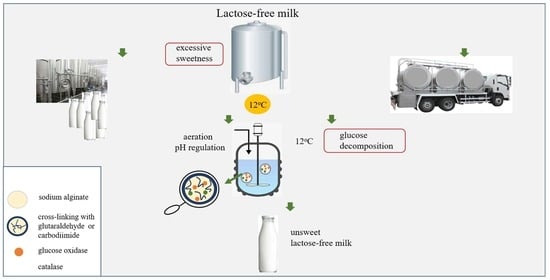
Critical Parameters in an Enzymatic Way to Obtain the Unsweet Lactose-Free Milk Using Catalase and Glucose Oxidase Co-Encapsulated into Hydrogel with Chemical Cross-Linking
Abstract
1. Introduction
2. Materials and Methods
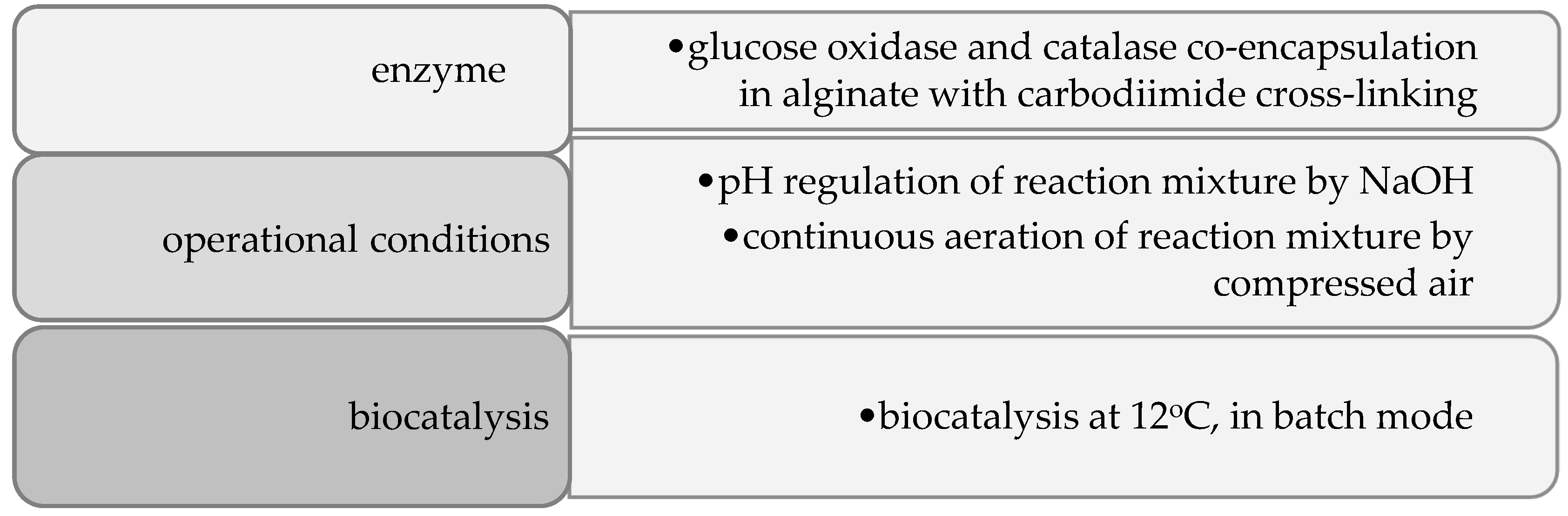
2.1. Materials
2.2. Methods
2.2.1. pH Regulation
2.2.2. Aeration
2.2.3. The Kinetic Characteristics of the Cascade with Glucose Oxidase and Catalase in the Native Form
2.2.4. Co-Encapsulation of Glucose Oxidase and Catalase
2.2.5. The Activity of Co-Encapsulated Glucose Oxidase and Catalase
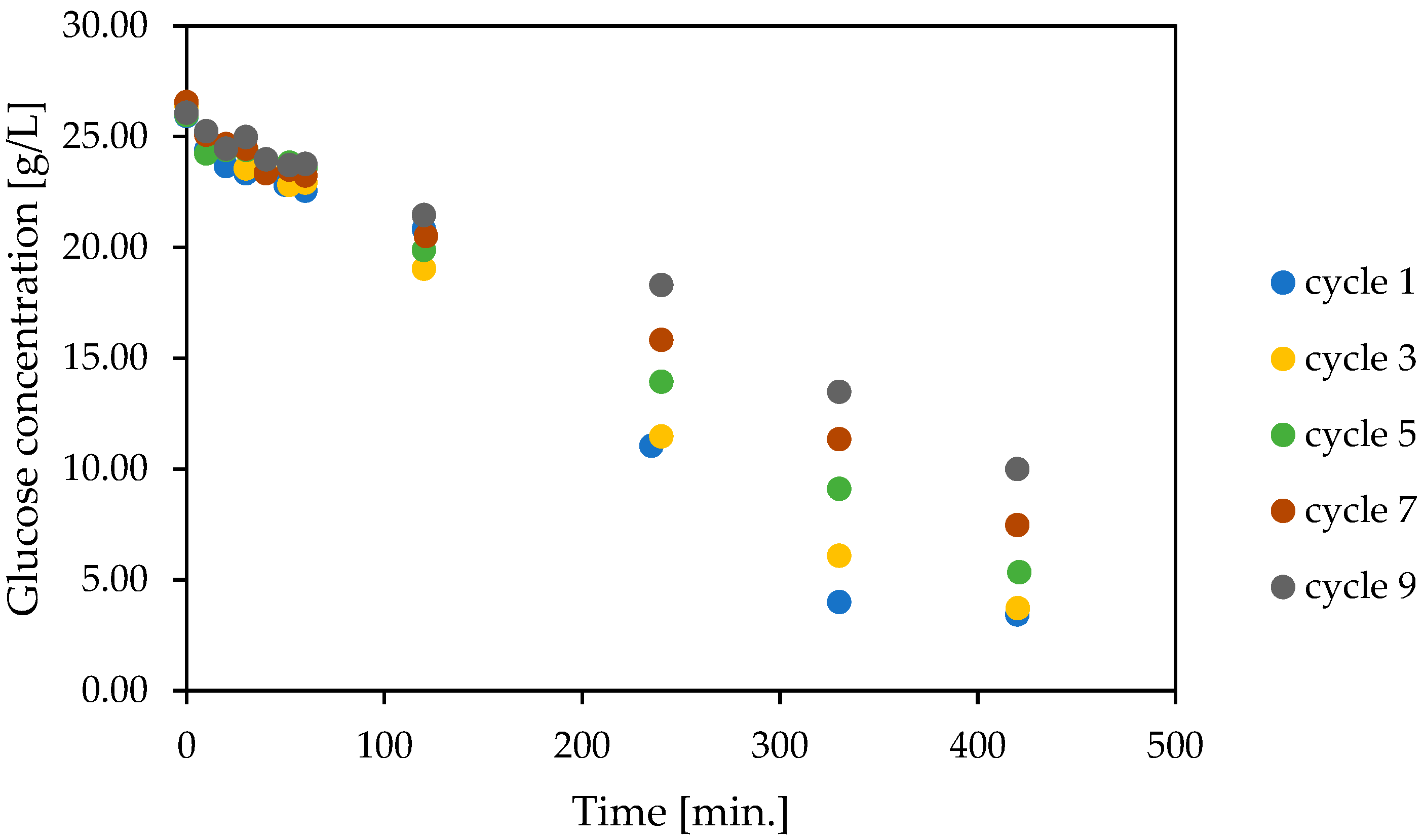
2.2.6. The Reusing of Co-Encapsulated Glucose Oxidase and Catalase
2.2.7. Glucose Oxidation in Lactose-Free Milk Catalyzed by Co-Encapsulated Glucose Oxidase and Catalase
3. Results
3.1. The Regulation of pH
3.2. The Aeration Impact
3.3. Catalase Impact
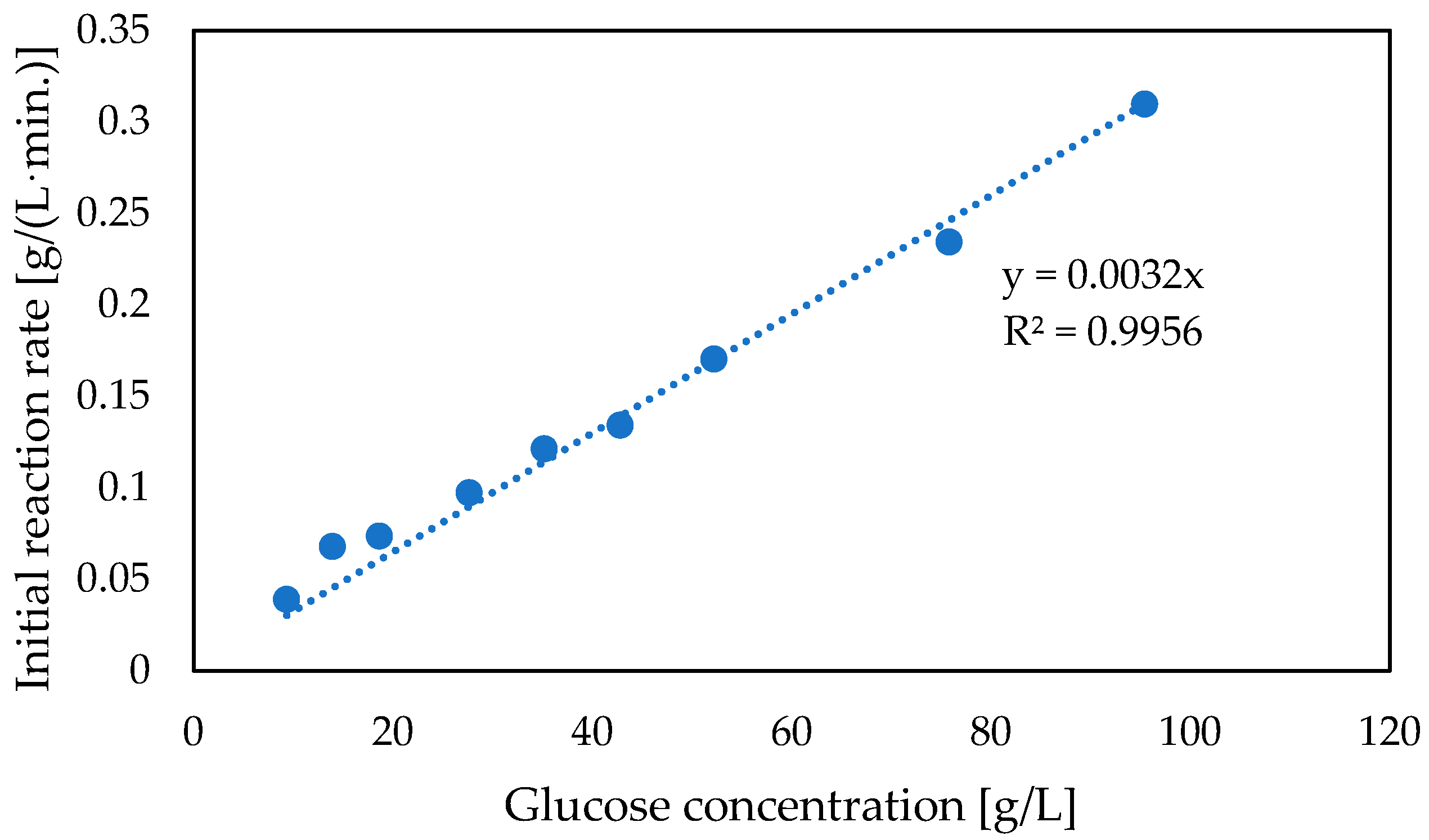
3.4. Two-Enzymatic Cascade with Native Glucose Oxidase and Catalase
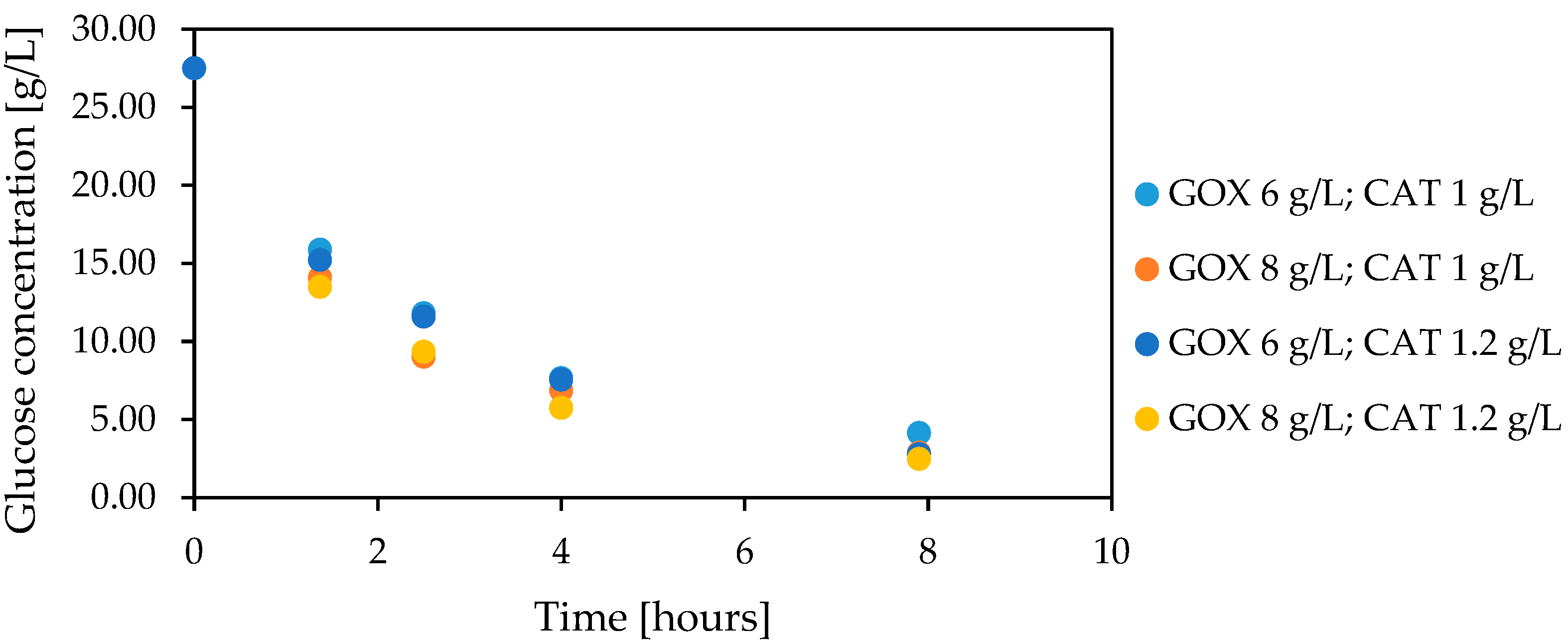
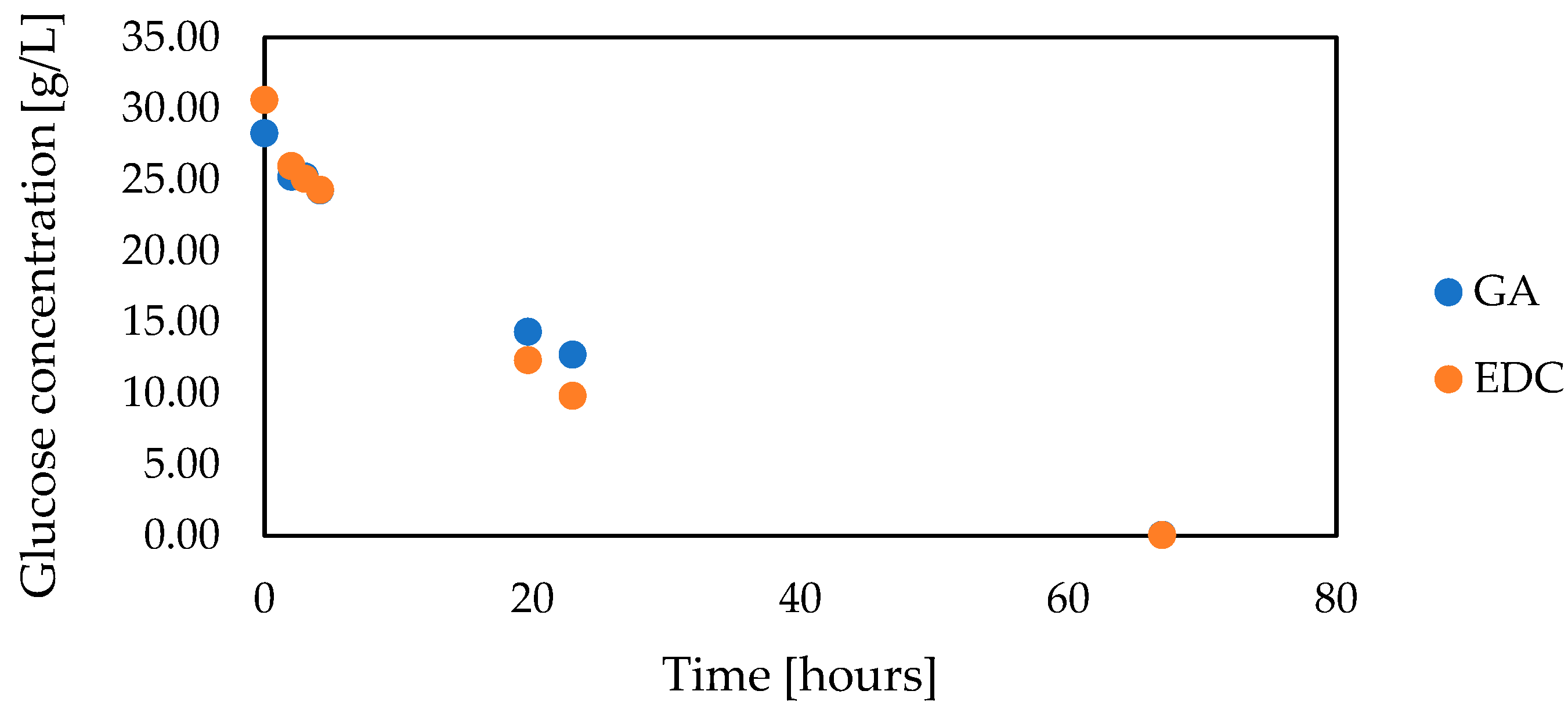
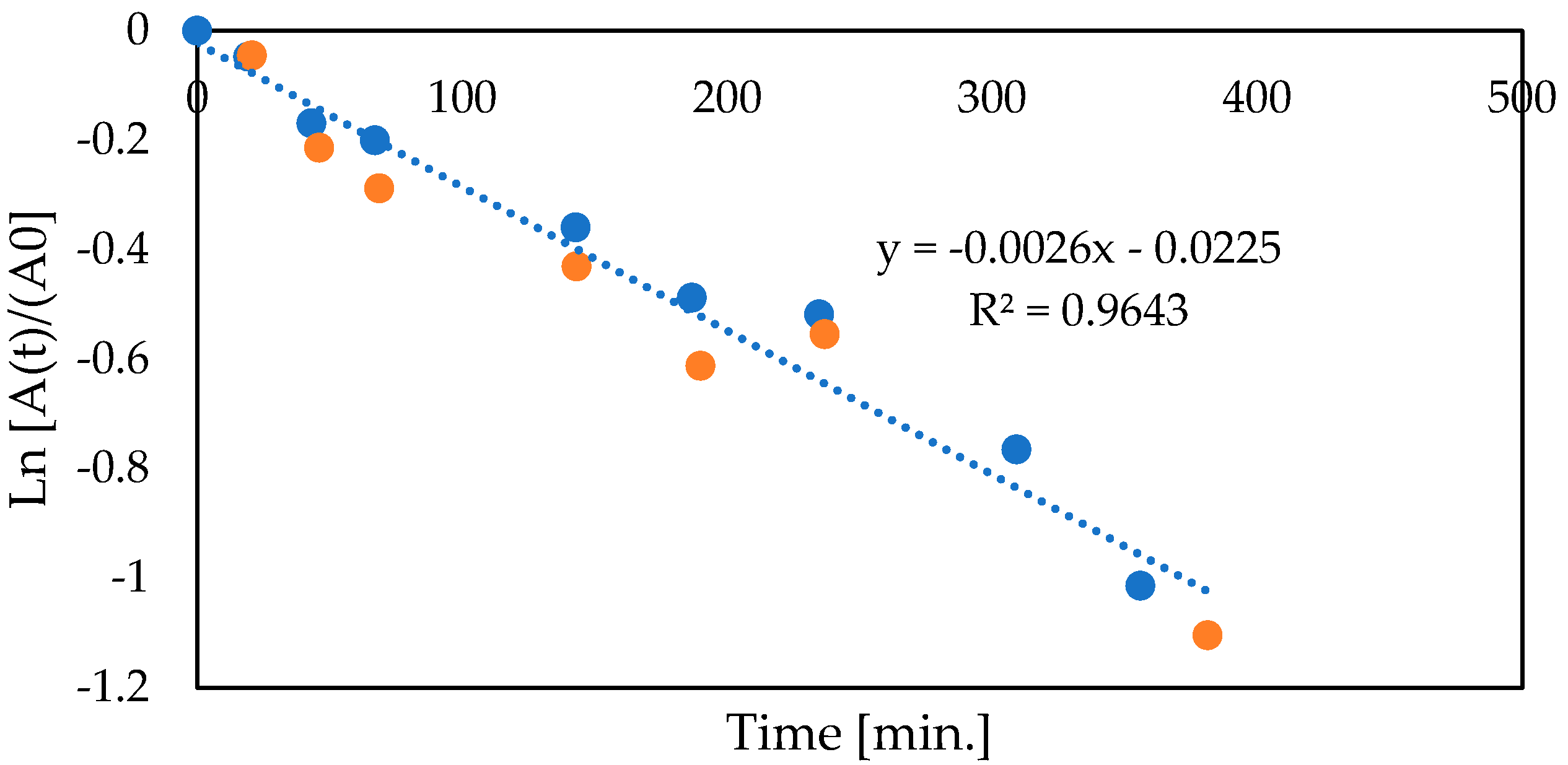
3.5. Co-Encapsulation of Glucose Oxidase and Catalase
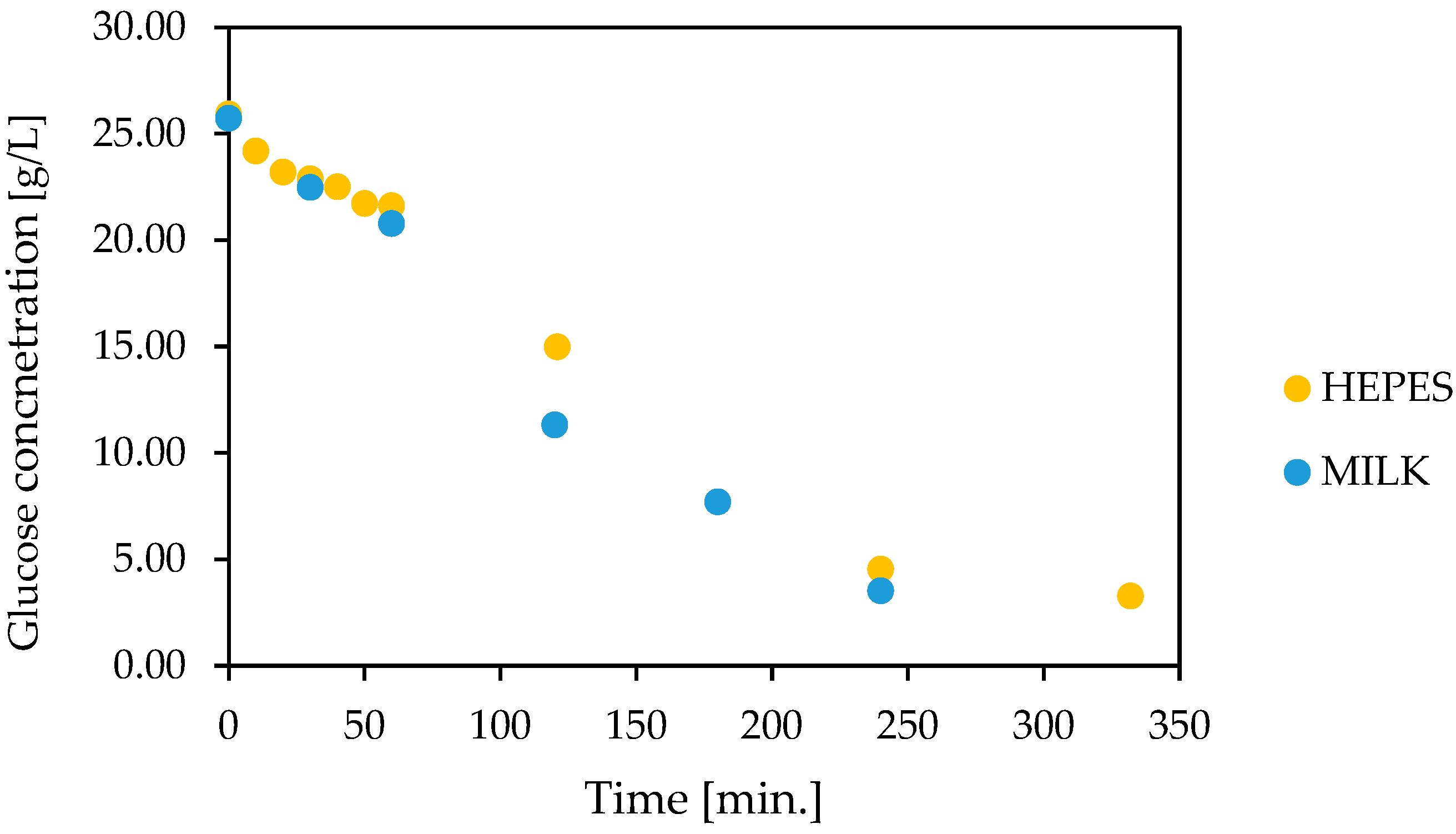
3.6. Glucose Oxidation in Lactose-Free Milk Catalyzed by Co-Encapsulated Glucose Oxidase and Catalase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose Intolerance: An Update on Its Pathogenesis, Diagnosis, and Treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef]
- Monti, L.; Negri, S.; Meucci, A.; Stroppa, A.; Galli, A.; Contarini, G. Lactose, Galactose and Glucose Determination in Naturally “Lactose Free” Hard Cheese: HPAEC-PAD Method Validation. Food Chem. 2017, 220, 18–24. [Google Scholar] [CrossRef]
- Rizzo, P.V.; Harwood, W.S.; Drake, M.A. Consumer Desires and Perceptions of Lactose-Free Milk. J. Dairy Sci. 2020, 103, 6950–6966. [Google Scholar] [CrossRef]
- Harju, M.; Kallioinen, H.; Tossavainen, O. Lactose Hydrolysis and Other Conversions in Dairy Products: Technological Aspects. Int. Dairy J. 2012, 22, 104–109. [Google Scholar] [CrossRef]
