Evolution of Free Amino Acids, Histamine and Volatile Compounds in the Italian Anchovies (Engraulis encrasicolus L.) Sauce at Different Ripening Times
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Process Description
2.2. Physical and Chemical Analysis
2.3. Free Amino Acids Determination
2.4. Histamine Determination
2.5. Analysis of Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Free Amino Acids
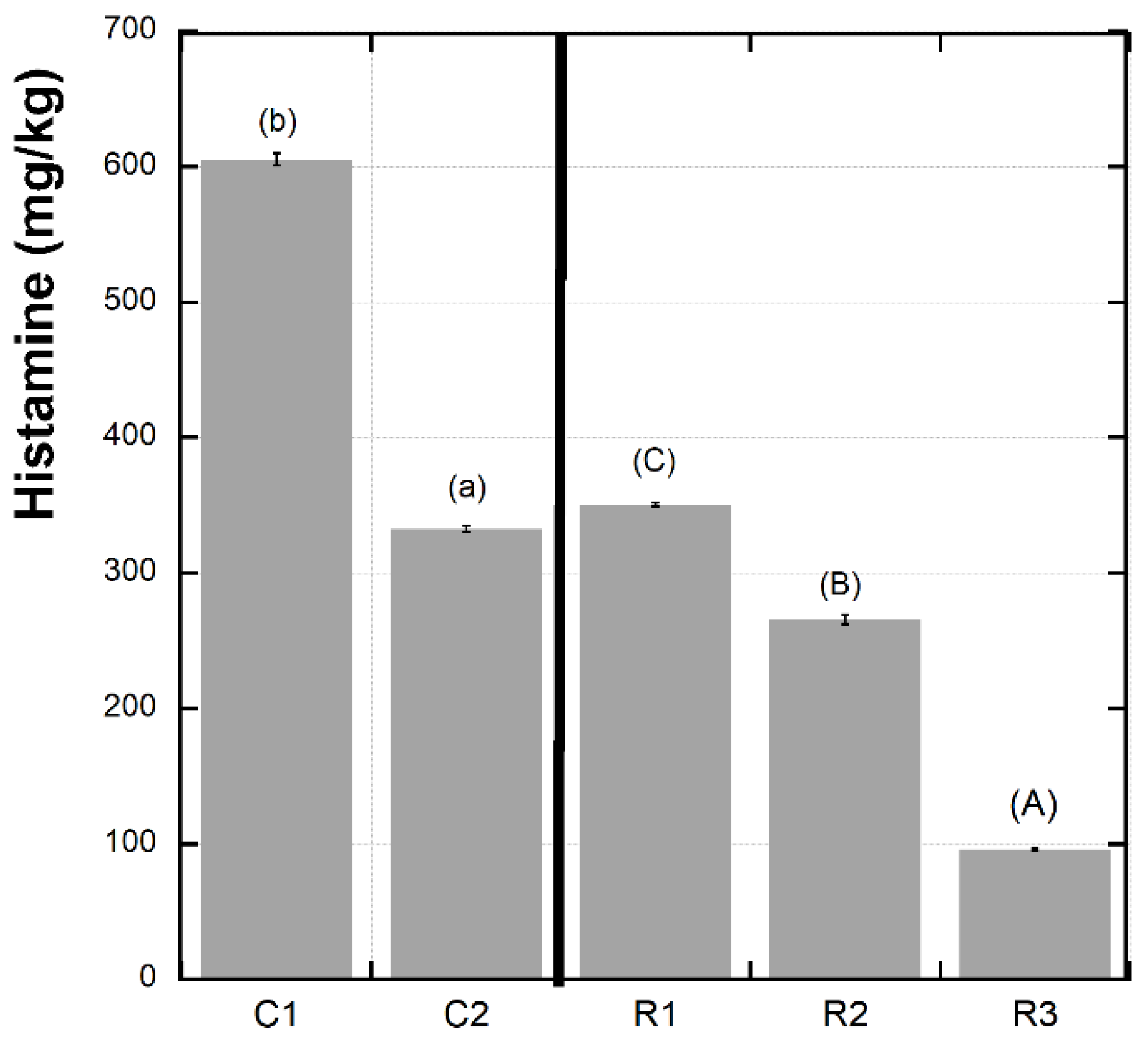
3.3. Histamine
3.4. Volatile Compounds
| Aroma Compounds | Sensory Threshold (ppb) | Descriptor | OAV | ||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | R1 | R2 | R3 | |||
| Alcohol | |||||||
| 1-Hexanol | 9 | Herbaceous, grass [32] | 2.80 | 1.62 | 2.26 | 2.43 | 5.63 |
| 1-Heptanol | 2.4 | Resin, floral, green [33] | 167.85 | 103.64 | 94.04 | 99.06 | 102.94 |
| 1-Nonanol | 45.5 | Dusty, oily [30] | 1.47 | 0.41 | n.c. | n.c. | n.c. |
| 1-Dodecanol | 1.50 | Floral, fruity, fatty [34] | 18.55 | 113.52 | 37.01 | 31.75 | 28.60 |
| Aldehydes | |||||||
| 1-Octanal | 0.70 | Honey, green [35] | 89.35 | 311.17 | 218.22 | n.c. | n.c. |
| Nonanal | 1 | Fatty, wax, soapy [36] | 352.72 | 269.01 | 1022.16 | 1016.34 | 56.75 |
| Decanal | 6 | Fruity, orange [33] | 30.63 | 36.69 | 3.28 | 3.29 | 10.77 |
| Phenylethanal | 5 | Hawthorne, honey [37] | 9.45 | 5.28 | 2.60 | 2.33 | n.c. |
| Acids | |||||||
| Butanoic acid | 240 | Cheesy [38] | 0.65 | 0.80 | 0.18 | 0.22 | 0.28 |
| Isovaleric acid | 30 | Cheesy [39] | 6.86 | 78.02 | 8.62 | 21.56 | 186.21 |
| Ketones | Soap, green [1] | ||||||
| Acetophenone | 65 | 1.33 | 0.71 | 2.54 | 1.53 | 1.22 | |
| others | |||||||
| Dimethyl trisulphide | 0.36 | Sulfur, rotten cabbage [40] | 64.07 | 76.17 | n.c. | n.c. | n.c. |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Zhang, J.; Zhu, Y.; Wang, X.; Shi, W. Volatile components present in different parts of grass carp. J. Food Biochem. 2018, 42, e12668. [Google Scholar] [CrossRef]
- Lowe, B. Fish sauces in ancient Rome: A historic perspective on the use of free glutamates to enhance flavor. In Proceedings of the 8th Pangborn Conference, ESN Workshop: Characteristics of Memory for Foods: Consequences for Sensory and Consumer Science, Florence, Italy, 27 July 2009. [Google Scholar]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor characteristics of shrimp sauces with different fermentation and storage time. LWT 2019, 110, 142–151. [Google Scholar] [CrossRef]
