Study on Volatile Profiles, Polycyclic Aromatic Hydrocarbons, and Acrylamide Formed in Welsh Onion (Allium fistulosum L.) Fried in Vegetable Oils at Different Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of WOF Samples
2.3. Analysis of Volatile Compounds
2.4. Analysis of PAHs
2.5. Analysis of Acrylamide
2.6. Statistical Analysis
3. Results and Discussion
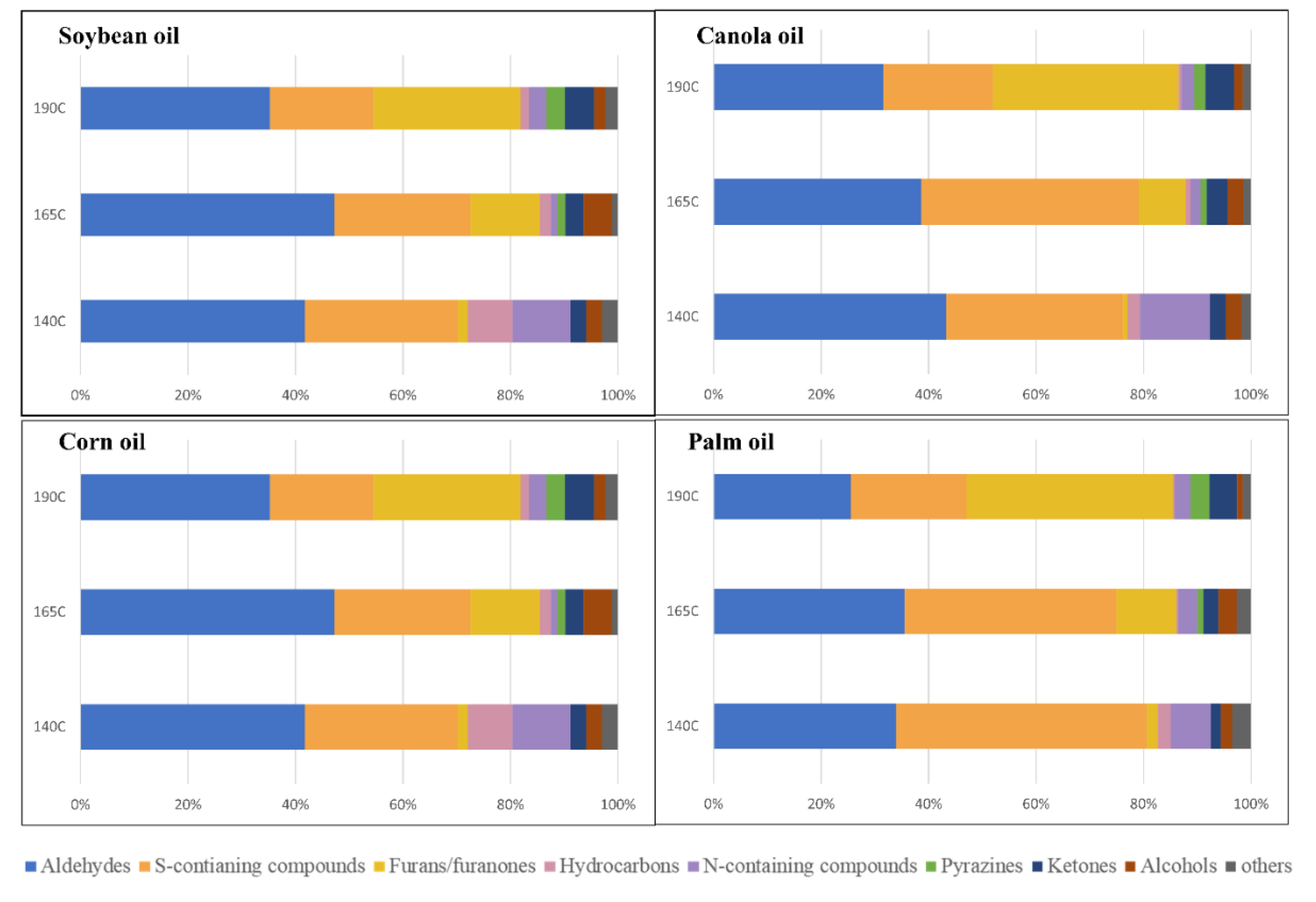
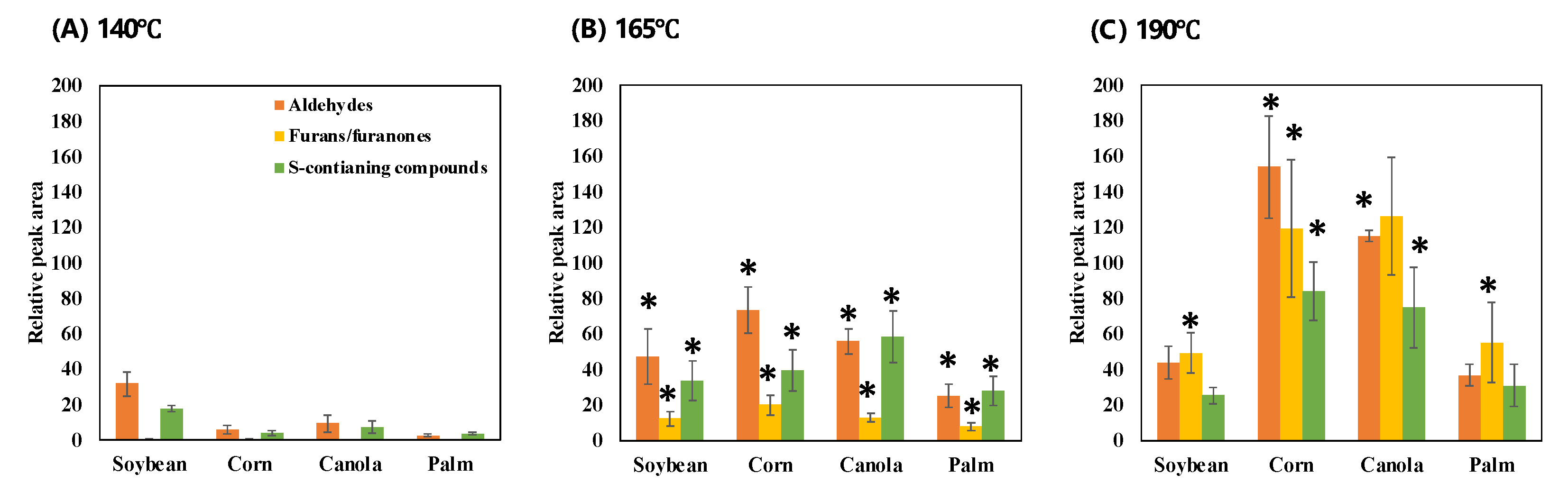
3.1. Volatile Changes of WOF According to Types of Vegetable Oil and Frying Temperatures
3.2. Hazardous Compounds in WOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Influence of different frying processes on the flavor characteristics and sensory profile of garlic oil. Molecules 2019, 24, 4456. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y. Effects of different vegetable oils on the aroma characteristics of deep-fried shallot flavoring evaluated by HS-SPME/GC-MS coupled with PLSR. J. Food Process. Preserv. 2020, 44, e14698. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, B.; Mao, X.; Chen, H.; Zhang, Y. Flavor formation in frying process of green onion (Allium Fistulosum L.) deep-fried oil. Food Res. Int. 2019, 121, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Gertz, C. Fundamentals of the frying process. Eur. J. Lipid Sci. Technol. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Menzio, J.; Tagliapietra, S. Polycyclic aromatic hydrocarbons in coffee samples: Enquiry into processes and analytical methods. Food Chem. 2021, 344, 128631. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Gertz, C.; Klostermann, S. Analysis of acrylamide and mechanisms of its formation in deep-fried products. Eur. J. Lipid Sci. Technol. 2002, 104, 762–771. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Totani, N.; Yawata, M.; Takada, M.; Moriya, M. Acrylamide content of commercial frying oil. J. Oleo Sci. 2007, 56, 103–106. [Google Scholar] [CrossRef][Green Version]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonça, C.B. Fatty acid composition of vegetable oils and fats. Bol. Cent. Pesqui. Process. Aliment. 2007, 25. [Google Scholar]