- Mahato, D.K.; Keast, R.; Liem, D.G.; Russell, C.G.; Cicerale, S.; Gamlath, S. Sugar Reduction in Dairy Food: An Overview with Flavoured Milk as an Example. Foods 2020, 9, 1400. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The Use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef]
- Czyzewska, K.; Trusek, A. Encapsulated NOLATM Fit 5500 Lactase—An Economically Beneficial Way to Obtain Lactose-Free Milk at Low Temperature. Catalysts 2021, 11, 527. [Google Scholar] [CrossRef]
- Trusek, A.; Dworakowska, D.; Czyzewska, K. 3D Enzymatic Preparations with Graphene Oxide Flakes and Hydrogel to Obtain Lactose-Free Products. Food Bioprod. Process 2020, 121, 224–229. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme Production of D-Gluconic Acid and Glucose Oxidase: Successful Tales of Cascade Reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Enzyme Immobilization and Co-Immobilization: Main Framework, Advances and Some Applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Kang, S.; Bae, Y.H. A Sulfonamide Based Glucose-Responsive Hydrogel with Covalently Immobilized Glucose Oxidase and Catalase. J. Control. Release 2003, 86, 115–121. [Google Scholar] [CrossRef]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In Situ Immobilization of Glucose Oxidase and Catalase in a Hybrid Interpenetrating Polymer Network by 3D Bioprinting and Its Application. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Ozyilmaz, G.; Tukel, S.S. Simultaneous Co-Immobilization of Glucose Oxidase and Catalase in Their Substrates. Appl. Biochem. Microbiol. 2007, 43, 29–35. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Li, D.; Jiang, Y. Co-Immobilization of Glucose Oxidase and Catalase in Silica Inverse Opals for Glucose Removal from Commercial Isomaltooligosaccharide. Int. J. Biol. Macromol. 2018, 107, 2034–2043. [Google Scholar] [CrossRef]
- Morthensen, S.T.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. Significance of Membrane Bioreactor Design on the Biocatalytic Performance of Glucose Oxidase and Catalase: Free vs. Immobilized Enzyme Systems. Biochem. Eng. J. 2017, 117, 41–47. [Google Scholar] [CrossRef]