- Curtis, R.I. Umami and the foods of classical antiquity. Am. J. Clin. Nutr. 2009, 90, 712S–718S. [Google Scholar] [CrossRef] [PubMed]
- Michihata, T.; Sado, Y.; Yano, T.; Enomoto, T. Preparation of ISHIRU (Fish Sauce) by a Quick Ripening Process and Changes in the Composition of Amino Acids, Oligopeptides and Organic Acids during Processing. Nippon. Shokuhin Kagaku Kogaku Kaishi 2000, 47, 369–377. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2020/1529 of 14 October 2020 Entering a Name in the Register of Protected Designations of Origin and Protected Geographical Indications (‘Colatura di alici di Cetara’ (PDO)); European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Su, G.; Zhao, H.; Wang, C.; Zhao, M. Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chem. 2014, 145, 126–134. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1019/2013 of 23 October 2013 Amending Annex I to Regulation (EC) No 2073/2005 as Regards Histamine in Fishery Products; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Cinquina, A.L.; Longo, F.; Calì, A.; De Santis, L.; Baccelliere, R.; Cozzani, R. Validation and comparison of analytical methods for the determination of histamine in tuna fish samples. J. Chromatogr. A 2004, 1032, 79–85. [Google Scholar] [CrossRef]
- Corona, O.; Liguori, L.; Albanese, D.; Di Matteo, M.; Cinquanta, L.; Russo, P. Quality and volatile compounds in red wine at different degrees of dealcoholization by membrane process. Eur. Food Res. Technol. 2019, 245, 2601–2611. [Google Scholar] [CrossRef]
- Mueda, R.T. Physico-chemical and color characteristics of saltfermented fish sauce from anchovy stolephorus commersonii. AACL Bioflux 2015, 8, 565–572. [Google Scholar]
- Russo, G.L.; Langellotti, A.L.; Genovese, A.; Martello, A.; Sacchi, R. Volatile compounds, physicochemical and sensory characteristics of Colatura di Alici, a traditional Italian fish sauce. J. Sci. Food Agric 2020, 100, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K. Effects of the addition of spleen of skipjack tuna (Katsuwonus pelamis) on the liquefaction and characteristics of fish sauce made from sardine (Sardinella gibbosa). Food Chem. 2006, 98, 440–452. [Google Scholar] [CrossRef]
- Thongthai, C.; Gildberg, A.J.A.f.f. Asian Fish Sauce as a Source of Nutrition. In Asian Functional Food; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 215–265. [Google Scholar]