- Johnson, D.R.; Decker, E.A. The role of oxygen in lipid oxidation reactions: A review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Lan, C.; Lin, P.; Kuo, Y. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard. Mater. 2017, 324, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Zhang, Y.; Shi, H.; Tang, J. Flavor chemistry of Chinese foods. Food Rev. Int. 1989, 5, 253–287. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, X.; Chen, Q.; Zhang, H.; Sun, H.; Zhang, W.; Li, C. Oxidative stabilities of olive and camellia oils: Possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT 2020, 118, 108858. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Fu, X.; Su, G.; Zhao, M. Reducing the influence of the thermally induced reactions on the determination of aroma-active compounds in soy sauce using SDE and GC-MS/O. Food Anal. Methods 2017, 10, 931–942. [Google Scholar] [CrossRef]
- Mayr, C.M.; Capone, D.L.; Pardon, K.H.; Black, C.A.; Pomeroy, D.; Francis, I.L. Quantitative analysis by GC-MS/MS of 18 aroma compounds related to oxidative off-flavor in wines. J. Agric. Food Chem. 2015, 63, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Becalski, A.; Seaman, S. Furan precursors in food: A model study and development of a simple headspace method for determination of furan. J. AOAC Int. 2005, 88, 102–106. [Google Scholar] [CrossRef]
- Shen, M.; Liu, Q.; Jiang, Y.; Nie, S.; Zhang, Y.; Xie, J.; Wang, S.; Zhu, F.; Xie, M. Influences of operating parameters on the formation of furan during heating based on models of polyunsaturated fatty acids. J. Food Sci. 2015, 80, T1432–T1437. [Google Scholar] [CrossRef]
- Fujita, M.; Endo, M.; Sano, M. Purification and characterization of alliin lyase from Welsh onion, Allium Fistulosum L. Agric. Biol. Chem. 1990, 54, 1077–1079. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl-cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Saito, K. S-Alk(en)yl-cysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Farkas, P.; Hradský, P.; Kovác, M. Novel flavour components identified in the steam distillate of onion (Allium Cepa L.). Z. Lebensm.-Unters. Forsch. 1992, 195, 459–462. [Google Scholar] [CrossRef]
- Chyau, C.; Mau, J. Effects of various oils on volatile compounds of deep-fried shallot flavouring. Food Chem. 2001, 74, 41–46. [Google Scholar] [CrossRef]
