Spectroscopic and Thermal Characterization of Extra Virgin Olive Oil Adulterated with Edible Oils
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Photoluminescence
3.2. Raman Spectroscopy
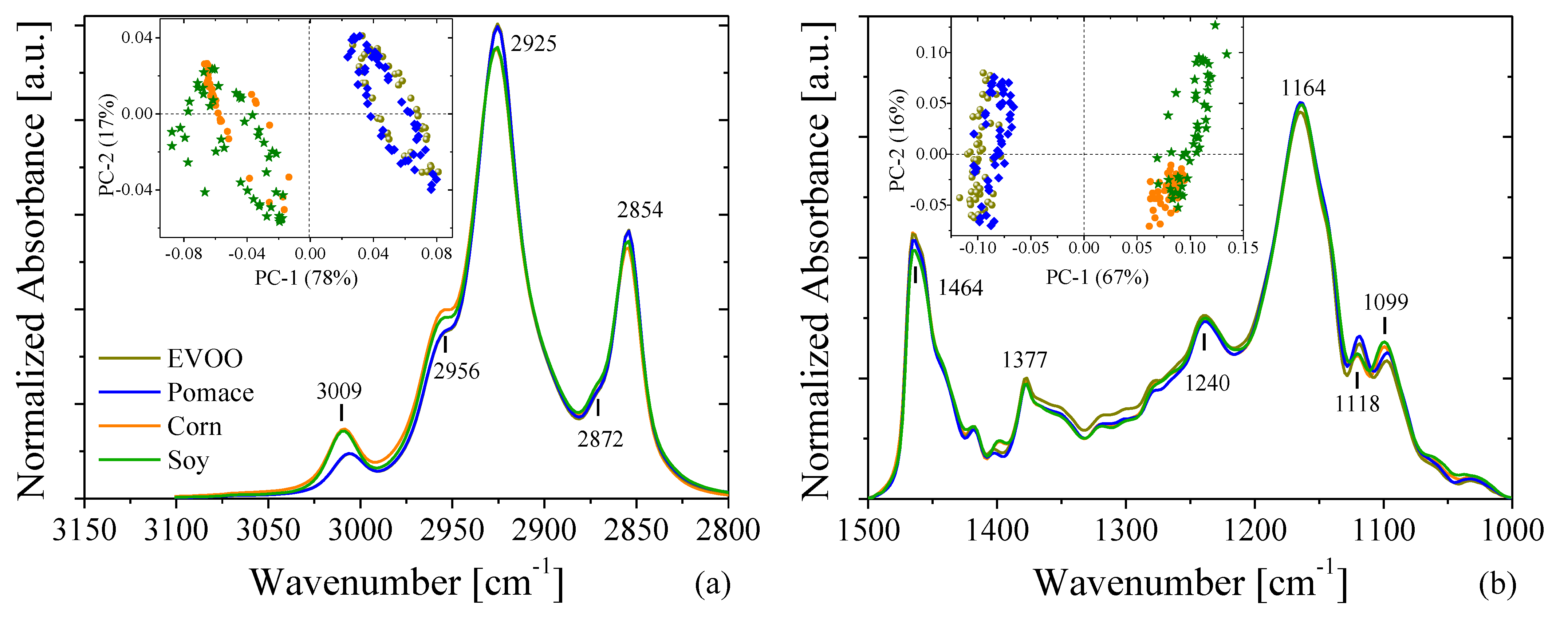
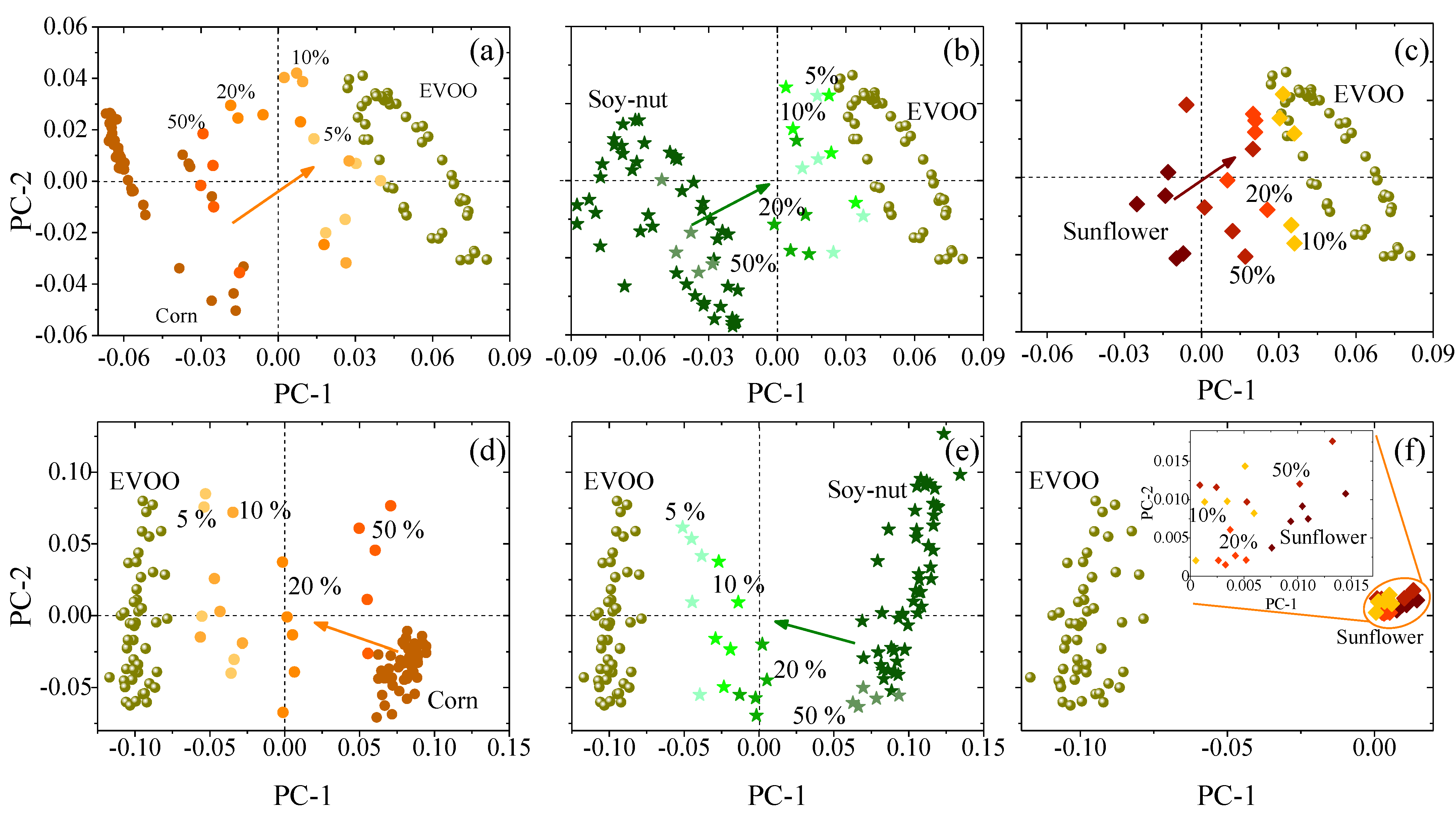
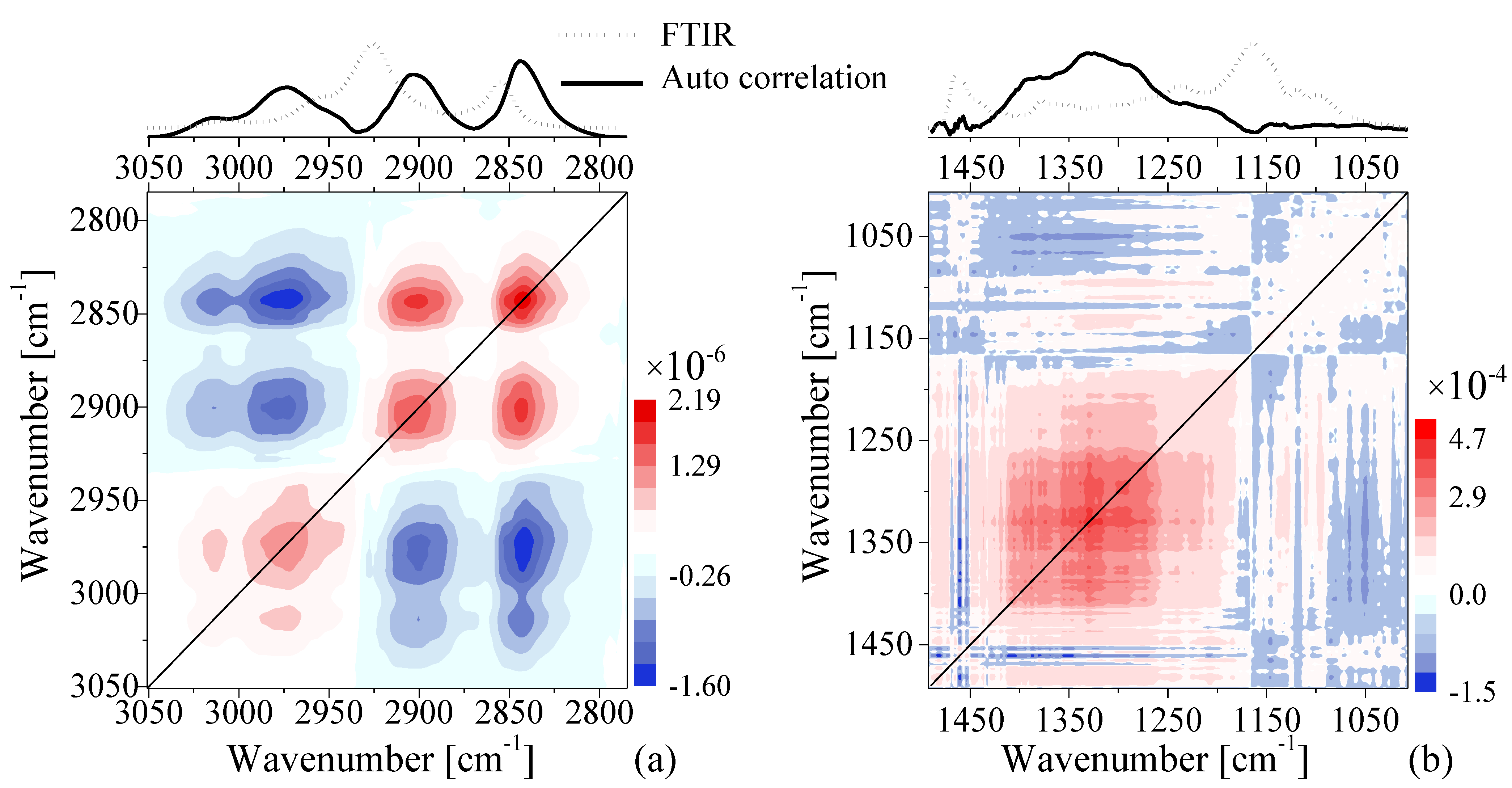
3.3. Fourier-Transform Infrared Spectroscopy
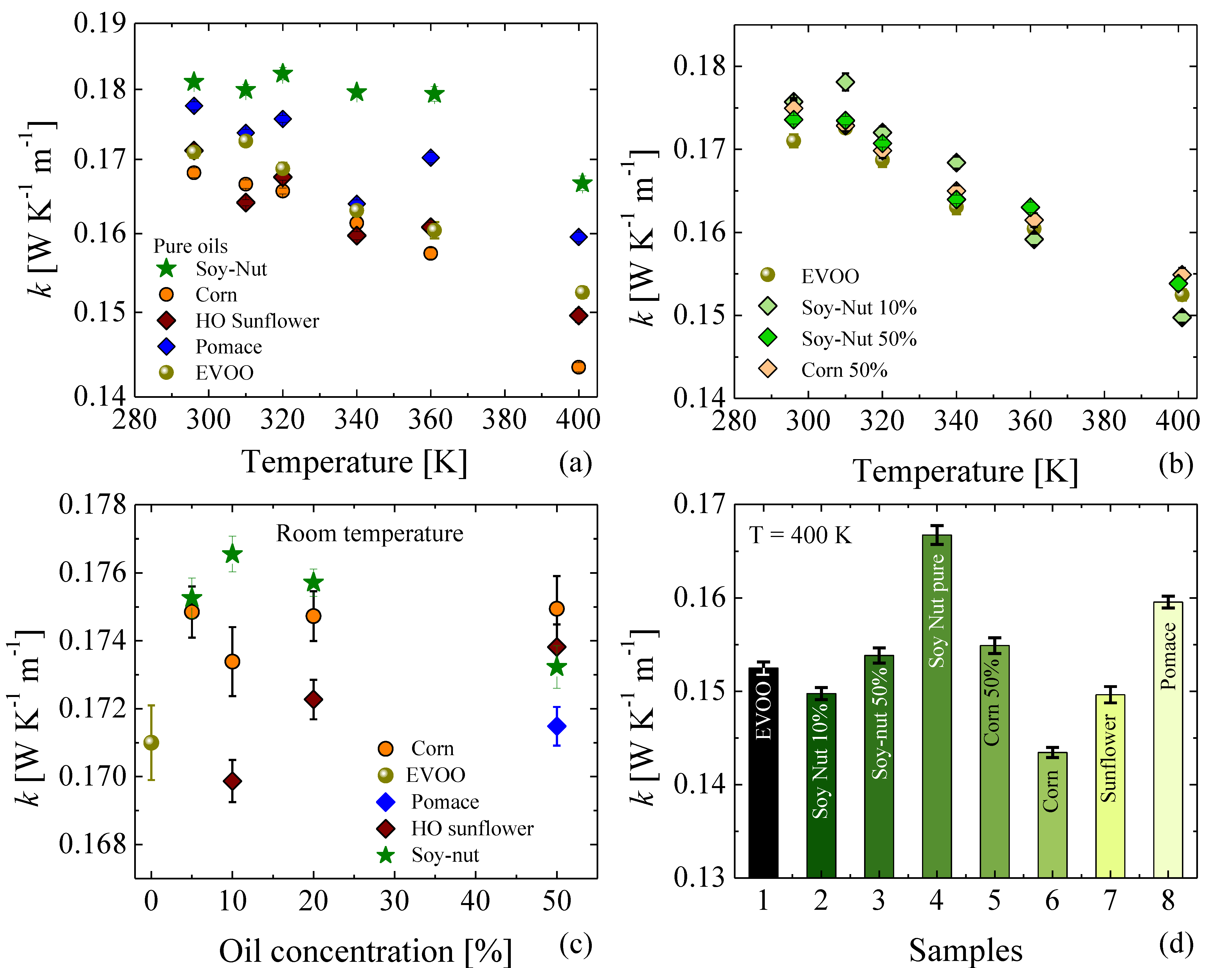
3.4. Thermal Conductivities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Codex Standars for Fats and Oils from Vegetable Sources. 1999. Available online: http://www.fao.org/docrep/004/y2774e/y2774e04.htm (accessed on 18 October 2021).