- Utami, R.; Wijaya, C.H.; Lioe, H.N. Taste of Water-Soluble Extracts Obtained from Over-Fermented Tempe. Int. J. Food Prop. 2016, 19, 2063–2073. [Google Scholar] [CrossRef]
- Xu, X.; Xu, R.; Song, Z.; Jia, Q.; Feng, T.; Huang, M.; Song, S. Identification of umami-tasting peptides from Volvariella volvacea using ultra performance liquid chromatography quadrupole time-of-flight mass spectrometry and sensory-guided separation techniques. J. Chromatogr. A 2019, 1596, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; In’t Veld, J.H. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sanceda, N.G.; Suzuki, E.; Ohashi, M.; Kurata, T. Histamine Behavior during the Fermentation Process in the Manufacture of Fish Sauce. J. Agric. Food Chem. 1999, 47, 3596–3600. [Google Scholar] [CrossRef]
- Martuscelli, M.; Crudele, M.A.; Gardini, F.; Suzzi, G. Biogenic amine formation and oxidation by Staphylococcus xylosus strains from artisanal fermented sausages. Lett. Appl. Microbiol. 2000, 31, 228–232. [Google Scholar] [CrossRef]
- Zaman, M.Z.; Bakar, F.A.; Selamat, J.; Bakar, J. Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J. Food Sci. 2010, 28, 440–449. [Google Scholar] [CrossRef]
- Zaman, M.Z.; Bakar, F.A.; Selamat, J.; Bakar, J.; Ang, S.S.; Chong, C.Y. Degradation of histamine by the halotolerant Staphylococcus carnosus FS19 isolate obtained from fish sauce. Food Control 2014, 40, 58–63. [Google Scholar] [CrossRef]
- Kuda, T.; Mihara, T.; Yano, T. Detection of histamine and histamine-related bacteria in fish-nukazuke, a salted and fermented fish with rice-bran, by simple colorimetric microplate assay. Food Control 2007, 18, 677–681. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Zhao, Y.; Wang, J.; Wei, Y.; Yang, D. Application of UHPLC-Q/TOF-MS-based metabolomics in the evaluation of metabolites and taste quality of Chinese fish sauce (Yu-lu) during fermentation. Food Chem. 2019, 296, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y.; Endo, K.; Shimoda, M. Identification of Distinctive Volatile Compounds in Fish Sauce. J. Agric. Food Chem. 2002, 50, 5412–5416. [Google Scholar] [CrossRef]
- Van Ba, H.; Ryu, K.S.; Lan, N.T.K.; Hwang, I. Influence of particular breed on meat quality parameters, sensory characteristics, and volatile components. Food Sci. Biotechnol. 2013, 22, 651–658. [Google Scholar] [CrossRef]