- Jayalekshmy, A.; Narayanan, C.; Mathew, A. Identification of volatile flavor compounds in roasted coconut. J. Am. Oil Chem. Soc. 1991, 68, 873–880. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Hbaieb, R.H.; Kotti, F.; Gargouri, M.; Msallem, M.; Vichi, S. Ripening and storage conditions of Chétoui and Arbequina olives: Part I. effect on olive oils volatiles profile. Food Chem. 2016, 203, 548–558. [Google Scholar] [CrossRef]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Perceptual interactions and characterisation of odour quality of binary mixtures of volatile phenols and 2-isobutyl-3-methoxypyrazine in a red wine matrix. J. Wine Res. 2020, 31, 49–66. [Google Scholar] [CrossRef]
- Neugebauer, A.; Schieberle, P.; Granvogl, M. Characterization of the Key Odorants Causing the Musty and Fusty/Muddy Sediment Off-Flavors in Olive Oils. J. Agric. Food Chem. 2021, 69, 14878–14892. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Ximba, B.J.; Opeolu, B.O. Polycyclic aromatic hydrocarbons (PAHs) in edible oil: Temperature effect on recovery from base hydrolysis product and health risk factor. Food Public Health 2014, 4, 23–30. [Google Scholar]
- Wongmaneepratip, W.; Vangnai, K. Effects of oil types and pH on carcinogenic polycyclic aromatic hydrocarbons (PAHs) in grilled chicken. Food Control 2017, 79, 119–125. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
| PAHs | Heating Temp. | Concentrations 1 (μg/kg) | |||
|---|---|---|---|---|---|
| Soybean Oil | Corn Oil | Canola Oil | Palm Oil | ||
| B[a]A | 140 °C | 0.65 ± 0.02 b 2 | N.D. a | N.D. a | N.D. a |
| 165 °C | N.D. 3 a | N.D. a | N.D. a | N.D. a | |
| 190 °C | 0.71 ± 0.01b | N.D. a | N.D. a | N.D. a | |
| CHR | 140 °C | 0.64 ± 0.02 b | N.D. a | N.D. a | N.D. a |
| 165 °C | N.D. a | N.D. a | N.D. a | N.D. a | |
| 190 °C | 0.61 ± 0.04 b | N.D. a | N.D. a | N.D. a | |
| B[b]F | 140 °C | 1.26 ± 0.17 b | N.D. a | N.D. a | Trace 4 a |
| 165 °C | N.D. a | N.D. a | N.D. a | 0.28 ± 0.01 b | |
| 190 °C | 1.39 ± 0.03 b | N.D. a | N.D. a | 0.26 ± 0.00 b | |
| B[k]F | 140 °C | 0.72 ± 0.05 b | N.D. a | N.D. a | N.D. a |
| 165 °C | N.D. a | N.D. a | N.D. a | N.D. a | |
| 190 °C | 0.75 ± 0.02 b | N.D. a | N.D. a | N.D. a | |
| B[a]P | 140 °C | 1.38 ± 0.05 b | N.D. a | N.D. a | trace a |
| 165 °C | 0.59 ± 0.04 a | N.D. a | N.D. a | trace a | |