- Godjevargova, T.; Dayal, R.; Turmanova, S. Gluconic Acid Production in Bioreactor with Immobilized Glucose Oxidase Plus Catalase on Polymer Membrane Adjacent to Anion-Exchange Membrane. Macromol. Biosci. 2004, 4, 950–956. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, P.; Huang, J.; Cai, J. Co-Immobilization of Glucose Oxidase and Catalase in Porous Magnetic Chitosan Microspheres for Production of Sodium Gluconate. Int. J. Chem. React. Eng. 2022, 20, 989–1001. [Google Scholar] [CrossRef]
- Agyei, D.; Shanbhag, B.K.; He, L. Enzyme Engineering (Immobilization) for Food Applications. In Improving and TailoingEnzymes for Food Quality and Functionality; Rickey, Y.Y., Ed.; Woodhead Publishing: Kidlington, UK, 2015; pp. 213–235. [Google Scholar] [CrossRef]
- Spök, A. Safety Regulations of Food Enzymes. Food Technol. Biotechnol. 2006, 44, 197–209. [Google Scholar]
- Kuddus, M. Cold-Active Enzymes in Food Biotechnology: An Updated Mini Review. J. Appl. Biol. Biotechnol. 2018, 6, 58–63. [Google Scholar] [CrossRef]
- Czyżewska, K.; Trusek, A. Catalytic Membrane with the Recombinant Catalase from Psychrotolerant Bacteria Serratia Sp. in Dairy Applications. Desalin. Water Treat. 2018, 128, 253–258. [Google Scholar] [CrossRef]
- Chan, A.W.; Whitney, R.A.; Neufeld, R.J. Semisynthesis of a Controlled Stimuli-Responsive Alginate Hydrogel. Biomacromolecules 2009, 10, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Jonović, M.; Žuža, M.; Ðorđević, V.; Šekuljica, N.; Milivojević, M.; Jugović, B.; Bugarski, B.; Knežević-Jugović, Z. Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins. Catalysts 2021, 11, 305. [Google Scholar] [CrossRef]
- Çolak, Ö.; Yaşar, A.; Çete, S.; Arslan, F. Glucose Biosensor Based on the Immobilization of Glucose Oxidase on Electrochemically Synthesized Polypyrrole-Poly(Vinyl Sulphonate) Composite Film by Cross-Linking with Glutaraldehyde. Artif. Cells Nanomed. Biotechnol. 2012, 40, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common Causes of Glucose Oxidase Instability in in Vivo Biosensing: A Brief Review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef]
