Effect of Apple Juice Enrichment with Selected Plant Materials: Focus on Bioactive Compounds and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Plant Materials
2.3. Apple Juice and Apple-Enriched Smoothies Production
- (i)
- Apple juice production. Apple fruits were hand-washed in distilled water, cut into halves and arils, and ground in a Thermomix appliance (Vorwerk, Wuppertal, Germany) for 20 s with 0.2 mL of Pectinex Smash XXL enzyme per 1 kg of fruit. Then, the mashes were pressed in a hydraulic press (pilot plant laminar press; 15 tons of pressure) to obtain the juice.
- (ii)
- The processing of strawberry tree and persimmon fruits, myrtle berries, saffron and feijoa flowers. Strawberry tree fruits and feijoa flowers were frozen, freeze-dried, and homogenized into powder using a closed laboratory mill to avoid hydration (IKA 11A; BIOSAN, Vilno, Lithuania). Myrtle berries were double-extracted with ethanol 96% (1:1, w/v) under sonication for 30 min. Then, the extract was filtered using a strainer and concentrated by vacuum distillation (Büchi Rotavapor R-114, Switzerland) until complete alcohol elimination was achieved. Saffron flowers, the by-product obtained after the removal of the stigmas, were squeezed (manual press) to obtain juice, which was centrifuged, filtered using 0.45 μm cellulose acetate filter, frozen and freeze-dried. Persimmon fruits were grounded, heated at 80 °C in a Thermomix appliance (Vorwerk, Wuppertal, Germany), mashed, and reduced down in a blender (Symbio, Zelmer, Rzeszów, Poland) to a thin purée. After that, the purée was cooled and used to produce juices.
- (iii)
- Mixing semi-finished products. Apple juice (AJ) and other plant semi-products were mixed in appropriate proportions (w/w; 95:5 for A. unedo fruits (AJ+C5), M. communis berry extract (AJ+M5), D. kaki purée (AJ+P5) and A. sellowiana flowers (AJ+F5), and 99.99:0.01 (AJ+S01) and 99.95:0.05 (AJ+S05) for C. sativus flower juice) (Figure S1). Then, all products were heated to 100 °C, hot-filled in glass jars (135 mL), pasteurised (10 min at 90 °C), and cooled to 20 °C. The seven different products were analysed immediately after processing.
2.4. Physico-Chemical Analyses
2.5. Determination of Sugar and Organic Acids Content
2.6. Colour Measurement
2.7. Identification and Quantification of Polyphenolic Compounds
2.8. Analysis of Polymeric Proanthocyanidins by Phloroglucinol Method
2.9. Determination of Total Phenolic Content (Folin–Ciocalteu Assay), Total Reducing Power (FRAP, CUPRAC Assays) and Free Radical Scavenging Activity (DPPH•, ABTS•+, ORAC Assays)
2.10. Consumer Evaluation of the Enriched Apple Smoothies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters of the Apple Juice and Enriched Apple Smoothies
3.2. Sugar and Organic Acids Content of the Apple Juice and Enriched Apple Smoothies
3.3. Identification and Quantification of Phenolic Compounds in the Apple Juice and Enriched Apple Smoothies
3.4. Antioxidant Activity of the Obtained Apple Juice and Enriched Apple Smoothies
3.5. Sensory Evaluation of the Obtained Apple Juice and Enriched Apple Smoothies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troggio, M.; Gleave, A.; Salvi, S.; Chagné, D.; Cestaro, A.; Kumar, S.; Crowhurst, R.N.; Gardiner, S.E. Apple, from genome to breeding. Tree Genet. Genomes 2012, 8, 509–529. [Google Scholar] [CrossRef]
- Technavio: Apple Market—Forecast and Analysis, Technavio, 2022–2026. Available online: https://www.prnewswire.com/news-releases/apple-market-2026-increasing-demand-for-superfoods-to-boost-growth---technavio-301628604.html (accessed on 15 November 2022).
