Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Microencapsulation by Spray Drying
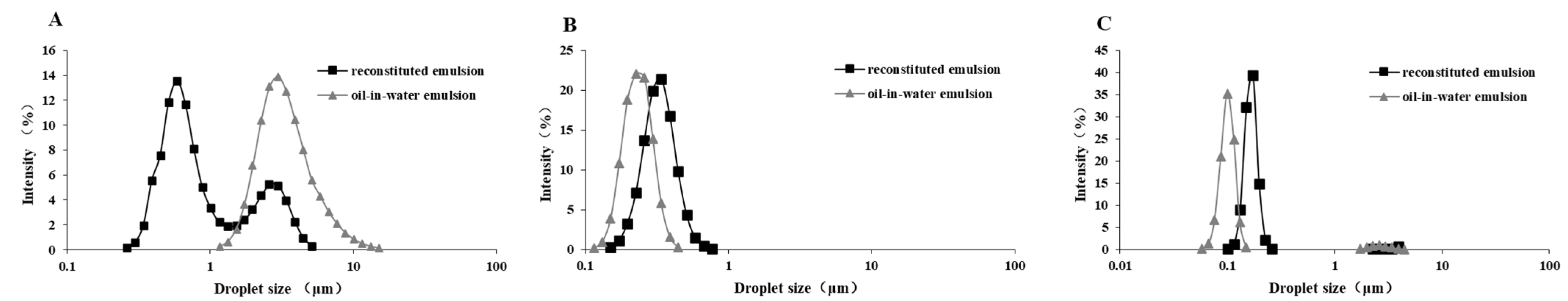
2.4. Feed Emulsion Droplet Size Analysis
2.5. Reconstituted Emulsion Droplet Size Analysis
2.6. Total Oil Content Determination
2.7. Surface Oil Content Determination
2.8. Water Activity and Moisture Content Determination
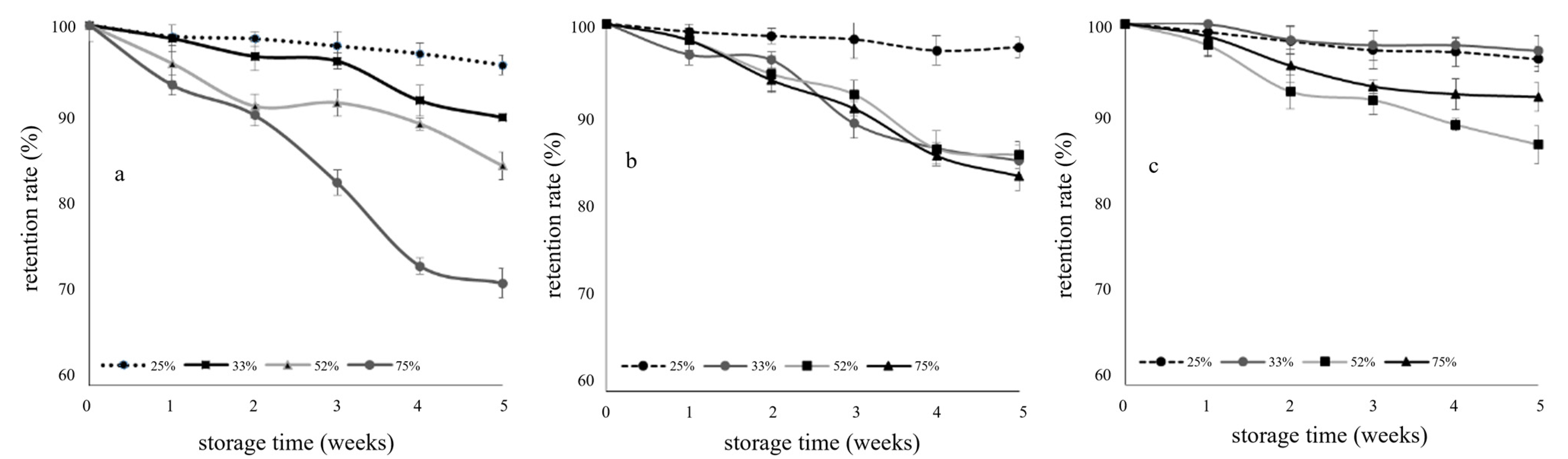
2.9. Storage Stability of Encapsulated Powders
2.9.1. Statistical Analysis
2.9.2. GC-MS Analysis
2.9.3. Morphology by Scanning Electron Microscopy (SEM)
2.9.4. Determination of Glass Transition Temperature (Tg) by Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. The Physical Properties of the Spray-Dried Powder
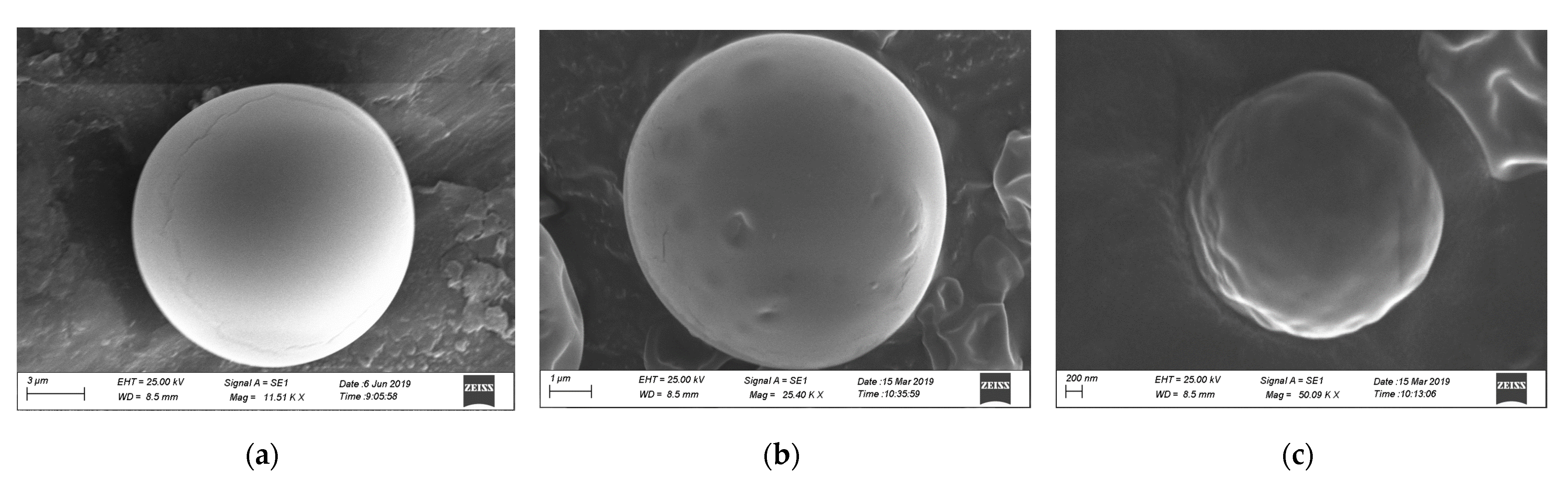
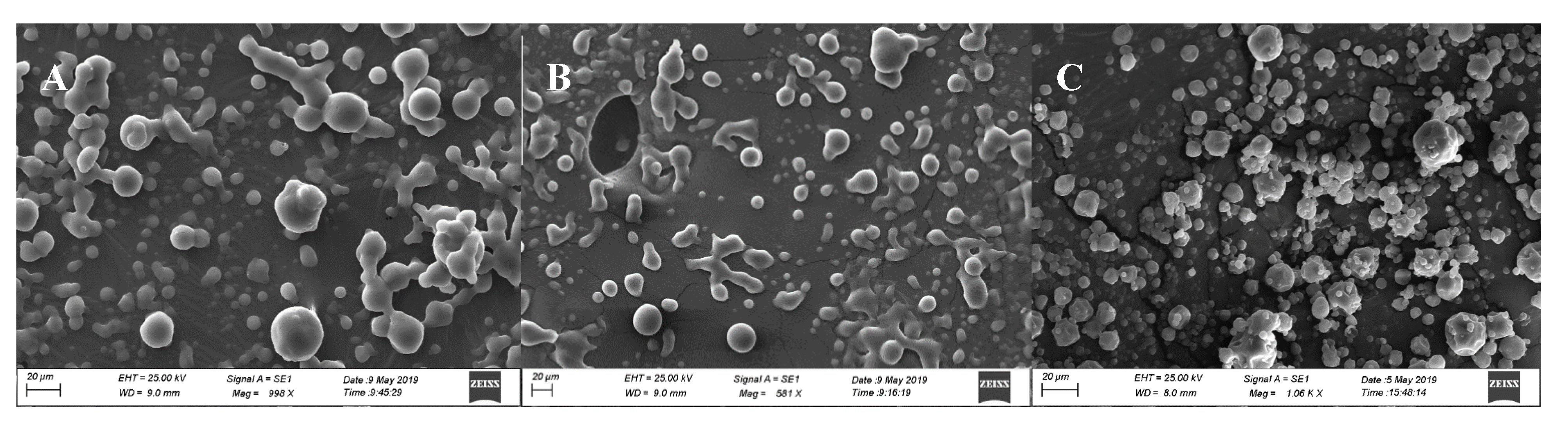
3.2. Morphological Characterization by Scanning Electron Microscopy (SEM)
3.3. Release Kinetics of D-Limonene from Spray-Dried Powders during Storage
3.4. Glass Transition Temperature
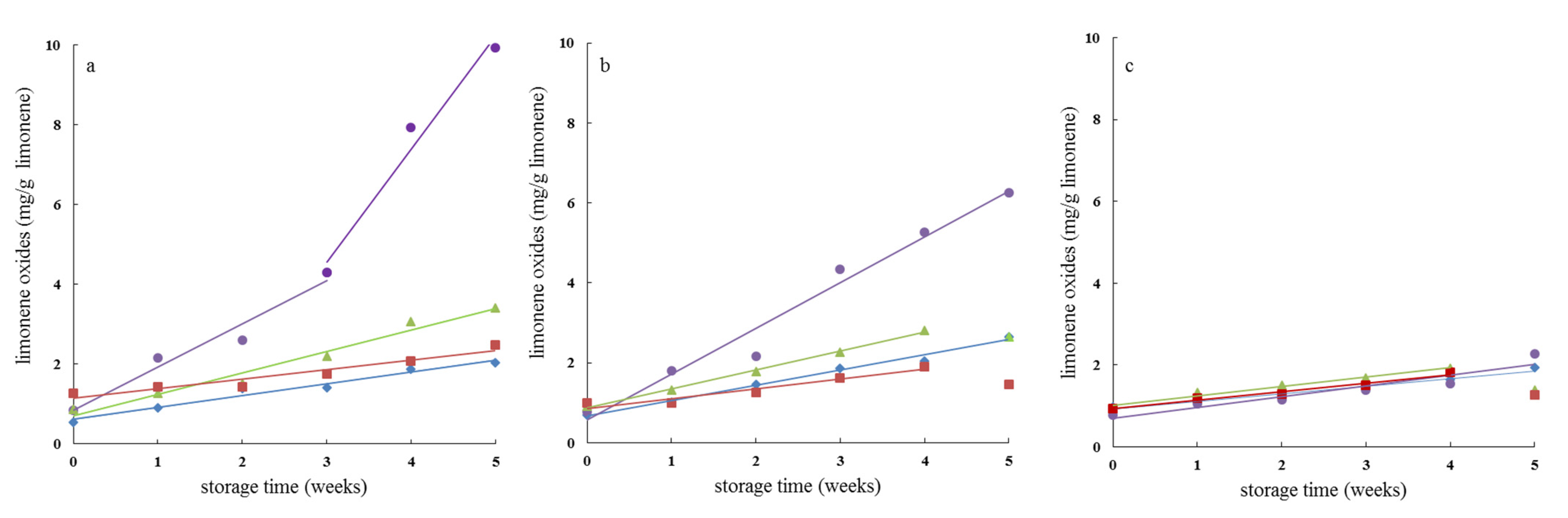
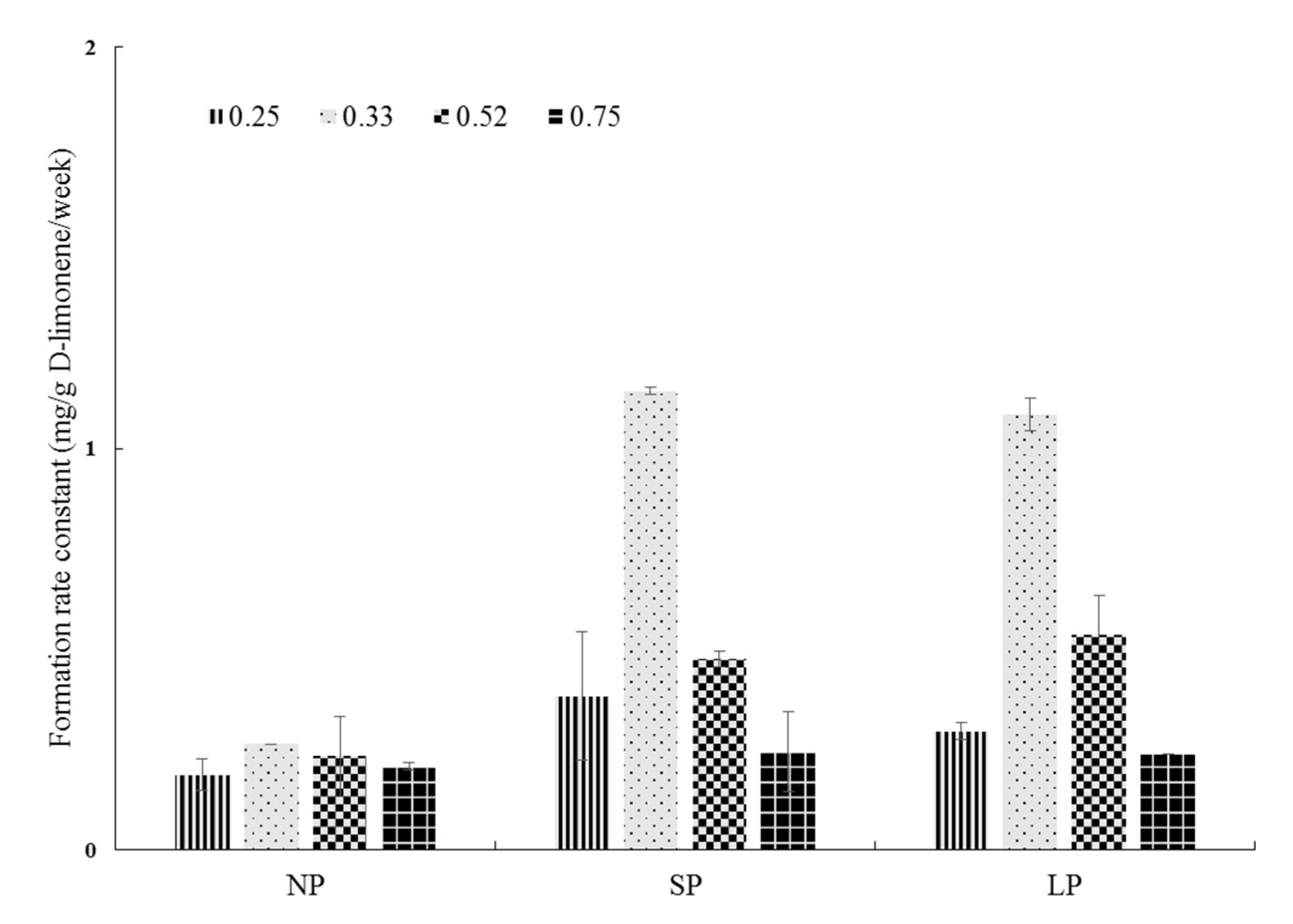
3.5. Oxidative Stability of D-Limonene in Spray-Dried Powders
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, S.A.; Pahmeyer, M.J.; Assadpour, E.; Jafari, S.M. Extraction and Purification of d-Limonene from Orange Peel Wastes: Recent Advances. Ind. Crops Prod. 2022, 177, 114484. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent Developments in Extraction and Encapsulation Techniques of Orange Essential Oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.S.; Arora, J.K.; Vatankhah, H.; Arora, J.K.; Vatankhah, H.; Taherian, A.R.; Rattan, N. Stability of Hydrocolloid Enriched Oil-in-Water Emulsions in Beverages Subjected to Thermal and Nonthermal Processing. J. Dispers. Sci. Technol. 