- House, J.L.; Anderson, E.M.; Ward, W.K. Immobilization Techniques to Avoid Enzyme Loss from Oxidase-Based Biosensors: A One-Year Study. J. Diabetes Sci. Technol. 2007, 1, 18–27. [Google Scholar] [CrossRef]
- Kouassi, G.K.; Irudayaraj, J.; McCarty, G. Activity of Glucose Oxidase Functionalized onto Magnetic Nanoparticles. Biomagn. Res. Technol. 2005, 3, 1. [Google Scholar] [CrossRef]
- Yee, Y.C.; Hashim, R.; Mohd Yahya, A.R.; Bustami, Y. Colorimetric Analysis of Glucose Oxidase-magnetic Cellulose Nanocrystals (CNCS) for Glucose Detection. Sensors 2019, 19, 2511. [Google Scholar] [CrossRef]
- Chirra, H.D.; Sexton, T.; Biswal, D.; Hersh, L.B.; Hilt, J.Z. Catalase-Coupled Gold Nanoparticles: Comparison between the Carbodiimide and Biotin-Streptavidin Methods. Acta Biomater. 2011, 7, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Akyilmaz, E.; Oyman, G.; Cınar, E.; Odabas, G. A New Polyaniline–Catalase–Glutaraldehyde-Modified Biosensor for Hydrogen Peroxide Detection. Prep. Biochem. Biotechnol. 2017, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Benea, I.C.; Bîtcan, I.; Péter, F.; Klébert, S.; Feczkó, T.; Károly, Z.; Biró, E. One-Pot Biocatalytic Conversion of Lactose to Gluconic Acid and Galacto-Oligosaccharides Using Immobilized β-Galactosidase and Glucose Oxidase. Catal. Today 2021, 366, 202–211. [Google Scholar] [CrossRef]
- Jasti, L.S.; Dola, S.R.; Fadnavis, N.W.; Addepally, U.; Daniels, S.; Ponrathnam, S. Co-Immobilized Glucose Oxidase and β-Galactosidase on Bovine Serum Albumin Coated Allyl Glycidyl Ether (AGE)–Ethylene Glycol Dimethacrylate (EGDM) Copolymer as a Biosensor for Lactose Determination in Milk. Enzyme Microb. Technol. 2014, 64–65, 67–73. [Google Scholar] [CrossRef]
- Parmar, A.; Sharma, V.; Arora, S.; Raju Panjagari, N. Activation of Lactoperoxidase System in Buffalo Milk Using Dual Enzyme (Lactase & Glucose Oxidase) and Its Effect on Milk Constituents. Int. J. Dairy Technol. 2022, 75, 348–360. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Ulrich, L.G.; Kornecki, J.F.; Fernandez-Lafuente, R.; Tardioli, P.W.; Ribeiro, M.P. Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor. Catalysts 2019, 9, 657. [Google Scholar] [CrossRef]