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Šulc, M.; Sus, J.; Pavlíková, O. Polyphenol content and antiradical activity in different apple varieties. J. Hortic. Sci. 2006, 33, 95–102. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Nagasako-Akazome, Y.; Kanda, T.; Ohtake, Y.; Shimasaki, H.; Kobayashi, T. Apple polyphenols influence cholesterol metabolism in healthy subject with relatively high body mass index. J. Oleo Sci. 2007, 56, 417–428. [Google Scholar] [CrossRef]
- Akazome, Y.; Kametani, N.; Kanda, T.; Shimasaki, H.; Kobayashi, S. Evaluation of safety of excessive intake and efficacy of long-term intake of beverages containing apple polyphenols. J. Oleo Sci. 2010, 6, 321–338. [Google Scholar] [CrossRef]
- Hyson, D.; Studenbaker-Hallman, D.; Davis, P.A.; Gershwin, M.E. Apple juice consumption reduces plasma low-density lipoprotein oxidation in healthy men and women. J. Med. Food 2000, 3, 159–166. [Google Scholar] [CrossRef]
- Tsao, R. Apples. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 239–248. [Google Scholar]
- Liu, Y.; Del Toro-Gipson, R.S.; Drake, M.A. Sensory properties and consumer acceptance of ready-to-drink vanilla protein beverages. J. Sens. Stud. 2021, 36, e12704. [Google Scholar] [CrossRef]
- Tireki, S. A review on packed non-alcoholic beverages: Ingredients, production, trends and future opportunities for functional product development. Trends Food Sci. Technol. 2021, 112, 442–454. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V.M. Quality stability and sensory attributes of apple juice processed by thermosonication pulsed electric field and thermal processing. Food Sci. Technol. Int. 2017, 23, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Will, F.; Roth, M.; Olk, M.; Ludwig, M.; Dietrich, H. Processing and analytical characterisation of pulp-enriched cloudy apple juices. LWT—Food Sci. Technol. 2008, 41, 2057–2063. [Google Scholar] [CrossRef]
- Siebert, K.J.; Lynn, P.Y. Haze-active protein and polyphenols in apple juice assessed by turbidimetry. J. Food Sci. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Duralija, B.; Putnik, P.; Brdar, D.; Bebek Markovinović, A.; Zavadlav, S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Bursać Kovačević, D. The perspective of Croatian old apple cultivars in extensive farming for the production of functional foods. Foods 2021, 10, 708. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Harholt, J.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Palatability and chemical safety of apple juice fortified with pomegranate peel extract. Food Funct. 2013, 4, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J.; Oszymiański, J.; Wojdyło, A. Effect of apple leaves addition on physicochemical properties of cloudy beverages. Ind. Crops Prod. 2013, 44, 413–420. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, A. Antioxidant activity, cytotoxic activity and metabolic profiling juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef]
- Montoro, P.; Serreli, G.; Gil, K.A.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. Evaluation of bioactive compounds and antioxidant capacity of edible feijoa (Acca sellowiana (O. Berg) Burret) flower extracts. J. Food Sci. Technol. 2020, 57, 2051–2060. [Google Scholar] [CrossRef]
- Kanur, N.; Kumari, A.; Agarwal, A.; Sabharwal, M.; Dipti, S. Utilisation of Diospyros kaki L. (persimmon) as a functional ingredient to produce functional foods: A review. Nutr. Food Sci. 2022, 52, 1083–1099. [Google Scholar]
- Biegańska-Mercik, R.; Radziejewska-Kubzdela, E.; Marecik, R. Characterization of phenolics, glucosinolates and antioxidant activity of beverages based on apple juice with addition of frozen and freeze-dried curly kale leaves (Brassica oleracea L. var. acephala L.). Food Chem. 2017, 230, 271–280. [Google Scholar] [CrossRef]
- Pernice, R.; Borriello, G.; Ferracane, R.; Borrelli, R.C.; Cennamo, F.; Ritieni, A. Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009, 112, 545–550. [Google Scholar] [CrossRef]
