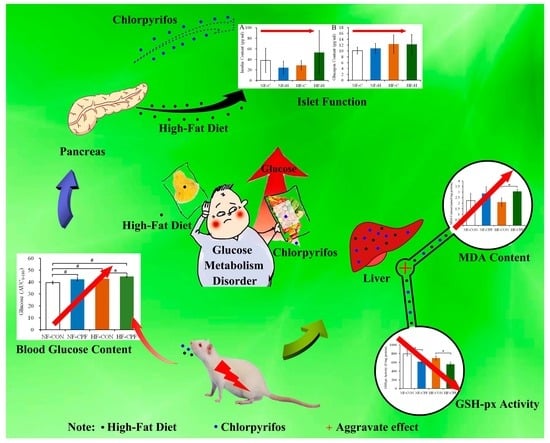
High-Fat Diet Aggravates the Disorder of Glucose Metabolism Caused by Chlorpyrifos Exposure in Experimental Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Design of the Experiment
2.3. Glucose Homeostasis Measurements
2.4. Detection of ALT, AST, and ALP Concentration in Serum
2.5. Antioxidant Function Determination of Liver
2.6. Detection of Glycogen and Glucose Content in Liver
2.7. Determination of Insulin and Glucagon Concentration in Serum
2.8. Statistical Analysis
3. Results
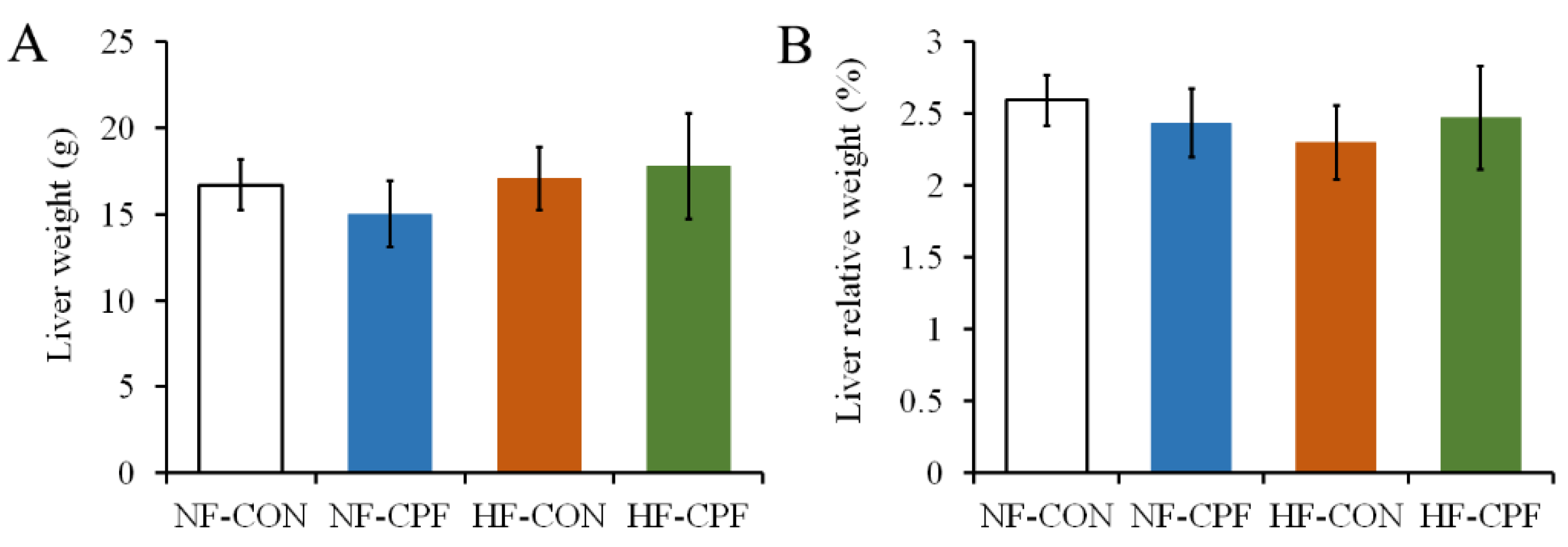
3.1. HF Diet CPF Did Not Affect Liver Weight
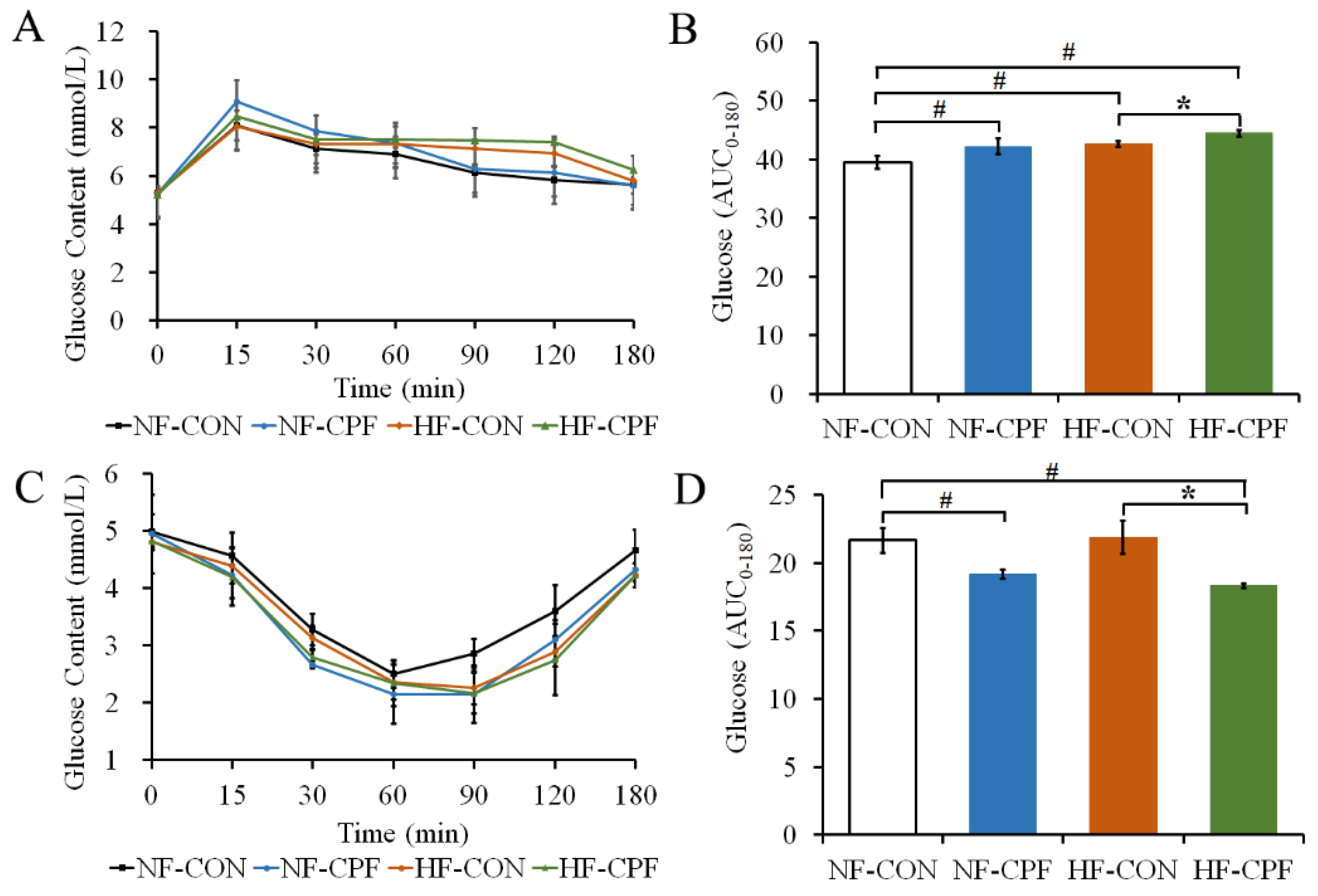
3.2. HF Diet Promoted a More Severe Glucose Intolerance for CPF Treatment
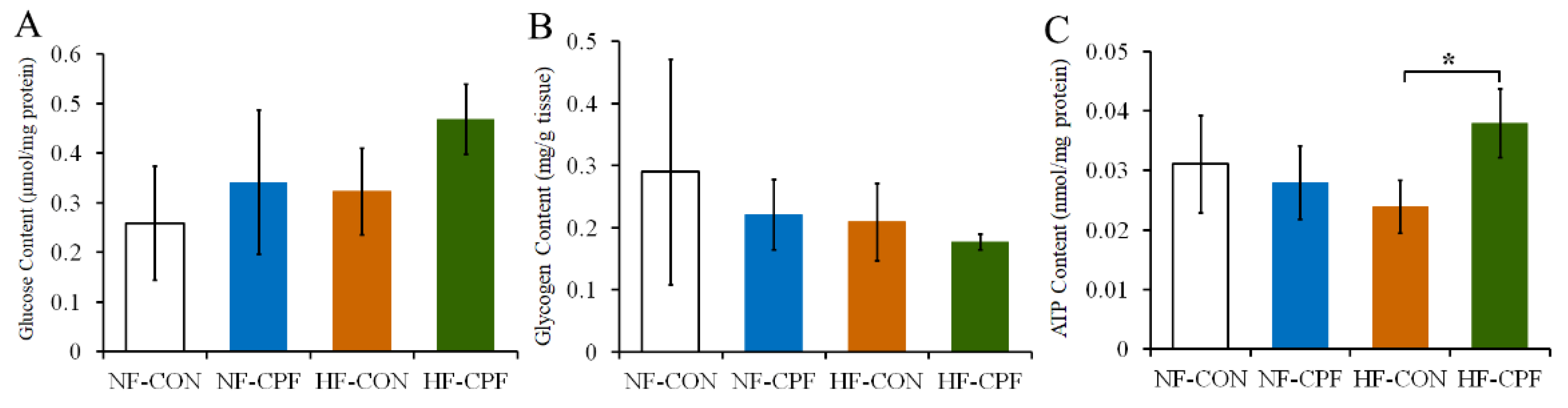
3.3. HF Diet and CPF Co-Treatment Led to the Disorder of Glucose Metabolism
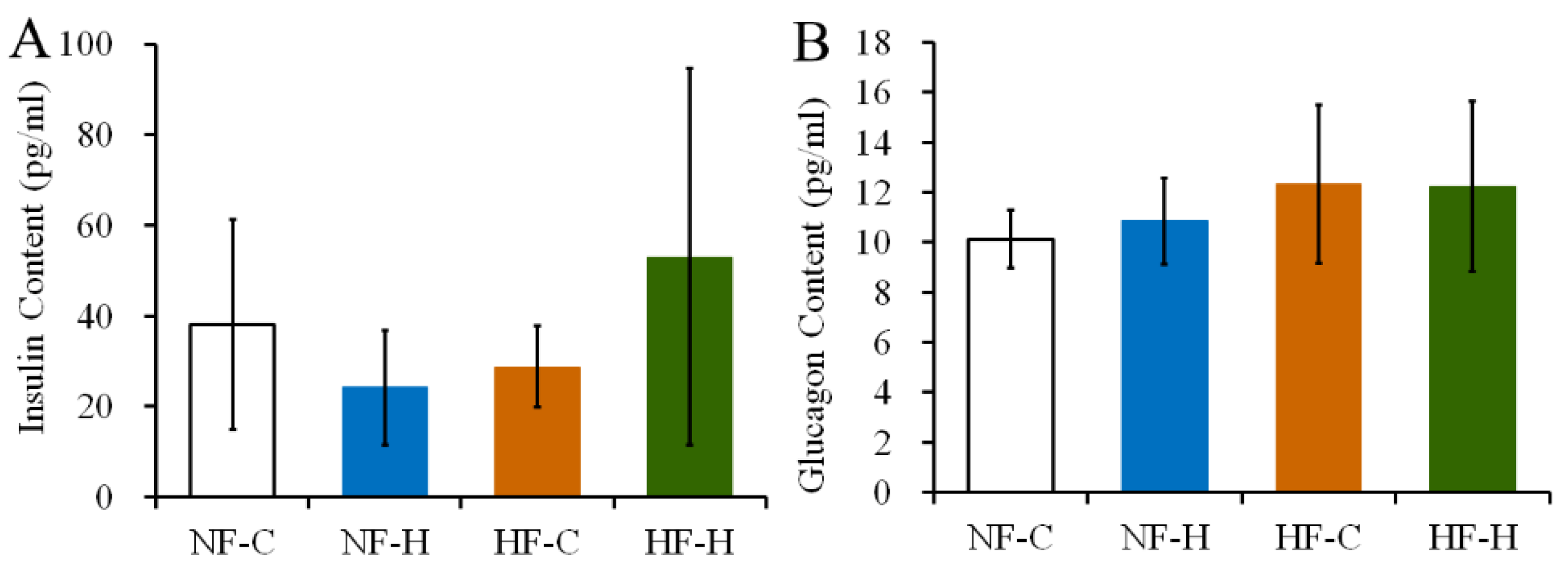
3.4. CPF Treatment Did Not Destroy the Function of Islet Cells in Rats under NF or HF Diet
3.5. CPF Treatment Damaged Liver Function of Rats under HF Diet
3.6. CPF and HF Diet Co-Treatment Damaged the Antioxidant Function of Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adedeji, T.G.; Jeje, S.O.; Omayone, T.P.; Agbonifo, W.O. Oxidative stress and inflammatory response to high dietary fat and carbonated soda intake in male and female Wistar rats. Nutrition 2022, 103, 111800. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Y.; Zhang, Y.; Qin, J.; Lei, X. Dietary experiences after bariatric surgery in patients with obesity: A qualitative systematic review. Obes. Surg. 2022, 32, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes—Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Pollack, C.C.; Onega, T.; Emond, J.A.; Vosoughi, S.; O’Malley, A.J.; McClure, A.C.; Rothstein, R.I.; Gilbert-Diamond, D. A national evaluation of geographic accessibility and provider availability of obesity medicine diplomates in the United States between 2011 and 2019. Int. J. Obes. 2022, 46, 669–675. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Xu, K.; Du, Y.; Liu, J.; Wang, J.; Jiang, Y. Fecal metabolomics reveals the positive effect of ethanol extract of propolis on T2DM mice. Food Sci. Hum. Wellness 2023, 12, 161–172. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Bai, Y.; Cheng, Z. Sex-specific and dose-response relationships of urinary cobalt and molybdenum levels with glucose levels and insulin resistance in US adults. J. Environ. Sci. 2023, 124, 42–49. [Google Scholar] [CrossRef]
- Beard, J.; Sladden, T.; Morgan, G.; Berry, G.; Brooks, L.; McMichael, A. Health impacts of pesticide exposure in a cohort of outdoor workers. Environ. Health Perspect. 2003, 111, 724–730. [Google Scholar] [CrossRef]
- Schreinemachers, D.M. Mortality from ischemic heart disease and diabetes mellitus (type 2) in four US wheat-producing states: A hypothesis-generating study. Environ. Health Perspect. 2006, 114, 186–193. [Google Scholar] [CrossRef]
- Guo, X.W.; Wang, H.; Song, Q.X.; Li, N.; Liang, Q.W.; Su, W.Y.; Liang, M.M.; Ding, X.X.; Sun, C.Y.; Lowe, S.; et al. Association between exposure to organophosphorus pesticides and the risk of diabetes among US Adults: Cross-sectional findings from the National Health and Nutrition Examination Survey. Chemosphere 2022, 301, 134471. [Google Scholar] [CrossRef]
- Kondakala, S.; Ross, M.K.; Chambers, J.E.; Howell, G.E., III. Effect of high fat diet on the toxicokinetics and toxicodynamics of chlorpyrifos following acute exposure in male C57BL/6J mice. J. Biochem. Mol. Toxicol. 2022, 36, e23028. [Google Scholar] [CrossRef] [PubMed]
- Anamika, S.; Kirty, P.; Singh Brar, D.; Avinash, T.; Vikas, N. A review on Api-products: Current scenario of potential contaminants and their food safety concerns. Food Control 2023, 145, 109499. [Google Scholar]