- Sankaran, K.; Godbole, S.S.; D’Souza, S.F. Preparation of Spray-Dried, Sugar-Free Egg Powder Using Glucose Oxidase and Catalase Coimmobilized on Cotton Cloth. Enzyme Microb. Technol. 1989, 11, 617–619. [Google Scholar] [CrossRef]
- Wingard, L.B.; Castner, J.F.; Yao, S.J.; Wolfson, S.K.; Drash, A.L.; Liu, C.C. Immobilized Glucose Oxidase in the Potentiometric Detection of Glucose. Appl. Biochem. Biotechnol. 1984, 9, 95–104. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Morita, S.; Okada, J. Oxidation of Glucose on Immobilized Glucose Oxidase. Chem. Pharm. Bull. 1982, 30, 782–788. [Google Scholar] [CrossRef][Green Version]
- Valentová, O.; Marek, M.; Švec, F.; Štamberg, J.; Vodrážka, Z. Comparison of Different Methods of Glucose Oxidase Immobilization. Biotechnol. Bioeng. 1981, 23, 2093–2104. [Google Scholar] [CrossRef]
- Miller, C.N. Use of dinitrosalicyle acid reagent for determination of reducing sugar. Anal. Chem. 1959, 81, 426–428. [Google Scholar] [CrossRef]
- Vogt, S.; Schneider, M.; Schafer-Eberwein, H.; Noll, G. Determination of the pH Dependent Redox Potential of Glucose Oxidase by Spectroelectrochemistry. Anal. Chem. 2014, 86, 7530–7535. [Google Scholar] [CrossRef] [PubMed]
- Czyzewska, K.; Trusek, A. Encapsulated Catalase from Serratia Genus for H2O2 Decomposition in Food Applications. Pol. J. Chem. Technol. 2018, 20, 39–43. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 39–43. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Madeeha, M.; Asgher, M.; Batool, N. Purification and Thermodynamic Characterization of Glucose Oxidase from a Newly Isolated Strain of Aspergillus niger. Can. J. Microbiol. 2006, 52, 519–524. [Google Scholar] [CrossRef]
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Brüschweiler, B.J.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Safety Evaluation of the Food Enzyme Glucose Oxidase from Aspergillus niger (Strain ZGL). EFSA J. 2019, 17, 519–524. [Google Scholar] [CrossRef]