- Cha, Y.J.; Cadwallader, K.R. Volatile Components in Salt-Fermented Fish and Shrimp Pastes. J. Food Sci 1995, 60, 19–24. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric characterization of aroma active compounds in fermented fish paste in comparison with fish sauce, fermented soy paste and sauce products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Rolls, E.T. Chapter 7-Taste and smell processing in the brain. In Handbook of Clinical Neurology; Doty, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 97–118. [Google Scholar]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Flavour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners B.V.: Utrecht, The Netherlands, 2003. [Google Scholar]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. Kent): Impact of drying on volatile composition. Int. J. Food Sci. 2016, 51, 789–800. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of Aged Red Wine Aroma Properties from Aroma Chemical Composition. Partial Least Squares Regression Models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Azodanlou, R.; Darbellay, C.; Luisier, J.-L.; Villettaz, J.-C.; Amadò, R. Changes in flavour and texture during the ripening of strawberries. Eur. Food Res. Technol. 2004, 218, 167–172. [Google Scholar] [CrossRef]
- Vigentini, I.; Maghradze, D.; Petrozziello, M.; Bonello, F.; Mezzapelle, V.; Valdetara, F.; Failla, O.; Foschino, R. Indigenous georgian wine-associated yeasts and grape cultivars to edit the wine quality in a precision oenology perspective. Front. Microbiol. 2016, 7, 352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, W.; Qian, M.C. Characterization of Aroma Compounds of Chinese “Wuliangye” and “Jiannanchun” Liquors by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
| Mobile Phase (0.250 mL/min) | Time/Potential Waveform | |||||
|---|---|---|---|---|---|---|
| Time (min) | H2O (%) | NaOH (%) | NaOAc (%) | Time (sec) | Potential (V) | Integration |
| 0.0 | 80 | 20 | 0 | 0.00 | +0.13 | |
| 2.0 | 80 | 20 | 0 | 0.04 | +0.13 | |
| 12.0 | 80 | 20 | 0 | 0.05 | +0.28 | |
| 16.0 | 68 | 32 | 0 | 0.11 | +0.28 | began |
| 24.0 | 36 | 24 | 40 | 0.12 | +0.60 | |
| 40.0 | 36 | 24 | 40 | 0.41 | +0.60 | |
| 40.1 | 20 | 80 | 0 | 0.42 | +0.28 | |
| 42.1 | 20 | 80 | 0 | 0.56 | +0.28 | end |
| 42.2 | 80 | 20 | 0 | 0.57 | −1.67 | |
| 62.0 | 80 | 20 | 0 | 0.58 | −1.67 | |