- Bayram, Y.; Sagdic, O. Antioxidant, color, and sensory properties of apple juices colored with saffron microcapsules. Lat. Am. Appl. Res. 2022, 52, 379–386. [Google Scholar] [CrossRef]
- Guerreiro, A.; Gago, C.; Miguel, G.; Faleiro, M.L.; Panagopoulos, T.; Antunes, M.D. Potential of strawberry tree fruit (Arbutus unedo L.) for fresh consumption and its behavior through storage. Acta Hortic. 2018, 1194, 941–946. [Google Scholar] [CrossRef]
- Lhaj, Z.A.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan strawberry tree (Arbutus unedo L.) fruits: Nutritional value and mineral composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Orrù, C.D. Myrtle (Myrtus communis L.) berries: Composition and properties. In Berries: Properties, Consumption and Nutrition, 2nd ed.; Tuberoso, C.I.G., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 145–155. [Google Scholar]
- Tuberoso, C.I.G.; Melis, M.; Angioni, A.; Pala, M.; Cabras, P. Myrtle hydroalcoholic extracts obtained from different selections of Myrtus communis L. Food Chem. 2007, 101, 806–811. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepo, B.; Eduardo-Figueira, M. From Diospyros kaki L. (persimmon) phytochemical profile and health impact to new product perspectives and waste valorization. Nutrients 2021, 13, 3283. [Google Scholar] [CrossRef]
- Anliker, M.D.; Reindl, J.; Vieths, S.; Wuthrich, B. Allergy caused by ingestion of persimmon (Diospyros kaki): Detection of specific IgE and cross-reactivity to profilin and carbohydrate determinants. J. Allergy Clin. Immunol. 2001, 107, 718–723. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran, J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar]
- Serrano-Díaz, J.S.; Estevan, C.; Sogorb, M.A.; Carmona, M.; Alonso, G.L.; Vilanova, E. Cyto-toxic effect against 3T3 fibroblasts cells of saffron floral bio-residues extracts. Food Chem. 2014, 147, 55–59. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.G.; Do Amarante, C.V.T.; Steffens, C.A.; Benincá, T.D.; Padilha, M. Postharvest quality of Feijoa flowers treated with different preservative solutions and 1-methylcyclopropene. Rev. Bras. Frutic. 2016, 38, e759. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- PN-EN 12145:2001; Fruit and Vegetable Juices—Determination of Total Dry Substance. Gravimetric Method for the Determination of Loss of Mass due to Drying. Polish Committee for Standardization: Warsaw, Poland, 2001.
- PN-EN 12143:2000; Fruit and Vegetable Juices—Determination of Soluble Matter by Refractometric Method. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-EN 12147:2000; Fruit and Vegetable Juices—Determination of Titratable Acidity. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-A-04019:1998; Food Products—Determination of Vitamin C Content. Polish Committee for Standardization: Warsaw, Poland, 1998.
- PN-EN 1135:1999; Fruit and Vegetable Juices. Determination of Ash Content. Polish Committee for Standardization: Warsaw, Poland, 1999.
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur. Food Res. Technol. 2019, 245, 929–938. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Teleszko, M.; Samoticha, J. Sensory attributes and changes of physicochemical properties during storage of smoothies prepared from selected fruits. LWT—Food Sci. Technol. 2016, 71, 102–109. [Google Scholar] [CrossRef]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Physicochemical characterisation of quince fruits for industrial use: Yield, turbidity, viscosity and colour properties of juices. Int. J. Food Sci. Technol. 2014, 49, 1818–1824. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef]
- Bektaşǒglu, B.; Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Novel hydroxyl radical scavenging antioxidant activity assay for water-soluble antioxidants using a modified CUPRAC method. Biochem. Biophys. Res. Commun. 2006, 345, 1194–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assay: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 8589:2009; Sensory Analysis—General Guidelines for the Design of Sensory Analysis Laboratories. ISO: Geneva, Switzerland, 2009.