| 190 °C | 1.42 ± 0.05 b | N.D. a | N.D. a | trace a | |
| I[c,d]P | 140 °C | 0.41 ± 0.05 b | N.D. a | N.D. a | 0.36 ± 0.03 b |
| 165 °C | trace a | N.D. a | N.D. a | 0.30 ± 0.03 ab | |
| 190 °C | 0.51 ± 0.04 c | N.D. a | N.D. a | 0.32 ± 0.01 a | |
| D[a,h]A | 140 °C | 0.15 ± 0.01 a | N.D. a | N.D. a | 0.16 ± 0.01 a |
| 165 °C | 0.17 ± 0.01 ab | N.D. a | N.D. a | 0.19 ± 0.05 a | |
| 190 °C | 0.19 ± 0.02 b | N.D. a | N.D. a | 0.19 ± 0.01 a | |
| B[g,h,i]P | 140 °C | 0.48 ± 0.06 b | N.D. a | N.D. a | 0.36 ± 0.02 a |
| 165 °C | 0.11 ± 0.00 a | N.D. a | N.D. a | 0.34 ± 0.05 a | |
| 190 °C | 0.53 ± 0.03 b | N.D. a | N.D. a | 0.37 ± 0.05 a | |
| Compound | Heating Temp. | Concentrations 1 (μg/kg) | |||
|---|---|---|---|---|---|
| Soy Bean Oil | Corn Oil | Canola Oil | Palm Oil | ||
| Acrylamide | 140 °C | N.D. 3 a 2 | N.D. a | N.D. a | N.D. a |
| 165 °C | 7.31 ± 0.80 b | N.D. a | N.D. a | N.D. a | |
| 190 °C | 14.72 ± 2.68 c | 21.74 ± 8.98 b | 25.38 ± 3.76 b | 36.46 ± 12.83 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Park, M.-K.; Mun, S.-J.; Jung, M.-Y.; Lee, S.-M.; Kim, Y.-S. Study on Volatile Profiles, Polycyclic Aromatic Hydrocarbons, and Acrylamide Formed in Welsh Onion (Allium fistulosum L.) Fried in Vegetable Oils at Different Temperatures. Foods 2022, 11, 1335. https://doi.org/10.3390/foods11091335
Kim H-M, Park M-K, Mun S-J, Jung M-Y, Lee S-M, Kim Y-S. Study on Volatile Profiles, Polycyclic Aromatic Hydrocarbons, and Acrylamide Formed in Welsh Onion (Allium fistulosum L.) Fried in Vegetable Oils at Different Temperatures. Foods. 2022; 11(9):1335. https://doi.org/10.3390/foods11091335
Chicago/Turabian StyleKim, Hye-Min, Min-Kyung Park, Soo-Jeong Mun, Mun-Yhung Jung, Sang-Mi Lee, and Young-Suk Kim. 2022. "Study on Volatile Profiles, Polycyclic Aromatic Hydrocarbons, and Acrylamide Formed in Welsh Onion (Allium fistulosum L.) Fried in Vegetable Oils at Different Temperatures" Foods 11, no. 9: 1335. https://doi.org/10.3390/foods11091335
APA StyleKim, H.-M., Park, M.-K., Mun, S.-J., Jung, M.-Y., Lee, S.-M., & Kim, Y.-S. (2022). Study on Volatile Profiles, Polycyclic Aromatic Hydrocarbons, and Acrylamide Formed in Welsh Onion (Allium fistulosum L.) Fried in Vegetable Oils at Different Temperatures. Foods, 11(9), 1335. https://doi.org/10.3390/foods11091335