| 0.59 | +0.93 | |||||
| 0.60 | +0.13 | |||||
| Samples | C1 | C2 | R1 | R2 | R3 |
|---|---|---|---|---|---|
| moisture % | 62.72 ± 0.47 a | 66.36 ± 0.50 b | 67.34 ± 0.40 C | 66.14 ± 0.50 B | 59.70 ± 0.45 A |
| pH | 5.41 ± 0.04 a | 5.81 ± 0.04 b | 5.73 ± 0.05 B | 5.54 ± 0.04 A | 5.84 ± 0.04 B |
| L* | 30.40 ± 0.23 b | 29.11 ± 0.22 a | 31.16 ± 0.62 B | 34.08 ± 0.26 C | 25.59 ± 0.20 A |
| a* | 12.74 ±0.10 b | 9.77 ± 0.08 a | 11.21 ± 0.11 B | 11.32 ± 0.09 B | 2.72 ± 0.02 A |
| b* | 7.61 ± 0.06 b | 5.50 ± 0.04 a | 11.73 ± 0.06 B | 13.63 ± 0.10 C | 0.23 ± 0.00 A |
| C | 14.84 ± 0.12 b | 11.21 ± 0.09 a | 16.22 ± 0.12 B | 17.74 ± 0.14 C | 2.73 ± 0.11 A |
| Hue | 30.85 ± 0.00 b | 29.37 ± 0.02 a | 46.30 ± 0.16 B | 50.21 ± 0.01 C | 4.82 ± 0.03 A |
| AMINO ACIDS | C1 | C2 | R1 | R2 | R3 | |
|---|---|---|---|---|---|---|
| Non ESSENTIAL | Arginine | 0.167 ± 0.033 a | 0.182 ± 0.025 a | 0.162 ± 0.020 A | 0.154 ± 0.013 A | 0.151 ± 0.044 A |
| Hydroxylysine | 0.210 ± 0.021 a | 0.270 ± 0.038 a | 0.206 ± 0.011 B | 0.115 ± 0.006 A | 0.201 ± 0.006 B | |
| Glutamine | 0.291 ± 0.002 b | 0.168 ± 0.017 a | 0.255 ± 0.003 B | 0.275 ± 0.002 C | 0.227 ± 0.011 A | |
| Alanine | 0.597 ± 0.013 b | 0.437 ± 0.037 a | 0.403 ± 0.012 A | 0.639 ± 0.041 C | 0.568 ± 0.001 B | |
| Glycine | 0.275 ± 0.136 a | 0.227 ± 0.009 a | 0.248 ± 0.004 A | 0.249 ± 0.026 A | 0.407 ± 0.006 B | |
| Serine | 0.369 ± 0.032 a | 0.358 ± 0.001 a | 0.262 ± 0.021 A | 0.342 ± 0.008 B | 0.477 ± 0.013 C | |
| Proline | 0.214 ± 0.011 b | 0.182 ± 0.001 a | 0.189 ± 0.010 A | 0.189 ± 0.018 A | 0.361 ± 0.024 B | |
| Glutamate | 0.697 ± 0.081 a | 0.642 ± 0.017 a | 0.555 ± 0.026 A | 0.562 ± 0.022 A | 0.727 ± 0.018 B | |
| Aspartate | 0.611 ± 0.039 b | 0.504 ± 0.027 a | 0.447 ± 0.016 A | 0.438 ± 0.005 A | 0.608 ± 0.019 B | |
| Tyrosine | 0.021 ± 0.003 a | 0.063 ± 0.009 b | 0.051 ± 0.001 B | 0.060 ± 0.001 C | 0.036 ± 0.003 A | |
| ESSENTIAL | Lysine | 0.572 ± 0.008 a | 0.546 ± 0.024 a | 0.415 ± 0.008 A | 0.419 ± 0.012 A | 0.543 ± 0.009 B |
| Threonine | 0.374 ± 0.023 a | 0.374 ± 0.015 a | 0.312 ± 0.010 A | 0.378 ± 0.002 B | 0.539 ± 0.002 C | |
| Valine | 0.367 ± 0.018 b | 0.326 ± 0.011 a | 0.360 ± 0.018 A | 0.354 ± 0.032 A | 0.510 ± 0.013 B | |
| Isoleucine | 0.325 ± 0.029 b | 0.265 ± 0.018 a | 0.295 ± 0.011 A | 0.246 ± 0.040 A | 0.256 ± 0.009 A | |
| Leucine | 0.423 ± 0.047 a | 0.371 ± 0.041 a | 0.467 ± 0.036 B | 0.455 ± 0.028 B | 0.285 ± 0.014 A | |