- Nowicka, P.; Wojdyło, A.; Teleszko, M. Effect of mixing different kinds of fruit juice with sour cherry puree on nutritional properties. J. Food Sci. Technol. 2017, 54, 114–129. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Ulrih, N.P. The physico-chemical properties of strawberry tree (Arbutus unedo L.) fruits. Croat. J. Food Sci. Technol. 2013, 5, 29–33. [Google Scholar]
- Zatylny, A.M.; Ziehl, W.D.; St-Pierre, R.G. Physicochemical properties of fruit of chokecherry (Prunus virginiana L.), highbush cranberry (Viburnum trilobum Marsh.), and black currant (Ribes nigrum L.) cultivars grown in Saskatchewan. Can. J. Plant Sci. 2005, 85, 425–429. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Bento, A.; Pereira, J.A. Arbutus unedo L. and its benefits on human health. J. Food Nutr. Res. 2011, 50, 73–85. [Google Scholar]
- Castellanos, D.A.; Polanía, W.; Herrera, A.O. Development of an equilibrium modified atmosphere packaging (EMAP) for feijoa fruits and modeling firmness and color evolution. Postharvest Biol. Technol. 2016, 120, 193–203. [Google Scholar] [CrossRef]
- Jaros, D.; Thamke, I.; Raddatz, H.; Rohm, H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009, 229, 55–61. [Google Scholar] [CrossRef]
- Konić-Ristić, A.; Šavikin, K.; Zdunić, G.; Janković, T.; Juranic, Z.; Menković, N.; Stanković, I. Biological activity and chemical composition of different berry juices. Food Chem. 2011, 125, 1412–1417. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Gonzales-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELab parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Izuchi, R.; Takahashi, H.; Inada, Y. Preparing a carotenoid polyphenol-enriched extract from the peel of persimmon, Diospyros kaki L.f. Biosci. Biotechnol. Biochem. 