- Osaili, T.M.; Al-Natour, M.Q.; Al-Abboodi, A.R.; Alkarasneh, A.Y.; El Darra, N.; Khazaal, S.; Holley, R. Detection and risk associated with organochlorine, organophosphorus, pyrethroid and carbamate pesticide residues in chicken muscle and organ meats in Jordan. Food Control 2023, 144, 109355. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al Sallagi, M.S.; Dhanasekaran, D.K.; Odeh WA, B.; Al Ali, H.J.; Al Ali, A.A.; Obaid, R.S. Pesticide residues in fresh fruits imported into the United Arab Emirates. Heliyon 2022, 8, e11946. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Amirabadizadeh, A.; Samarghandian, S.; Mehrpour, O. Impact of chlorpyrifos on blood glucose concentration in an animal model: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2020, 27, 2474–2481. [Google Scholar] [CrossRef]
- Leonel Javeres, M.N.; Raza, S.; Judith, N.; Anwar, F.; Habib, R.; Batool, S.; Nurulain, S.M. Mixture of Organophosphates Chronic Exposure and Pancreatic Dysregulations in Two Different Population Samples. Front. Public Health 2020, 8, 534902. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Wang, B.; Tsakiridis, E.E.; Zhang, S.; Llanos, A.; Desjardins, E.M.; Yabut, J.M.; Green, A.E.; Day, E.A.; Smith, B.K.; Lally, J.S.V.; et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Djekkoun, N.; Depeint, F.; Guibourdenche, M.; El Sabbouri, H.E.K.; Corona, A.; Rhazi, L.; Gay-Queheillard, J.; Rouabah, L.; Hamdad, F.; Bach, V.; et al. Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring. Toxics 2022, 10, 138. [Google Scholar] [CrossRef]
- Ndonwi, E.N.; Atogho-Tiedeu, B.; Lontchi-Yimagou, E.; Shinkafi, T.S.; Nanfa, D.; Balti, E.V.; Katte, J.C.; Mbanya, A.; Matsha, T.; Mbanya, J.C.; et al. Metabolic effects of exposure to pesticides during gestation in female Wistar rats and their offspring: A risk factor for diabetes? Toxicol. Res. 2020, 36, 249–256. [Google Scholar] [CrossRef]
- Abdollahi, M.; Donyavi, M.; Pournourmohammadi, S.; Saadat, M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2004, 137, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Yi-Ching, C.; Man-Hui, P.; Yi-Tien, C.; Yu-Chen, H. Dietary exposure to chlorpyrifos affects systemic and hepatic immune-cell phenotypes in diabetic mice. Toxicology 2021, 452, 152698. [Google Scholar]