- Suri, S.; Kumar, V.; Prasad, R.; Tanwar, B.; Goyal, A.; Kaur, S.; Gat, Y.; Kumar, A.; Kaur, J.; Singh, D. Considerations for Development of Lactose-Free Food. J. Nutr. Intermed. Metab. 2019, 15, 27–34. [Google Scholar] [CrossRef]
- Nigam, P.S. Microbial Enzymes with Special Characteristics for Biotechnological Applications. Biomolecules 2013, 3, 597–611. [Google Scholar] [CrossRef]
- Singh, J.; Verma, N. Glucose Oxidase from Aspergillus niger: Production, Characterization and Immobilization for Glucose Oxidation. Adv. Appl. Sci. Res. 2013, 4, 250–257. [Google Scholar]
- Adányi, N.; Szabó, E.E.; Váradi, M. Multi-Enzyme Biosensors with Amperometric Detection for Determination of Lactose in Milk and Dairy Products. Eur. Food Res. Technol. 1999, 209, 220–226. [Google Scholar] [CrossRef]
- Ammam, M.; Fransaer, J. Two-Enzyme Lactose Biosensor Based on β-Galactosidase and Glucose Oxidase Deposited by AC-Electrophoresis: Characteristics and Performance for Lactose Determination in Milk. Sens. Actuators B Chem. 2010, 148, 583–589. [Google Scholar] [CrossRef]
- Gursoy, S.; Yildiz, A.; Cogal, G.C.; Gursoy, O. A Novel Lactose Biosensor Based on Electrochemically Synthesized 3,4-Ethylenedioxythiophene/Thiophene (EDOT/Th) Copolymer. Open Chem. 2020, 18, 974–985. [Google Scholar] [CrossRef]
- Hartmeier, W. Glucose Oxidation with Immobilized Glucose Oxidase-Catalase. U.S. Patent US 4460686A, 29 April 1982. [Google Scholar]
- Vroemen, A.J.; Beverini, M. Enzymatic Production of Gluconic Acid or Its Salts. Canadian Patent CA2194859C, 13 May 1996. [Google Scholar]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Chakraborty, A.; Can, A.S. Calcium Gluconate. In Handbook of Nuclear, Biological, and Chemical Agent Exposures; StatPearls: Treasure Island, FL, USA, 2022; pp. 55–57. [Google Scholar] [CrossRef]
- Tao, Z.; Raffel, R.A.; Souid, A.; Goodisman, J. Kinetic Studies on Enzyme-Catalyzed Reactions: Oxidation of Glucose, Decomposition of Hydrogen Peroxide and Their Combination. Biophys. J. 2009, 96, 2977–2988. [Google Scholar] [CrossRef]