2009, 73, 2793–2795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2016, 199, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Teleszko, M.; Sokół-Łętowska, A. Composition and quantification of major polyphenolic compounds, antioxidant activity and colour properties of quince and mixed quince jams. Int. J. Food Sci. Nutr. 2013, 64, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F. Internal fruit quality of figs (Ficus carica L.) in the Northern Mediterranean region. Ital. J. Food Sci. 2008, 20, 255–262. [Google Scholar]
- Tappy, L. Quality of sugars and sugar-containing foods. Cereal Foods World 2018, 63, 107–113. [Google Scholar]
- Seymour, E.M.; Singer, A.A.M.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef]
- Theron, M.M.; Rykers Lues, J.F. Organic Acids and Food Preservation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 1–318. [Google Scholar]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Gromova, L.V. Effect of phloretin and phloridzin on properties of digestion and absorption in the rat small intestine. J. Evol. Biochem. Physiol. 2006, 42, 454–460. [Google Scholar] [CrossRef]
- Barbosa, A.C.L.; Pinto, M.; Da, S.; Sarkar, D.; Ankolekar, C.; Shetty, K. Influence of varietal and pH on antihyperglycemia and antihypertension properties of longterm stored apples using in vitro assay models. J. Food Biochem. 2011, 36, 479–493. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Evaluation of spectrophotometric methods for antioxidant compound measurement in relation to total antioxidant capacity in beverages. Food Chem. 2010, 120, 607–614. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S. Fruit and vegetable-based beverages—Nutritional properties and health benefits. In Natural Beverages, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge MA, USA, 2019; pp. 303–338. [Google Scholar]
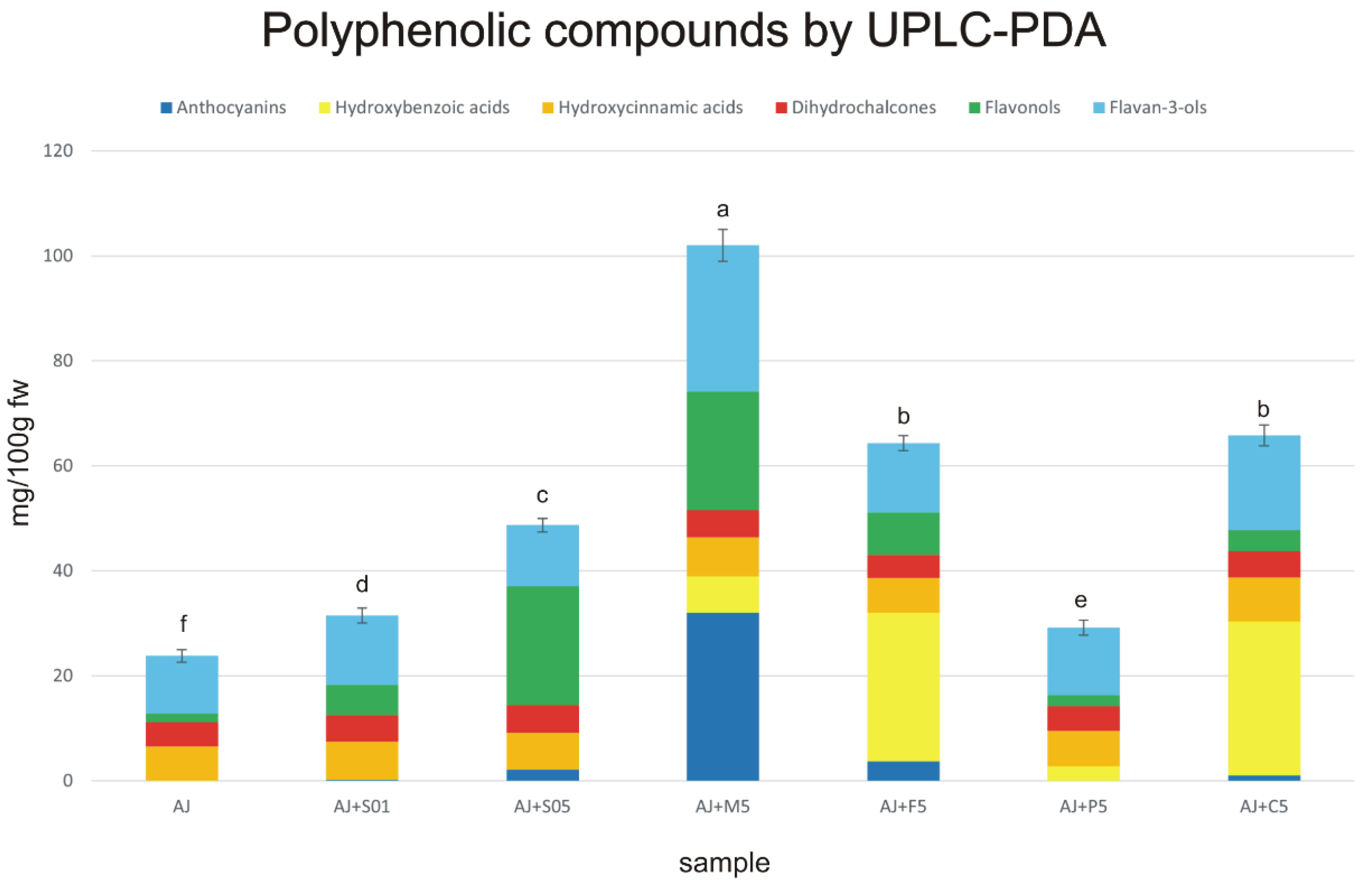