- Li, J.; Pang, G.; Ren, F.; Fang, B. Chlorpyrifos-induced reproductive toxicity in rats could be partly relieved under high-fat diet. Chemosphere 2019, 229, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Wang, H.-P.; Liang, Y.-J.; Long, D.-X.; Chen, J.-X.; Hou, W.-Y.; Wu, Y.-J. Metabolic profiles of serum from rats after subchronic exposure to chlorpyrifos and carbaryl. Chem. Res. Toxicol. 2009, 22, 1026–1033. [Google Scholar] [CrossRef]
- Kulawiec, D.G.; Zhou, T.; Knopp, J.L.; Chase, J.G. Continuous glucose monitoring to measure metabolic impact and recovery in sub-elite endurance athletes. Biomed. Signal Process. Control 2021, 70, 103059. [Google Scholar] [CrossRef]
- Picard, A.; Berney, X.; Castillo-Armengol, J.; Tarussio, D.; Jan, M.; Sanchez-Archidona, A.R.; Croizier, S.; Thorens, B. Hypothalamic Irak4 is a genetically controlled regulator of hypoglycemia-induced glucagon secretion. Mol. Metab. 2022, 61, 101479. [Google Scholar] [CrossRef]
- Leonel Javeres, M.N.; Habib, R.; Judith Laure, N.; Abbas Shah, S.T.; Valis, M.; Kuca, K.; Muhammad Nurulain, S. Chronic Exposure to Organophosphates Pesticides and Risk of Metabolic Disorder in Cohort from Pakistan and Cameroon. Int. J. Environ. Res. Public Health 2021, 18, 2310. [Google Scholar] [CrossRef]
- Basiri, S.; Esmaily, H.; Vosough-Ghanbari, S.; Mohammadirad, A.; Yasa, N.; Abdollahi, M. Improvement by Satureja khuzestanica essential oil of malathion-induced red blood cells acetylcholinesterase inhibition and altered hepatic mitochondrial glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities. Pestic. Biochem. Physiol. 2007, 89, 124–129. [Google Scholar] [CrossRef]
- Rothman, D.L.; Magnusson, I.; Cline, G.; Gerard, D.; Kahn, C.R.; Shulman, R.G.; Shulman, G.I. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1995, 92, 983–987. [Google Scholar] [CrossRef]
