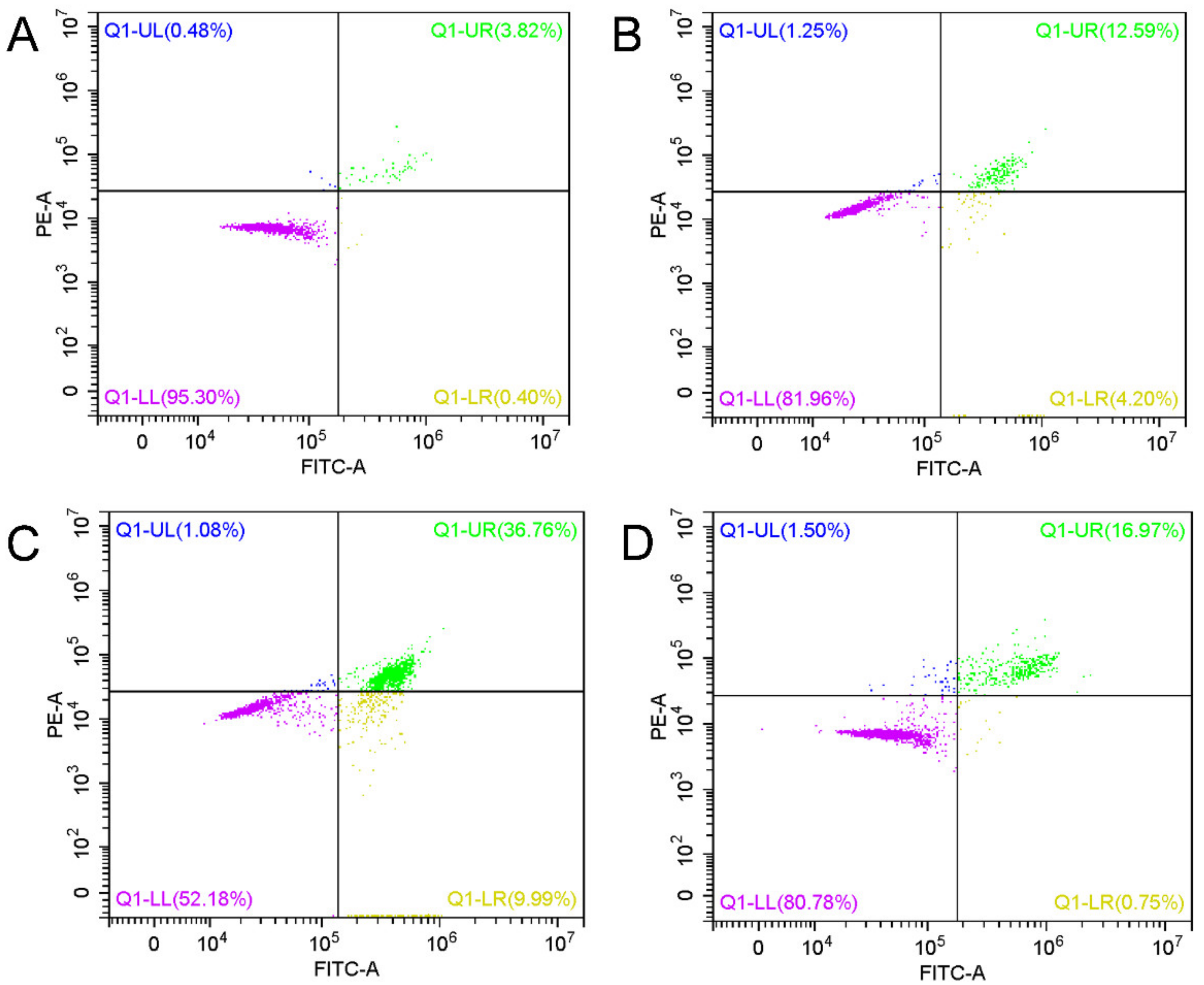
2.7. Analysis of Cell Apoptosis
2.8. Activities of Caspase-3 and -9
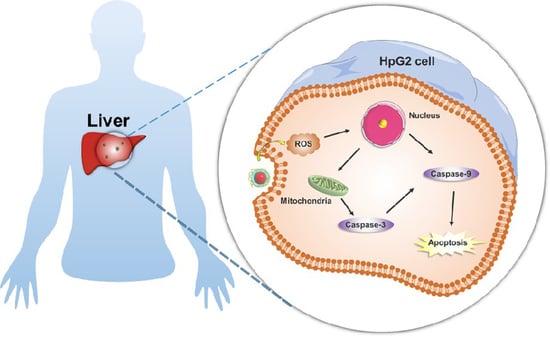
2.9. ROS Production
3. Results
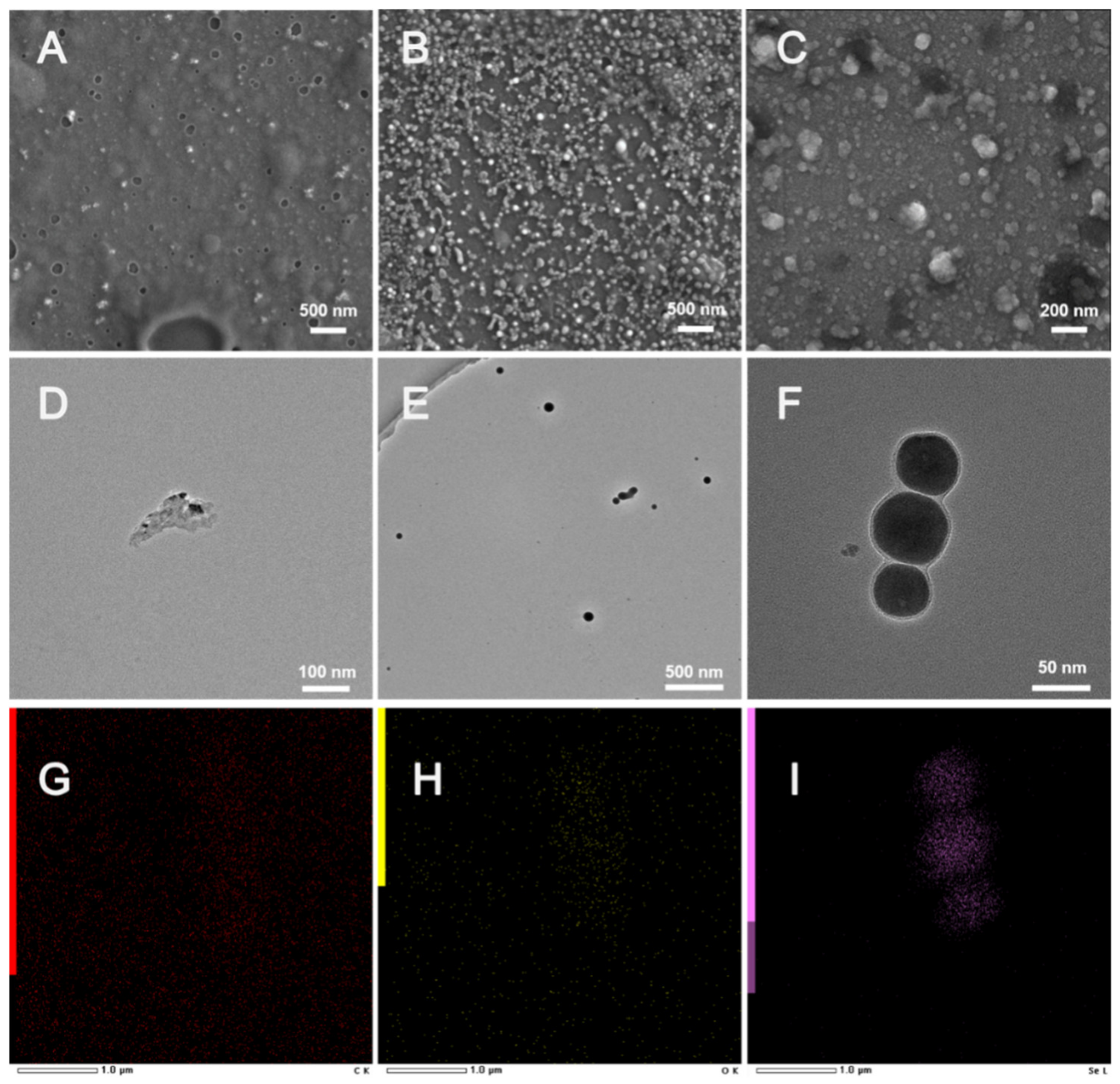
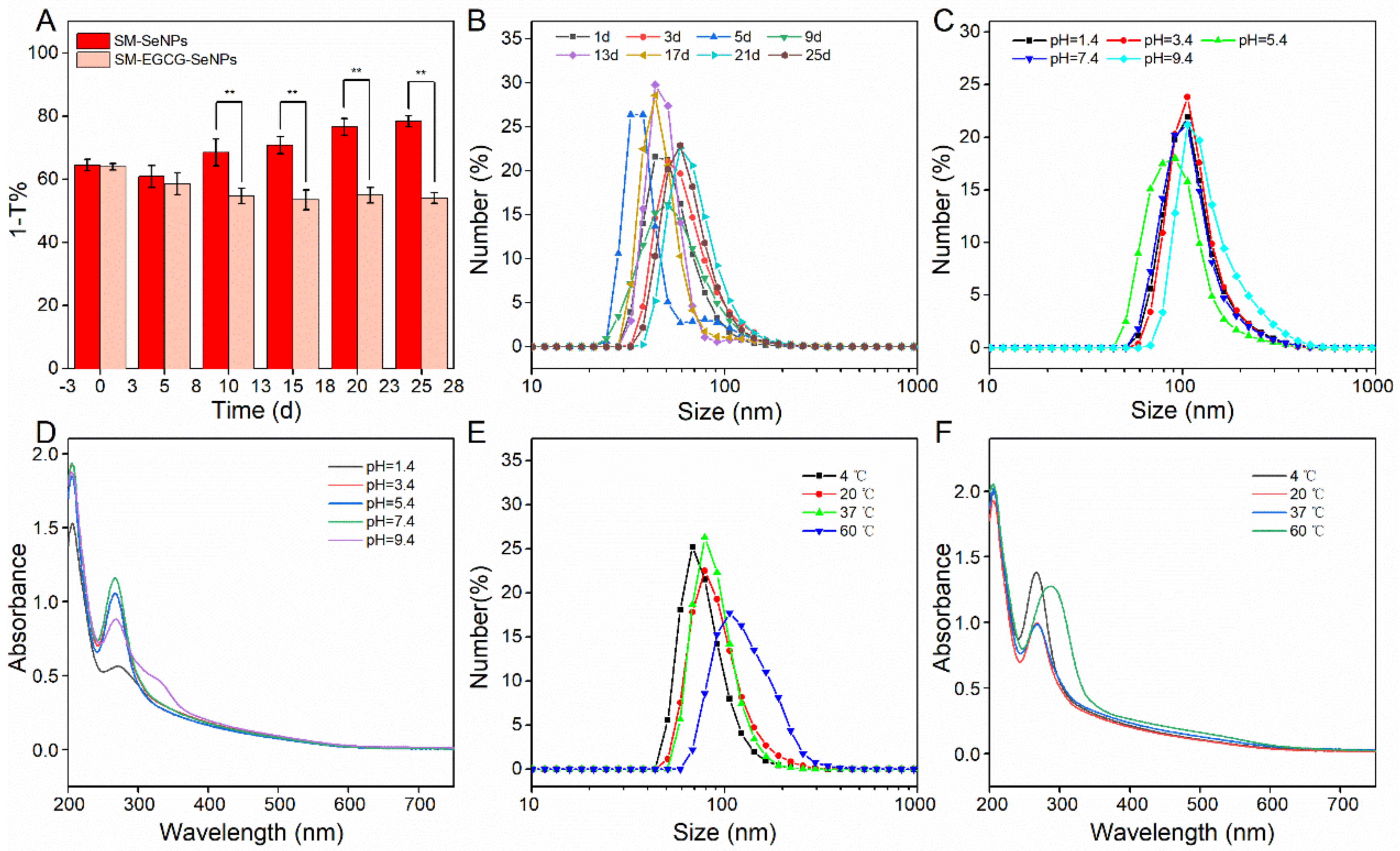
3.1. Characterization of SM-EGCG-SeNPs
3.2. Evaluation of Antioxidant Activity of SM-EGCG-SeNPs
3.3. Antiproliferative Effect of SM-EGCG-SeNPs
3.4. Cell Apoptosis Induction Caused by SM-EGCG-SeNPs in HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/beta-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, Y.; Zhao, G.; Sun, H.; Che, H.; Leng, X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 231, 115689. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, W.; Zhang, Z.; Ling, X.; Lin, H.; Peng, L.; Chen, T. Highly uniform synthesis of selenium nanoparticles with EGFR targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 11177–11193. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, Q.; Zhao, Z.; He, L.; Chan, L.; Liu, Y.; Chen, Y.; Bai, M.; Pan, T.; Qu, Y.; et al. Functionalized selenium nanosystem as radiation sensitizer of I-125 seeds for precise cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 25857–25869. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, J.; Luk, K.H.; Cheung, S.T.; Wong, K.; Chen, T. Potentiation of in vivo anticancer efficacy of selenium nanoparticles by mushroom polysaccharides surface decoration. J. Agric. Food Chem. 2019, 67, 2865–2876. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Healthc. Mater. 2021, 10, 16. [Google Scholar] [CrossRef]
- Hu, S.; Hu, W.; Li, Y.; Li, S.; Tian, H.; Lu, A.; Wang, J. Construction and structure-activity mechanism of polysaccharide nano-selenium carrier. Carbohydr. Polym. 2020, 236, 2100598. [Google Scholar] [CrossRef]
- Nie, T.; Wu, H.; Wong, K.; Chen, T. Facile synthesis of highly uniform selenium nanoparticles using glucose as the reductant and surface decorator to induce cancer cell apoptosis. J. Mater. Chem B 2016, 4, 2351–2358. [Google Scholar] [CrossRef]