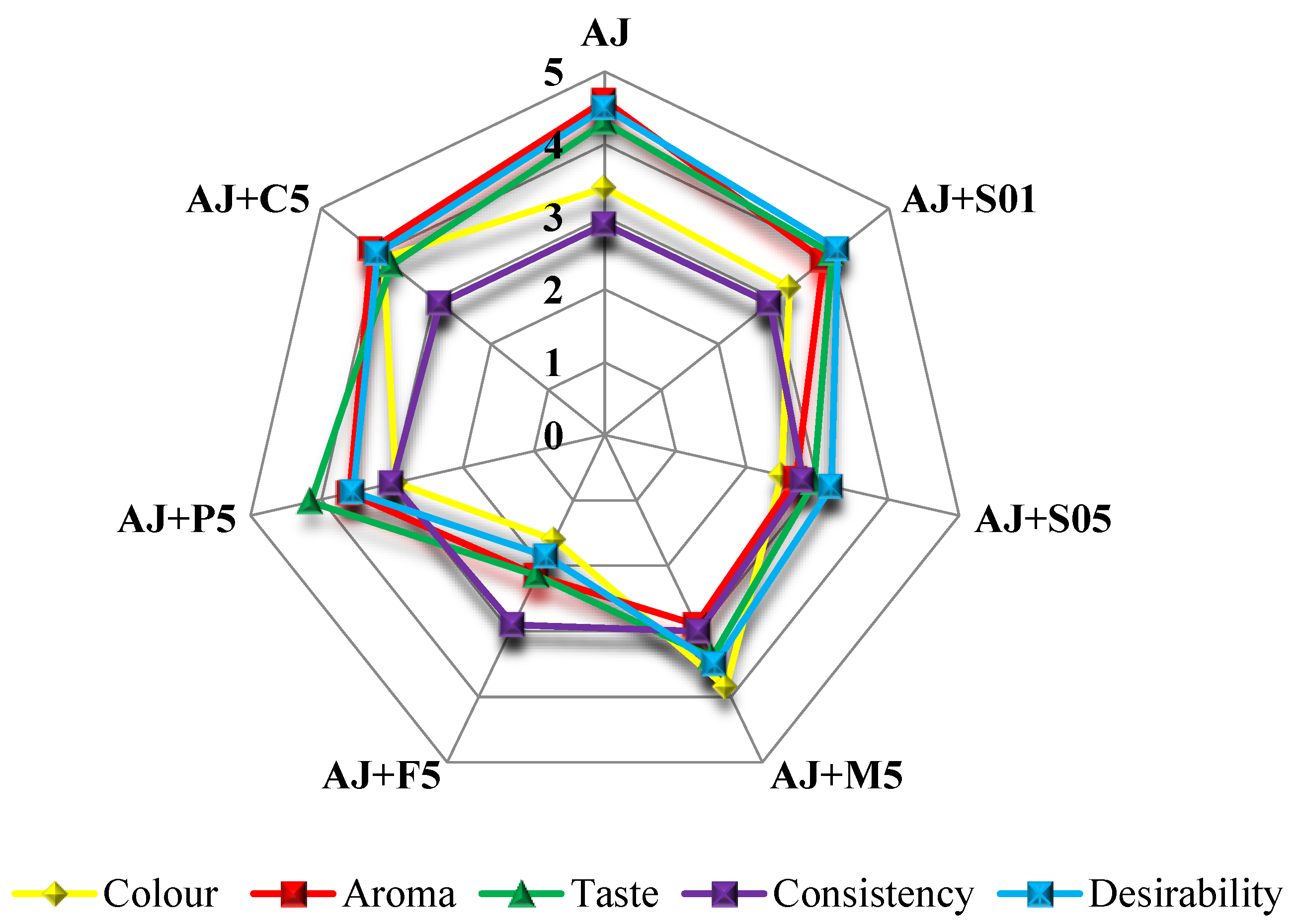
| Parameter | Sample | ||||||
|---|---|---|---|---|---|---|---|
| AJ | AJ+S01 | AJ+S05 | AJ+M5 | AJ+F5 | AJ+P5 | AJ+C5 | |
| Dry matter (g/100 g fw) | 13.54 ± 0.00 f | 13.88 ± 0.07 e | 14.48 ± 0.07 d | 14.85 ± 0.09 c | 17.59 ± 0.01 a | 14.49 ± 0.31 d | 16.97 ± 0.03 b |
| Ashes (g/100 g fw) | 0.28 ± 0.04 cd | 0.23 ± 0.00 d | 0.34 ± 0.03 cd | 0.33 ± 0.02 c | 0.53 ± 0.04 a | 0.28 ± 0.02 c | 0.31 ± 0.00 b |
| Total soluble solids (TSS, °Brix) | 13.20 ± 0.00 g | 13.50 ± 0.02 f | 14.10 ± 0.03 d | 14.60 ± 0.01 c | 16.00 ± 0.01 b | 13.80 ± 0.02 e | 16.10 ± 0.00 a |
| Total acidity (TA, g of MA #/100 g fw) | 0.42 ± 0.01 c | 0.44 ± 0.01 bc | 0.45 ± 0.00 b | 0.45 ± 0.01 b | 0.49 ± 0.01 a | 0.44 ± 0.03 bc | 0.50 ± 0.01 a |
| TSS/TA | 32.20 | 30.68 | 31.33 | 31.74 | 32.65 | 30.00 | 31.57 |
| pH | 3.31 ± 0.03 e | 3.52 ± 0.01 d | 3.58 ± 0.02 c | 3.68 ± 0.01 b | 4.00 ± 0.02 a | 3.59 ± 0.01 c | 3.56 ± 0.04 cd |
| Vitamin C (mg/100 g fw) | 0.90 ± 0.01 b | 0.64 ± 0.01 c | 0.65 ± 0.01 c | 0.65 ± 0.01 c | 0.95 ± 0.01 b | 0.68 ± 0.04 c | 23.68 ± 0.23 a |
| Colour parameters § | |||||||
| L* | 50.54 ± 0.03 b | 49.60 ± 0.05 c | 44.13 ± 0.06 d | 29.25 ± 0.00 f | 37.29 ± 0.13 e | 54.75 ± 0.06 a | 54.92 ± 0.21 a |
| a* | 1.49 ± 0.00 g | 2.74 ± 0.02 f | 6.11 ± 0.03 d | 7.78 ± 0.00 b | 7.30 ± 0.06 c | 2.83 ± 0.02 e | 12.36 ± 0.05 a |
| b* | 16.43 ± 0.05 c | 15.53 ± 0.02 d | 13.88 ± 0.01 e | −1.48 ± 0.00 g | 8.75 ± 0.03 f | 18.13 ± 0.12 b | 24.99 ± 0.02 a |
| ΔE* | - | 1.80 ± 0.00 f | 8.30 ± 0.02 d | 28.52 ± 0.03 a | 16.38 ± 0.11 b | 4.73 ± 0.03 e | 14.51 ± 0.02 c |
| Parameter | Sample | ||||||
|---|---|---|---|---|---|---|---|
| AJ | AJ+S01 | AJ+S05 | AJ+M5 | AJ+F5 | AJ+P5 | AJ+C5 | |
| Sugar content (g/100 g fw) | |||||||