| Methionine | 0.286 ± 0.035 b | 0.196 ± 0.017 a | 0.154 ± 0.021 A | 0.158 ± 0.015 A | 0.153 ± 0.005 A | |
| Cysteine | 0.041 ± 0.009 a | 0.040 ± 0.001 a | 0.036 ± 0.001 B | 0.032 ± 0.001 A | 0.044 ± 0.001 C | |
| Histidine | 0.406 ± 0.034 a | 0.428 ± 0.010 a | 0.345 ± 0.020 B | 0.288 ± 0.017 A | 0.383 ± 0.024 B | |
| Phenylalanine | 0.298 ± 0.020 b | 0.174 ± 0.020 a | 0.217 ± 0.017 A | 0.209 ± 0.009 A | 0.265 ± 0.007 B | |
| TOTAL | 6.54 ± 0.59 a | 5.75 ± 0.34 a | 5.40 ± 0.26 A | 5.56 ± 0.30 A | 6.74 ± 0.23 B |
| C1 | C2 | R1 | R2 | R3 | |
|---|---|---|---|---|---|
| Ethyl octanoate | 64.65 ± 1.91 b | 51.37 ± 1.52 a | 38.37 ± 0.98 A | 39.89 ± 1.18 A | 56.20 ± 1.66 B |
| Ethyl nonanoate | 25.79 ± 0.74 a | 57.91 ± 1.65 b | 18.22 ± 0.56 A | 17.75 ± 0.51 A | 53.54 ± 1.53 B |
| Butyryl lactone | 62.90 ± 1.59 b | 49.63 ± 1.25 a | 22.23 ± 0.66 A | 28.44 ± 0.72 cC | 24.06 ± 0.61 B |
| Ethyl phenylacetate | n.d. | 19.41 ± 0.93 | n.d. | 13.25 ± 0.51 aA | 165.15 ± 7.04 B |
| -Valerolactone | 61.75 ± 2.95 a | 104.53 ± 4.99 b | 32.55 ± 1.67 A | 34.94 ± 1.67 A | 71.07 ± 3.39 B |
| Ethyldodecanoate | 5.97 ± 0.28 | n.d. | 9.27 ± 0.22 B | 7.58 ± 0.29 A | 12.64 ± 0.54 C |
| Total Esters | 221.07 ± 7.46 a | 282.85 ± 10.33 b | 120.64 ± 4.09 A | 141.86 ± 4.87 B | 382.66 ± 14.76 C |
| 1-Hexanol | 25.17 ± 0.82 b | 14.62 ± 0.48 a | 20.33 ± 0.62 A | 21.84 ± 0.71 A | 50.64 ± 1.65 B |
| 1-Heptanol | 402.83 ± 9.18 b | 248.73 ± 5.67 a | 225.70 ± 3.27 A | 237.75 ± 5.42 B | 247.05 ± 5.63 B |
| 2-Ethyl-Hexanol | 49.26 ± 1.10 a | 66.27 ± 1.48 b | 198.18 ± 6.12 C | 126.26 ± 2.82 B | 76.27 ± 1.70 A |
| 1-Octanol | 34.29 ± 1.88 b | 11.56 ± 0.58 a | 96.05 ± 3.05 B | 95.45 ± 2.93 B | 81.50 ± 2.50 A |
| 1-Nonanol | 67.00 ± 1.50 b | 18.50 ± 0.41 a | n.d. | n.d. | n.d. |
| a-Terpineol (terpenoid) | 21.84 ± 0.48 | n.d. | n.d. | n.d. | n.d. |
| Phenylethylalcohol | 12.92 ± 0.29 a | 250.06 ± 5.58 b | 31.78 ± 0.83 A | 57.95 ± 1.29 B | 211.92 ± 4.73 C |
| 1-Dodecanol | 27.82 ± 1.03 a | 170.28 ± 6.29 b | 55.52 ± 4.22 B | 47.62 ± 1.76 A | 42.90 ± 1.59 A |
| Total Alcohols | 641.14 ± 16.28 a | 780.02 ± 20.49 b | 627.56 ± 18.11 B | 586.88 ± 14.93 A | 710.27 ± 17.80 C |
| 1-Octanal | 62.54 ± 1.76 a | 217.82 ± 6.12 b | 152.76 ± 6.22 | n.d. | n.d. |
| Nonanal | 352.72 ± 11.20 b | 269.01 ± 8.54 a | 1022.16 ± 15.38 B | 1016.34 ± 32.28 B | 56.75 ± 1.80 A |