2020, 43, 94–104. [Google Scholar] [CrossRef]
- Clark, J.P. Orange Juice Processing. Food Technol. 2003, 57, 50–51. [Google Scholar]
- Yılmaz, B.; Karavana, H.A. Application of Chitosan-Encapsulated Orange Oil onto Footwear Insock Leathers Spray Drying Technique for An Environmentally Sustainable Antibacterial Formulation. Johns. Matthey Technol. Rev. 2020, 64, 443–451. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Hernández-Sánchez, H.; Zambrano-Zaragoza, M.L.; Gutiérrez-López, G.F.; Real, A.D.; Quintanar-Guerrero, D.; Velasco-Bejarano, B. Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules. Foods. 2020, 9, 1884. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; El-Kalyoubi, M.; Ali, H.; El Mageed, M.A.; Khallaf, M.; Moawad, S. Effects of Carriers on Spray-Dried Flavors and Their Functional Characteristics. Pak. J. Biol. Sci. 2021, 23, 257–263. [Google Scholar] [CrossRef]
- Ramos, F.D.M.; Silveira Júnior, V.; Prata, A.S. Physical Aspects of Orange Essential Oil-Contaning Particles after Vacuum Spray Drying Processing. Food Chem. 2021, 12, 100142. [Google Scholar] [CrossRef]
- O’Toole, M.G.; Henderson, R.M.; Soucy, P.A.; Fasciotto, B.H.; Hoblitzell, P.J.; Keynton, R.S.; Ehringer, W.; Gobin, A. Curcumin Encapsulation in Submicrometer Spray-Dried Chitosan/Tween 20 Particles. Biomacromolecules 2012, 13, 2309–2314. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of Folic Acid in Food Hydrocolloids through Nanospray Drying and Electrospraying for Nutraceutical Applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef]