| Fructose | 6.73 ± 0.23 d | 6.77 ± 0.15 d | 9.34 ± 0.10 c | 11.47 ± 0.05 a | 10.93 ± 0.42 b | 11.02 ± 0.01 b | 11.72 ± 0.22 a |
| Sorbitol | 0.07 ± 0.00 d | 0.07 ± 0.01 d | 0.10 ± 0.01 c | 0.12 ± 0.02 bc | 0.11 ± 0.00 c | 0.14 ± 0.01 ab | 0.16 ± 0.02 a |
| Glucose | 0.97 ± 0.05 e | 1.02 ± 0.10 e | 1.33 ± 0.00 d | 2.57 ± 0.01 a | 2.03 ± 0.02 c | 0.98 ± 0.00 e | 2.40 ± 0.00 b |
| Sucrose | 0.32 ± 0.02 d | 0.32 ± 0.01 d | 0.36 ± 0.02 c | 0.23 ± 0.00 e | 0.50 ± 0.01 b | 0.34 ± 0.01 cd | 0.57 ± 0.02 a |
| Total | 8.10 ± 0.03 g | 8.17 ± 0.04 f | 11.13 ± 0.03 e | 14.40 ± 0.03 b | 13.56 ± 0.02 c | 12.48 ± 0.00 d | 14.85 ± 0.03 a |
| Organic acid content (g/100 g fw) | |||||||
| Oxalic | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.01 b | 0.20 ± 0.03 a | 0.02 ± 0.01 b | 0.02 ± 0.00 b |
| Citric | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.29 ± 0.03 a | 0.02 ± 0.00 b | 0.03 ± 0.00 b |
| Tartaric | 0.01 ± 0.00 b | nd | nd | nd | nd | 0.06 ± 0.01 a | nd |
| Malic | 0.48 ± 0.01 d | 0.51 ± 0.02 cd | 0.52 ± 0.03 bc | 0.56 ± 0.02 ab | 0.57 ± 0.02 a | 0.50 ± 0.02 cd | 0.58 ± 0.03 a |
| Quinic | 0.44 ± 0.01 d | 0.44 ± 0.01 d | 0.52 ± 0.01 bc | 0.53 ± 0.03 b | 0.48 ± 0.06 bcd | 0.47 ± 0.04 cd | 0.84 ± 0.01 a |
| Ascorbic | tr | tr | tr | tr | tr | tr | tr |
| Shikimic | tr | tr | tr | 0.01 ± 0.00 a | 0.02 ± 0.00 a | tr | tr |
| Fumaric | tr | tr | tr | tr | tr | tr | tr |
| Total | 0.93 ± 0.01 e | 0.97 ± 0.02 e | 1.06 ± 0.06 d | 1.14 ± 0.03 c | 1.56 ± 0.00 a | 1.06 ± 0.01 d | 1.47 ± 0.02 b |
| Sugar/organic acids | 8.62 | 8.34 | 10.40 | 12.52 | 8.64 | 11.66 | 10.03 |
| Parameter | Sample | ||||||
|---|---|---|---|---|---|---|---|
| AJ | AJ+S01 | AJ+S05 | AJ+M5 | AJ+F5 | AJ+P5 | AJ+C5 | |
| TP mg GAE/100 g fw | 82.25 ± 2.36 f | 91.19 ± f 6.78 ef | 98.08 ± 7.79 e | 132.20 ± 9.43 c | 179.84 ± 9.95 b | 194.06 ± 1.39 a | 115.89 ± 4.57 d |