| Decanal | 183.76 ± 8.06 a | 220.12 ± 9.66 b | 19.71 ± 1.05 A | 19.74 ± 0.87 A | 64.63 ± 2.84 B |
| Benzaldehyde | 84.19 ± 2.38 a | 123.15 ± 3.48 b | 76.95 ± 3.55 A | 114.16 ± 3.22 B | 288.39 ± 8.14 C |
| Phenylethanal | 47.27 ± 1.06 b | 26.39 ± 0.59 a | 12.99 ± 0.78 B | 11.63 ± 0.26 A | n.d. |
| 2-Undecenal | 11.99 ± 0.52 | n.d. | 98.74 ± 3.15 B | 36.55 ± 1.40 A | n.d. |
| Total Aldehydes | 742.47 ± 24.97 a | 856.49 ± 28.39 b | 1383.31 ± 30.13 C | 1198.43 ± 38.03 B | 409.77 ± 12.78 A |
| Acetic acid | 1040.52 ± 38.73 b | 660.35 ± 24.58 a | 66.29 ± 1.99 A | 77.28 ± 2.88 A | 517.09 ± 19.25 B |
| Propionic acid | 61.66 ± 1.95 a | 157.16 ± 4.96 b | 13.88 ± 0.77 A | 15.29 ± 0.48 A | 61.49 ± 1.94 B |
| Isobutyric acid | 214.89 ± 8.10 a | 314.22 ± 11.83 b | 29.56 ± 0.15 A | 37.82 ± 1.42 A | 439.05 ± 16.54 B |
| Butanoic acid | 157.10 ± 5.81 a | 193.14 ± 7.14 b | 49.27 ± 2.11 A | 53.96 ± 1.99 A | 67.77 ± 2.50 B |
| Isovaleric acid | 205.95 ± 7.98 a | 2340.67 ± 90.65 b | 258.67 ± 5.24 A | 646.82 ± 25.05 B | 5586.36 ± 216.35 C |
| Pentanoic acid | 27.76 ± 1.20 a | 60.85 ± 2.63 b | 24.58 ± 0.44 A | 27.58 ± 1.19 B | 35.63 ± 1.54 C |
| Hexanoic acid | 85.91 ± 3.17 a | 351.91 ± 12.99 b | 77.25 ± 3.18 A | 84.50 ± 3.12 A | 276.22 ± 10.10 B |
| Eptanoic acid | 27.82 ± 1.06 a | 63.39 ± 2.43 b | 58.86 ± 1.58 B | 62.64 ± 2.40 B | 31.05 ± 1.19 A |
| Octanoic acid | 62.90 ± 1.90 a | 158.84 ± 4.81 b | 69.64 ± 1.11 A | 71.60 ± 2.17 A | 76.34 ± 2.31 B |
| Nonanoic acid | 65.46 ± 2.25 a | 154.61 ± 5.32 b | 408.52 ± 9.66 B | 489.74 ± 16.85 C | 116.00 ± 3.99 A |
| Decanoic acid | 108.52 ± 4.46 a | 184.27 ± 7.58 b | 130.83 ± 4.33 B | 123.74 ± 5.09 B | 104.32 ± 4.29 A |
| Benzoic acid | 136.86 ± 5.81 a | 229.96 ± 9.76 b | 56.87 ± 2.81 A | 68.06 ± 2.89 A | 407.59 ± 17.29 B |
| Dodecanoic acid | 107.20 ± 3.67 a | 232.96 ± 7.98 b | 136.55 ± 4.21 C | 93.61 ± 3.20 B | 73.84 ± 2.53 A |
| Phenylacetic acid | n.d. | 26.80 ±1.03 a | n.d. | n.d. | 516.09 ± 21.99 |
| Tetradecanoic acid | 383.27 ± 18.11 a | 821.60 ± 38.82 b | 589.10 ± 18.22 C | 332.15 ± 15.69 B | 288.16 ± 13.61 A |
| Pentadecanoic acid | 65.78 ± 2.17 a | 133.14 ± 4.40 b | 68.78 ± 3.25 C | 62.15 ± 2.05 B | 42.85 ± 1.42 A |
| Hexadecanoic acid | 1912.37 ± 72.37 a | 2727.94 ± 103.23 b | 1478.50 ± 34.68 C | 1066.51 ± 40.36 B | 797.53 ± 30.18 A |
| Heptadecanoic acid | 56.41 ± 2.15 a | 89.03 ± 3.40 b | 49.33 ± 0.25 B | 53.08 ± 2.03 C | 16.34 ± 0.62 A |