- Karmali, K.; Karmali, A.; Teixeira, A.; Curto, M.J.M. Assay for Glucose Oxidase from Aspergillus niger and Penicillium amagasakiense by Fourier Transform Infrared Spectroscopy. Anal. Biochem. 2004, 333, 320–327. [Google Scholar] [CrossRef]
- Ruiz, E.; Busto, M.D.; Ramos-gómez, S.; Palacios, D.; Pilar-Izquierdo, M.C. Food Bioscience Encapsulation of Glucose Oxidase in Alginate Hollow Beads to Reduce the Fermentable Sugars in Simulated Musts. Food Biosci. 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Yang, Y.M.; Wang, J.W.; Tan, R.X. Immobilization of Glucose Oxidase on Chitosan-SiO2 Gel. Enzyme Microb. Technol. 2004, 34, 126–131. [Google Scholar] [CrossRef]
- Pečar, D.; Vasić-Rački, Đ.; Presečki, A.V. Immobilization of Glucose Oxidase on Eupergit C: Impact of Aeration, Kinetic and Operational Stability Studies of Free and Immobilized Enzyme. Chem. Biochem. Eng. Q. 2018, 32, 511–522. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. Average and Local Oxygen Transfer Properties in Bubble Column with Axial Distribution of Immobilized Glucose Oxidase Gel Beads. Chem. Eng. Sci. 2000, 55, 5405–5414. [Google Scholar] [CrossRef]
- Verlaan, P. Modelling and Characterization of an Airlift-Loop Bioreactor; Agricultural University: Wageningen, The Netherlands, 1987. [Google Scholar]
- Miłek, J.; Kwiatkowska-Marks, S.; Wójcik, M. Immobilization of Catalase from Aspergillus niger in Calcium Alginate Gel. Chemik 2011, 65, 305–308. [Google Scholar]
- Blandino, A.; Macías, M.; Cantero, D. Immobilization of Glucose Oxidase within Calcium Alginate Gel Capsules. Process Biochem. 2001, 36, 601–606. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.X.; Zhou, H.M. Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules. Int. J. Mol. Sci. 2011, 12, 3042–3054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Srivastava, R.; Brown, Q.; McShane, M.J. Combined Physical and Chemical Immobilization of Glucose Oxidase in Alginate Microspheres Improves Stability of Encapsulation and Activity. Bioconjugate Chem. 2005, 16, 1451–1458. [Google Scholar] [CrossRef]