- Pi, J.; Jin, H.; Liu, R.; Song, B.; Wu, Q.; Liu, L.; Jiang, J.; Yang, F.; Cai, H.; Cai, J.; et al. Pathway of cytotoxicity induced by folic acid modified selenium nanoparticles in MCF-7 cells. Appl. Microbiol. Biotechnol. 2013, 97, 1051–1062. [Google Scholar] [CrossRef]
- Yu, S.; Luk, K.; Cheung, S.; Kwok, K.; Wong, K.; Chen, T. Polysaccharide-protein complex-decorated selenium nanosystem as an efficient bone-formation therapeutic. J. Mater. Chem. B 2018, 6, 5215–5219. [Google Scholar] [CrossRef]
- Li, Q.; Chen, T.; Yang, F.; Liu, J.; Zheng, W. Facile and controllable one-step fabrication of selenium nanoparticles assisted by L-cysteine. Mater. Lett. 2010, 64, 614–617. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Yu, Q.; Yang, L.; Sun, D.; Zhou, Y.; Liu, J. Epigallocatechin-3-gallate (EGCG)-stabilized selenium nanoparticles coated with tet-1 peptide to reduce amyloid-beta aggregation and cytotoxicity. ACS Appl. Mater. 2014, 6, 8475–8487. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, Z.; Zhang, Y.; Mu, J.; Chen, L.; Li, B.; Lin, X. Construction, characterization, and bioactive evaluation of nano-selenium stabilized by green tea nano-aggregates. LWT Food Sci. Technol. 2020, 129, 109475. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A study of controlled uptake and release of anthocyanins by oxidized starch microgels. J. Agric. Food Chem. 2013, 61, 5880–5887. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Shi, M.; Zhao, L.; Li, W.; Chen, Y.; Lu, M.; Wu, J.; Yuan, Q.; Li, Y. Absorption of whey protein isolated (WPI)-stabilized beta-carotene emulsions by oppositely charged oxidized starch microgels. Food Res. Int. 2015, 67, 315–322. [Google Scholar] [CrossRef]
- Li, Y.; Vries, R.; Kleijn, M.; Slaghek, T. Lysozyme uptake by oxidized starch polymer microgels. Biomacromolecules 2010, 11, 1754–1762. [Google Scholar] [CrossRef]