- da Silva, M.T.S.; Pinto, J.C. Influence of Encapsulated Aroma Compounds on The Formation and Morphology of Gelatin Microparticles. Macromol. Symp. 2019, 383, 1800061. [Google Scholar] [CrossRef]
- Reineccius, G.A.; Yan, C. Factors Controlling the Deterioration of Spray Dried Flavourings and Unsaturated Lipids. Flavour Frag. J. 2016, 31, 5–21. [Google Scholar] [CrossRef]
- Subramaniam, A.; Veazey, R.L.; Schober, A.; Rada, A.; Rong, Y.; Van Sleeuwen, R.M.T. Orange Oil Stability in Spray Dry Delivery Systems. Carbohydrate 2013, 97, 352–357. [Google Scholar] [CrossRef]
- Drapala, K.P.; Auty, M.A.E.; Mulvihill, D.M.; O’Mahony, J.A. Influence of Emulsifier Type on the Spray-Drying Properties of Model Infant Formula Emulsions. Food Hydrocoll. 2017, 69, 56–66. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Rodríguez-Rojo, S.; Salgado, M.; Cocero, M.J.; Talebi-Jahromi, K.; Amoabediny, G. Insecticidal Activity of Spray Dried Microencapsulated Essential Oils of Rosmarinus Officinalis and Zataria Multiflora against Tribolium Confusum. Crop Prot. 2020, 128, 104996. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Sheu, T.Y.; Rosenberg, M. Microencapsulation by Spray Drying Ethyl Caprylate in Whey Protein and Carbohydrate Wall Systems. J. Food Sci. 1995, 60, 98–103. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Microencapsulation by Spray Drying: Influence of Emulsion Size on the Retention of Volatile Compounds. J. Food Sci. 2003, 68, 2256–2262. [Google Scholar] [CrossRef]