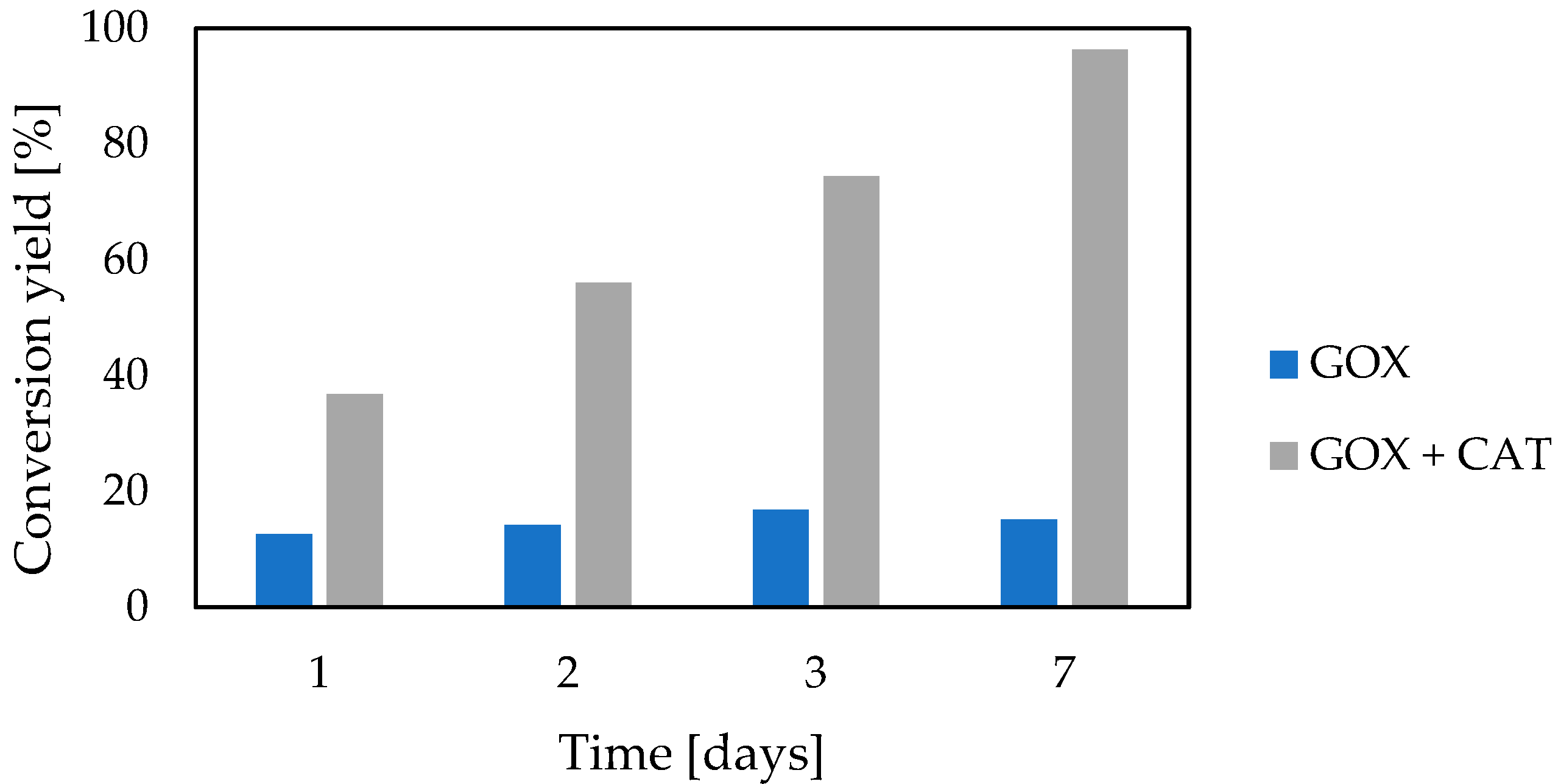
| Time [h] | GOX | GOX + NaOH | GOX + CaCO3 |
|---|---|---|---|
| pH | |||
| 0 | 6.59 | 6.65 | 6.68 |
| 1 | 6.36 | 6.84 | 6.61 |
| 3 | 6.0 | 6.71 | 6.54 |
| 24 | 3.69 | 6.62 | 6.35 |
| Type of Aeration | Mixing | Catalase Action | Compressed Air | |||
|---|---|---|---|---|---|---|
| Enzymes | GOX | GOX + CAT | GOX + CAT + Aeration | |||
| Time [min] | O2 [mg/L] | α [%] | O2 [mg/L] | α [%] | O2 [mg/L] | α [%] |
| 0 | 10.48 | 0 | 10.45 | 0 | 10.60 | 0 |
| 1 | 0.88 | 3.3 | 3.36 | 6.45 | 18.61 | 10.05 |
| 15 | 0.45 | 4.88 | 0.70 | 8.17 | >20.0 | 18.37 |
| 60 | 0.43 | 13.48 | 0.64 | 16.49 | >20.0 | 69.74 |
| 180 | 0.50 | 15.1 | 0.56 | 21.6 | >20.0 | 82.02 |
| Type of Capsules | Alginate | Alginate + GA | Alginate + EDC |
|---|---|---|---|
| Time [h] | Glucose Conversion [%] | ||
| 0 | 0 | 0 | 0 |
| 5.3 | 63.84 | 32.35 | 16.13 |
| 22 | 98.6 | 77.31 | 35.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czyzewska, K.; Trusek, A. Critical Parameters in an Enzymatic Way to Obtain the Unsweet Lactose-Free Milk Using Catalase and Glucose Oxidase Co-Encapsulated into Hydrogel with Chemical Cross-Linking. Foods 2023, 12, 113. https://doi.org/10.3390/foods12010113
Czyzewska K, Trusek A. Critical Parameters in an Enzymatic Way to Obtain the Unsweet Lactose-Free Milk Using Catalase and Glucose Oxidase Co-Encapsulated into Hydrogel with Chemical Cross-Linking. Foods. 2023; 12(1):113. https://doi.org/10.3390/foods12010113
Chicago/Turabian StyleCzyzewska, Katarzyna, and Anna Trusek. 2023. "Critical Parameters in an Enzymatic Way to Obtain the Unsweet Lactose-Free Milk Using Catalase and Glucose Oxidase Co-Encapsulated into Hydrogel with Chemical Cross-Linking" Foods 12, no. 1: 113. https://doi.org/10.3390/foods12010113
APA StyleCzyzewska, K., & Trusek, A. (2023). Critical Parameters in an Enzymatic Way to Obtain the Unsweet Lactose-Free Milk Using Catalase and Glucose Oxidase Co-Encapsulated into Hydrogel with Chemical Cross-Linking. Foods, 12(1), 113. https://doi.org/10.3390/foods12010113