- Li, Y.; Kadam, S.; Abee, T.; Slaghek, T.M.; Timmermans, J.W.; Stuart, M.A.C.; Norde, W.; Kleijn, M.J. Antimicrobial lysozyme-containing starch microgel to target and inhibit amylase-producing microorganisms. Food Hydrocoll. 2012, 28, 28–35. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (-)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappa B/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; McClements, D.; Zou, L.; Liu, X.; Liu, F. Co-encapsulation of epigallocatechin gallate (EGCG) and curcumin by two proteins-based nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Q.; Qin, X.; Bhavsar, D.; Yang, L.; Chen, Q.; Zheng, W.; Chen, L.; Liu, J. Improving the anticancer efficacy of laminin receptor-specific therapeutic ruthenium nanoparticles (RuBB-Loaded EGCG-RuNPs) via ROS-dependent apoptosis in SMMC-7721 cells. ACS Appl. Mater. Interfaces 2016, 8, 15000–15012. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.; Santos, J.F.; Bitoque, D.; Silva, G.A.; Marletta, A.; Nunes, V.A.; Ribeiro, P.A.; Silva, J.C.; Raposo, M. Polycaprolactone/gelatin nanofiber membranes containing EGCG-loaded liposomes and their potential use for skin regeneration. ACS Appl. Biol. Mater. 2019, 2, 4790–4800. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Liu, T.; Xu, H.; Meng, Y.; Li, W.; Xu, X.; Zhang, L. Construction of highly stable selenium nanoparticles embedded in hollow nanofibers of polysaccharide and their antitumor activities. Nano Res. 2017, 10, 3775–3789. [Google Scholar] [CrossRef]
- Su, J.; Guo, Q.; Chen, Y.; Mao, L.; Gao, Y.; Yuan, F. Utilization of beta-lactoglobulin- (-)-Epigallocatechin-3-gallate(EGCG) composite colloidal nanoparticles as stabilizers for lutein pickering emulsion. Food Hydrocoll. 2020, 98, 105293. [Google Scholar]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (-)-epigallocatechin-3-gallate. J. Agric. Food. 2013, 61, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, D.; Lv, X.; Liu, X.; Xu, W.; Chen, L.; Cai, J.; Din, Z.; Cheng, S. Green synthesis of robust selenium nanoparticles via polysaccharide-polyphenol interaction: Design principles and structure-bioactivity relationship. ACS Sustain. Chem. Eng. 2022, 10, 2052–2062. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, F.; Zheng, S.; Huang, X.; Zhang, J.; Zhang, P.; Fei, P. Preparation of lipid-Soluble bilberry anthocyanins through acylation with cinnamic acids and their antioxidation activities. J. Agric. Food Chem. 2020, 68, 7467–7473. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Peng, Y.; Gao, L.; Wang, F.; Wang, L.; Wei, X. A multifunctional layered nickel silicate nanogenerator of synchronous oxygen self-supply and superoxide radical generation for hypoxic tumor therapy. ACS Nano 2022, 16, 974–983. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat beta-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical stability and functional properties of selenium nanoparticles stabilized by chitosan, carrageenan, and gum Arabic. Carbohydr. Polym. 2021, 255, 117379. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical and functional properties of chitosan-stabilized selenium nanoparticles under different processing treatments. Food Chem. 2020, 331, 127378. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Shekhar, S.; Kumar, V.; Jana, N.R.; Jana, N.R. Efficient inhibition of protein aggregation, disintegration of aggregates, and lowering of cytotoxicity by green tea polyphenol-based self-assembled polymer nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 20309–20318. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Zhang, W.; Zhang, Y.; Zhu, W.; Liu, Y.; Zhang, D.; Wang, J. pH-Assisted surface functionalization of selenium nanoparticles with curcumin to achieve enhanced cancer chemopreventive activity. RSC Adv. 2016, 6, 72213–72223. [Google Scholar] [CrossRef]