- Salek-Maghsoudi, A.; Hassani, S.; Momtaz, S.; Shadboorestan, A.; Ganjali, M.R.; Ghahremani, M.H.; Hosseini, R.; No-rouzi, P.; Abdollahi, M. Biochemical and molecular evidence on the role of vaspin in early detection of the insulin re-sistance in a rat model of high-fat diet and use of diazinon. Toxicology 2019, 411, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Hassoun, E.A.; Stohs, S.J. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 1995, 104, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ogutcu, A.; Uzunhisarcikli, M.; Kalender, S.; Durak, D.; Bayrakdar, F.; Kalender, Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pestic. Biochem. Physiol. 2006, 86, 93–98. [Google Scholar] [CrossRef]
- Possamai, F.P.; Fortunato, J.J.; Feier, G.; Agostinho, F.R.; Quevedo, J.; Wilhelm Filho, D.; Dal-Pizzol, F. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ. Toxicol. Pharmacol. 2007, 23, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Uchendu, C.; Ambali, S.F.; Ayo, J.O.; Esievo, K.A.N. Chronic co-exposure to chlorpyrifos and deltamethrin pesticides induces alterations in serum lipids and oxidative stress in Wistar rats: Mitigating role of alpha-lipoic acid. Environ. Sci. Pollut. Res. 2018, 25, 19605–19611. [Google Scholar] [CrossRef]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Akturk, O.; Demirin, H.; Sutcu, R.; Yilmaz, N.; Koylu, H.; Altuntas, I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol. Toxicol. 2006, 22, 455–461. [Google Scholar] [CrossRef]
- Wang, X.; Shen, M.; Zhou, J.; Jin, Y. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2019, 216, 19–28. [Google Scholar] [CrossRef]
- Acker, C.I.; Nogueira, C.W. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere 2012, 89, 602–608. [Google Scholar] [CrossRef]
- Ismail, A.A.; Hendy, O.; Rasoul, G.A.; Olson, J.R.; Bonner, M.R.; Rohlman, D.S. Acute and Cumulative Effects of Repeated Exposure to Chlorpyrifos on the Liver and Kidney Function among Egyptian Adolescents. Toxics 2021, 9, 137. [Google Scholar] [CrossRef]