- Risch, S.J.; Reineccius, G.A. Spray-dried orange oil—Effect of emulsion size on flavor retention and shelf life. Flavor Encapsulation 1988, 8, 67–77. [Google Scholar] [CrossRef]
- Murúa-Pagola, B.; Beristain-Guevara, C.I.; Martínez-Bustos, F. Preparation of Starch Derivatives Using Reactive Extrusion and Evaluation of Modified Starches as Shell Materials for Encapsulation of Flavoring Agents by Spray Drying. J. Food Eng. 2009, 91, 380–386. [Google Scholar] [CrossRef]
- Finney, J.; Buffo, R.; Reineccius, G.A. Effects of Type of Atomization and Processing Temperatures on the Physical Properties and Stability of Spray-Dried Flavors. J. Food Sci. 2002, 67, 1108–1114. [Google Scholar] [CrossRef]
- Tomazelli Júnior, O.; Kuhn, F.; Padilha, P.J.M.; Vicente, L.R.M.; Costa, S.W.; Boligon, A.A.; Scapinello, J.; Nesi, C.N.; Magro, J.D.; Castellví, S.L. Microencapsulation of Essential Thyme Oil by Spray Drying and Its Antimicrobial Evaluation Against Vibrio Alginolyticus and Vibrio Parahaemolyticus. Braz. J. Bio. 2018, 78, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Ferrando, M.; Aceña-Muñoz, L.; De Lamo-Castellví, S.; Güell, C. Fish Oil Microcapsules from O/W Emulsions Produced by Premix Membrane Emulsification. Food Bioproc. Tech. 2013, 6, 3088–3101. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC (Association of Official Analytical Chemists) International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- SoottitantAwat, A.; Yoshii, H.; Furuta, T.; OhgAwara, M.; Forssell, P.; Partanen, R.; Poutanen, K.; Linko, P. Effect of Water Activity on the Release Characteristics and Oxidative Stability of D-Limonene Encapsulated by Spray Drying. J. Agric. Food Chem. 2004, 52, 1269–1276. [Google Scholar] [CrossRef]
- Yoshii, H.; SoottitantAwat, A.; Liu, X.D.; Atarashi, T.; Furuta, T.; Aishima, S.; Ohgawara, M.; Linko, P. Flavor Release from Spray-Dried Maltodextrin/Gum Arabic or Soy Matrices as a Function of Storage Relative Humidity. Innov. Food Sci. Emerg. 2001, 2, 55–61. [Google Scholar] [CrossRef]
- Gaculajr, M.C.; Kubala, J.J. Statistical Models for Shelf Life Failures. Food Sci. 1975, 40, 404–409. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Tian, G.; Lu, C.; Zhao, S.; Bao, Y.; McClements, D.J.; Xiao, H.; Zheng, J. Effects of Preheating and Storage Temperatures on Aroma Profile and Physical Properties of Citrus-Oil Emulsions. J. Agric. Food Chem. 2017, 65, 7781–7789. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Li, D.H.; Wu, H.J.; Huang, W.; Guo, L.; Dou, H.T. Microcapsule of Sweet Orange Essential Oil Encapsulated in Beta-Cyclodextrin Improves the Release Behaviors In Vitro and In Vivo. Eur. J. Lipid Sci. Tech. 2018, 120, 1700521. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Applications of Microfluidization in Emulsion-Based Systems, Nanoparticle Formation, and Beverages. Trends Food Sci. Technol. 2021, 116, 609–625. [Google Scholar] [CrossRef]
- Zhang, J.; Reineccius, G.A. Factors Controlling the Turbidity of Submicron Emulsions Stabilized by Food Biopolymers and Natural Surfactant. LWT Food Sci. Technol. 2016, 71, 162–168. [Google Scholar] [CrossRef]