- Liao, W.; Yu, Z.; Lin, Z.; Lei, Z.; Ning, Z.; Regenstein, J.M.; Yang, J.G.; Ren, J. Biofunctionalization of selenium nanoparticle with dictyophora indusiata polysaccharide and its antiproliferative activity through death-receptor and mitochondria-mediated apoptotic pathways. Sci. Rep. 2015, 5, 18629. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In Vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. Acs Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef]
- Kulkarni, A.; Rao, P.; Natarajan, S.; Goldman, A.; Sabbisetti, V.S.; Khater, Y.; Korimerla, N.; Chandrasekar, V.; Mashelkar, R.A.; Sengupta, S.; et al. Reporter nanoparticle that monitors its anticancer efficacy in real time. Proc. Natl. Acad. Sci. USA 2016, 113, 2104–2113. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Yao, T.; Zhou, L.; Xiao, C.; Wang, Z.; Zhai, J.; Xing, J.; Chen, J.; Tan, G.; et al. Wireless electrochemotherapy by selenium-doped piezoelectric biomaterials to enhance cancer cell apoptosis. ACS Appl. Mater. Interfaces 2020, 12, 34505–34513. [Google Scholar] [CrossRef]
- Luo, H.; Yang, Y.; Duan, J.; Wu, P.; Jiang, Q.; Xu, C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013, 4, 3. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Liu, Y.; Hu, Y.; Zhang, D.; Xu, W.; Chen, L.; He, J.; Cheng, S.; Cai, J. Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity. Foods 2023, 12, 13. https://doi.org/10.3390/foods12010013
Zhou J, Liu Y, Hu Y, Zhang D, Xu W, Chen L, He J, Cheng S, Cai J. Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity. Foods. 2023; 12(1):13. https://doi.org/10.3390/foods12010013
Chicago/Turabian StyleZhou, Jiaojiao, Yuantao Liu, Yili Hu, Die Zhang, Wei Xu, Lei Chen, Jiangling He, Shuiyuan Cheng, and Jie Cai. 2023. "Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity" Foods 12, no. 1: 13. https://doi.org/10.3390/foods12010013
APA StyleZhou, J., Liu, Y., Hu, Y., Zhang, D., Xu, W., Chen, L., He, J., Cheng, S., & Cai, J. (2023). Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity. Foods, 12(1), 13. https://doi.org/10.3390/foods12010013