| Octadecanoic acid | 567.29 ± 25.96 a | 837.46 ± 38.32 b | 352.88 ± 13.13 B | 347.01 ± 15.88 B | 230.32 ± 10.54 A |
| Total Acids | 5287.66 ± 206.84 a | 9738.36 ± 381.84 b | 3919.36 ± 107.11 A | 3713.55 ± 144.74 A | 9684.03 ± 378.28 B |
| Acetophenone | 86.71 ± 1.94 b | 46.20 ± 1.03 a | 165.42 ± 4.02 C | 99.65 ± 2.22 B | 79.11 ± 1.77 A |
| 1,2-Cyclopentanedione | 52.66 ± 1.18 b | 4.29 ± 0.10 a | n.d. | 12.58 ± 0.28 | n.d. |
| 2-Pentadecanone | 30.82 ± 1.06 a | 67.15 ± 2.31 b | 18.16 ± 0.34 B | 21.23 ± 0.73 C | 15.70 ± 0.54 A |
| Total Ketones | 170.19 ± 4.17 b | 117.64 ± 3.43 a | 183.58 ± 4.36 C | 133.45 ± 3.23 B | 94.81 ± 2.30 A |
| Pyridine | 133.77 ± 2.99 a | 176.15 ± 3.93 b | 182.11 ± 1.40 A | 187.35 ± 4.18 B | 236.05 ± 5.27 C |
| Nitrogen-containing | |||||
| Dimethyl trisulfide | 23.07 ± 0.86 a | 27.42 ± 1.03 b | n.d. | n.d. | n.d. |
| Sulfur-containing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona, O.; Cinquanta, L.; Li Citra, C.; Mazza, F.; Ferrantelli, V.; Cammilleri, G.; Marconi, E.; Cuomo, F.; Messia, M.C. Evolution of Free Amino Acids, Histamine and Volatile Compounds in the Italian Anchovies (Engraulis encrasicolus L.) Sauce at Different Ripening Times. Foods 2023, 12, 126. https://doi.org/10.3390/foods12010126
Corona O, Cinquanta L, Li Citra C, Mazza F, Ferrantelli V, Cammilleri G, Marconi E, Cuomo F, Messia MC. Evolution of Free Amino Acids, Histamine and Volatile Compounds in the Italian Anchovies (Engraulis encrasicolus L.) Sauce at Different Ripening Times. Foods. 2023; 12(1):126. https://doi.org/10.3390/foods12010126
Chicago/Turabian StyleCorona, Onofrio, Luciano Cinquanta, Caterina Li Citra, Francesca Mazza, Vincenzo Ferrantelli, Gaetano Cammilleri, Emanuele Marconi, Francesca Cuomo, and Maria Cristina Messia. 2023. "Evolution of Free Amino Acids, Histamine and Volatile Compounds in the Italian Anchovies (Engraulis encrasicolus L.) Sauce at Different Ripening Times" Foods 12, no. 1: 126. https://doi.org/10.3390/foods12010126
APA StyleCorona, O., Cinquanta, L., Li Citra, C., Mazza, F., Ferrantelli, V., Cammilleri, G., Marconi, E., Cuomo, F., & Messia, M. C. (2023). Evolution of Free Amino Acids, Histamine and Volatile Compounds in the Italian Anchovies (Engraulis encrasicolus L.) Sauce at Different Ripening Times. Foods, 12(1), 126. https://doi.org/10.3390/foods12010126