- Chen, C.C.; Wagner, G. Vitamin E Nanoparticle for Beverage Applications. Chem. Eng. Res. Des. 2004, 82, 1432–1437. [Google Scholar] [CrossRef]
- Jafari, S.M.; Mcclements, D.J. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Fathi, F.; Ebrahimi, S.N.; Matos, L.C.; Oliveira, M.B.P.P.; Alves, R.C. Emerging Drying Techniques for Food Safety and Quality: A review. Compr. Rev. Food Sci. F 2022, 21, 1125–1160. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Linke, T.; Hinrichs, J.; Kohlus, R. Factors Determining the Surface Oil Concentration of Encapsulated Lipid Particles-Impact of The Spray Drying Conditions. Dry. Technol. 2021, 39, 173–186. [Google Scholar] [CrossRef]
- Márquez-Gómez, M.; Galicia-García, T.; Márquez-Meléndez, R.; Ruiz-Gutiérrez, M.; Quintero-Ramos, A. Spray-Dried Microencapsulation of Orange Essential Oil Using Modified Rice Starch as Wall Material. J. Food Process. Pres. 2018, 42, e13428. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Dumoulin, E.D.; Richard, H.M.J.; Noleau, I.; Lebert, A.M. Flavor Encapsulation by Spray Drying: Application to Citral and Linalyl Acetate. Food Sci. 1992, 57, 217–221. [Google Scholar] [CrossRef]

| Sample Name | LELP | SESP | NENP |
|---|---|---|---|
| Emulsion size (μm) | 3.38 a | 0.22 c | 0.20 d |
| Reconstituted emulsion size (μm) | 1.17 a | 0.31 c | 0.17 d |
| Total oil content (g/20 g powder) | 1.39 ± 0.009 a | 1.18 ± 0.008 b | 0.928 ± 0.008 c |