- Dairi, S.; Carbonneau, M.-A.; Galeano-Diaz, T.; Remini, H.; Dahmoune, F.; Aoun, O.; Belbahi, A.; Lauret, C.; Cristol, J.-P.; Madani, K. Antioxidant Effects of Extra Virgin Olive Oil Enriched by Myrtle Phenolic Extracts on Iron-Mediated Lipid Peroxidation under Intestinal Conditions Model. Food Chem. 2017, 237, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is Olive Oil Good for You? A Systematic Review and Meta-Analysis on Anti-Inflammatory Benefits from Regular Dietary Intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Lampousi, A.-M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive Oil in the Prevention and Management of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies and Intervention Trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Hruby, A.; Salas-Salvadó, J.; Martínez-González, M.A.; Sun, Q.; Willett, W.C.; Hu, F.B. Olive Oil Consumption and Risk of Type 2 Diabetes in US Women. Am. J. Clin. Nutr. 2015, 102, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlFaris, N.A.; Ba-Jaber, A.S. Effects of a Low-energy Diet with and without Oat Bran and Olive Oil Supplements on Body Mass Index, Blood Pressure, and Serum Lipids in Diabetic Women: A Randomized Controlled Trial. Food Sci. Nutr. 2020, 8, 3602–3609. [Google Scholar] [CrossRef]
- Njike, V.Y.; Ayettey, R.; Treu, J.A.; Doughty, K.N.; Katz, D.L. Post-Prandial Effects of High-Polyphenolic Extra Virgin Olive Oil on Endothelial Function in Adults at Risk for Type 2 Diabetes: A Randomized Controlled Crossover Trial. Int. J. Cardiol. 2021, 330, 171–176. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of Hydroxytyrosol on Cardiovascular Biomarkers in Experimental Diabetes Mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Sayec, M.L.; Serreli, G.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Olive Oil Phenols and Their Metabolites Modulate Nitric Oxide Balance in Human Aortic Endothelial Cells. Proc. Nutr. Soc. 2021, 80, E34. [Google Scholar] [CrossRef]
- Olive World Olive Oil & Health. Available online: https://www.internationaloliveoil.org/olive-world/olive-oil-health/ (accessed on 11 February 2021).