- Li, F.; Xiang, B.; Jin, Y.; Li, C.; Ren, S.; Wu, Y.; Li, J.; Luo, Q. Hepatotoxic effects of inhalation exposure to polycyclic aromatic hydrocarbons on lipid metabolism of C57BL/6 mice. Environ. Int. 2020, 134, 105000. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.S.I.; Bizerra, P.F.V.; Mito, M.S.; Constantin, R.P.; Klosowski, E.M.; De Souza BT, L.; Constantin, R.P. The metabolic and toxic acute effects of phloretin in the rat liver. Chem. Biol. Interact. 2022, 364, 110054. [Google Scholar] [CrossRef] [PubMed]
- Seesen, M.; Pratchayasakul, W.; Pintana, H.; Chattipakorn, N.; Chattipakorn, S.C. Exposure to organophosphates in association with the development of insulin resistance: Evidence from in vitro, in vivo, and clinical studies. Food Chem. Toxicol. 2022, 168, 113389. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Glucose-6-Phosphate Dehydrogenase Deficiency Activates Endothelial Cell and Leukocyte Adhesion Mediated via the TGFβ/NADPH Oxidases/ROS Signaling Pathway. Int. J. Mol. 2020, 21, 7474. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Zhang, Z.; Cheng, W.; Liu, G.; Zhao, G. High-Fat Diet Aggravates the Disorder of Glucose Metabolism Caused by Chlorpyrifos Exposure in Experimental Rats. Foods 2023, 12, 816. https://doi.org/10.3390/foods12040816
Li J, Li X, Zhang Z, Cheng W, Liu G, Zhao G. High-Fat Diet Aggravates the Disorder of Glucose Metabolism Caused by Chlorpyrifos Exposure in Experimental Rats. Foods. 2023; 12(4):816. https://doi.org/10.3390/foods12040816
Chicago/Turabian StyleLi, Jinwang, Xiude Li, Zhihui Zhang, Weilong Cheng, Guangmin Liu, and Guoping Zhao. 2023. "High-Fat Diet Aggravates the Disorder of Glucose Metabolism Caused by Chlorpyrifos Exposure in Experimental Rats" Foods 12, no. 4: 816. https://doi.org/10.3390/foods12040816
APA StyleLi, J., Li, X., Zhang, Z., Cheng, W., Liu, G., & Zhao, G. (2023). High-Fat Diet Aggravates the Disorder of Glucose Metabolism Caused by Chlorpyrifos Exposure in Experimental Rats. Foods, 12(4), 816. https://doi.org/10.3390/foods12040816