| Surface oil (mg oil/100 g powder) | 11.4 ± 0.0529 a | 2.97 ± 0.0152 c | 2.30 ± 0.0231 d |
| Loading capacity (%) | 99.6 ± 0.605 a | 87.7 ± 0.772 b | 77.7 ± 0.700 d |
| Encapsulation efficiency (%) | 95.9 ± 0.000 c | 98.7 ± 0.000 a | 98.8 ± 0.000 a |
| Water activity | 0.24 ± 0.000 c | 0.32 ± 0.002 a | 0.24 ± 0.000 c |
| Moisture content | 3.55 ± 0.025 a | 2.95 ± 0.029 d | 3.45 ± 0.015 b |
| Tg/°C | ||||
|---|---|---|---|---|
| 25% | 33% | 52% | 75% | |
| LP | 62.03 ± 0.22 b | 61.39 ± 0.27 b | 51.31 ± 0.24 a | 42.92 ± 0.33 a |
| SP | 88.07 ± 0.22 c | 61.65 ± 0.22 b | 46.42 ± 0.21 b | 42.44 ± 0.23 a |
| NP | 100.25 ± 0.20 a | 74.45 ± 0.20 a | 48.72 ± 0.24 b | 42.84 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Meng, Z.; Luo, Z.; Duan, H.; Ramaswamy, H.S.; Wang, C. Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis). Foods 2023, 12, 116. https://doi.org/10.3390/foods12010116
Peng Q, Meng Z, Luo Z, Duan H, Ramaswamy HS, Wang C. Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis). Foods. 2023; 12(1):116. https://doi.org/10.3390/foods12010116
Chicago/Turabian StylePeng, Qun, Ziyi Meng, Ziyang Luo, Hanying Duan, Hosahalli S. Ramaswamy, and Chao Wang. 2023. "Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis)" Foods 12, no. 1: 116. https://doi.org/10.3390/foods12010116
APA StylePeng, Q., Meng, Z., Luo, Z., Duan, H., Ramaswamy, H. S., & Wang, C. (2023). Effect of Emulsion Particle Size on the Encapsulation Behavior and Oxidative Stability of Spray Microencapsulated Sweet Orange Oil (Citrus aurantium var. dulcis). Foods, 12(1), 116. https://doi.org/10.3390/foods12010116