| CUPRAC mmol Fe2+/100 g fw | 1.81 ± 0.09 f | 4.77 ± 0.06 c | 3.08 ± 0.04 e | 3.61 ± 0.21 d | 6.01 ± 0.04 b | 7.04 ± 0.09 a | 3.05 ± 0.02 e |
| FRAP mmol Trolox/100 g fw | 0.58 ± 0.00 e | 1.00 ± 0.01 d | 0.65 ± 0.01 e | 1.15 ± 0.04 c | 1.77 ± 0.02 a | 1.44 ± 0.01 b | 0.95 ± 0.11 d |
| ORAC mmol Trolox/100 g fw | 2.17 ± 0.17 c | 2.23 ± 0.10 c | 2.81 ± 0.19 b | 3.07 ± 0.16 b | 3.51 ± 0.23 a | 2.96 ± 0.13 b | 2.10 ± 0.15 c |
| DPPH• mmol Trolox/100 g fw | 0.47 ± 0.01 f | 0.89 ± 0.03 c | 0.50 ± 0.03 f | 0.73 ± 0.04 d | 1.26 ± 0.01 a | 1.05 ± 0.04 b | 0.60 ± 0.02 e |
| ABTS•+ mmol Trolox/100 g fw | 0.48 ± 0.00 e | 1.11 ± 0.01 c | 0.57 ± 0.00 e | 1.14 ±0.01 c | 2.21 ± 0.01 a | 1.82 ± 0.13 b | 0.93 ± 0.03 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, K.A.; Wojdyło, A.; Nowicka, P.; Montoro, P.; Tuberoso, C.I.G. Effect of Apple Juice Enrichment with Selected Plant Materials: Focus on Bioactive Compounds and Antioxidant Activity. Foods 2023, 12, 105. https://doi.org/10.3390/foods12010105
Gil KA, Wojdyło A, Nowicka P, Montoro P, Tuberoso CIG. Effect of Apple Juice Enrichment with Selected Plant Materials: Focus on Bioactive Compounds and Antioxidant Activity. Foods. 2023; 12(1):105. https://doi.org/10.3390/foods12010105
Chicago/Turabian StyleGil, Katarzyna Angelika, Aneta Wojdyło, Paulina Nowicka, Paola Montoro, and Carlo Ignazio Giovanni Tuberoso. 2023. "Effect of Apple Juice Enrichment with Selected Plant Materials: Focus on Bioactive Compounds and Antioxidant Activity" Foods 12, no. 1: 105. https://doi.org/10.3390/foods12010105
APA StyleGil, K. A., Wojdyło, A., Nowicka, P., Montoro, P., & Tuberoso, C. I. G. (2023). Effect of Apple Juice Enrichment with Selected Plant Materials: Focus on Bioactive Compounds and Antioxidant Activity. Foods, 12(1), 105. https://doi.org/10.3390/foods12010105