- Casadei, E.; Valli, E.; Panni, F.; Donarski, J.; Farrús Gubern, J.; Lucci, P.; Conte, L.; Lacoste, F.; Maquet, A.; Brereton, P.; et al. Emerging Trends in Olive Oil Fraud and Possible Countermeasures. Food Control 2021, 124, 107902. [Google Scholar] [CrossRef]
- van Ruth, S.M.; Huisman, W.; Luning, P.A. Food Fraud Vulnerability and Its Key Factors. Trends Food Sci. Technol. 2017, 67, 70–75. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive Oil Quality and Authenticity: A Review of Current EU Legislation, Standards, Relevant Methods of Analyses, Their Drawbacks and Recommendations for the Future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive Oil Authentication: A Comparative Analysis of Regulatory Frameworks with Especial Emphasis on Quality and Authenticity Indices, and Recent Analytical Techniques Developed for Their Assessment. A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857. [Google Scholar] [CrossRef]
- Tena, N.; Wang, S.C.; Aparicio-Ruiz, R.; García-González, D.L.; Aparicio, R. In-Depth Assessment of Analytical Methods for Olive Oil Purity, Safety, and Quality Characterization. J. Agric. Food Chem. 2015, 63, 4509–4526. [Google Scholar] [CrossRef]
- Kakouri, E.; Revelou, P.-K.; Kanakis, C.; Daferera, D.; Pappas, C.S.; Tarantilis, P.A. Authentication of the Botanical and Geographical Origin and Detection of Adulteration of Olive Oil Using Gas Chromatography, Infrared and Raman Spectroscopy Techniques: A Review. Foods 2021, 10, 1565. [Google Scholar] [CrossRef]
- Barros, I.H.A.S.; Paixão, L.S.; Nascimento, M.H.C.; Lacerda, V.; Filgueiras, P.R.; Romão, W. Use of Portable Raman Spectroscopy in the Quality Control of Extra Virgin Olive Oil and Adulterated Compound Oils. Vib. Spectrosc. 2021, 116, 103299. [Google Scholar] [CrossRef]
- de Lima, T.K.; Musso, M.; Bertoldo Menezes, D. Using Raman Spectroscopy and an Exponential Equation Approach to Detect Adulteration of Olive Oil with Rapeseed and Corn Oil. Food Chem. 2020, 333, 127454. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.-Y.; Yang, I.-S.; Chen, X.-B. Raman Spectroscopy Analysis of Free Fatty Acid in Olive Oil. Appl. Sci. 2019, 9, 4510. [Google Scholar] [CrossRef] [Green Version]
- Giovenzana, V.; Beghi, R.; Romaniello, R.; Tamborrino, A.; Guidetti, R.; Leone, A. Use of Visible and near Infrared Spectroscopy with a View to On-Line Evaluation of Oil Content during Olive Processing. Biosyst. Eng. 2018, 172, 102–109. [Google Scholar] [CrossRef]
- Yousef, I.; Ribó, L.; Crisol, A.; Šics, I.; Ellis, G.; Ducic, T.; Kreuzer, M.; Benseny-Cases, N.; Quispe, M.; Dumas, P.; et al. MIRAS: The Infrared Synchrotron Radiation Beamline at ALBA. Synchrotron Radiat. News 2017, 30, 4–6. [Google Scholar] [CrossRef]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Oh, D.W.; Jain, A.; Eaton, J.K.; Goodson, K.E.; Lee, J.S. Thermal Conductivity Measurement and Sedimentation Detection of Aluminum Oxide Nanofluids by Using the 3ω Method. Int. J. Heat Fluid Flow 2008, 29, 1456–1461. [Google Scholar] [CrossRef]
- Lubner, S.D.; Choi, J.; Wehmeyer, G.; Waag, B.; Mishra, V.; Natesan, H.; Bischof, J.C.; Dames, C. Reusable Bi-Directional 3ω Sensor to Measure Thermal Conductivity of 100-Μm Thick Biological Tissues. Rev. Sci. Instrum. 2015, 86, 014905. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, J.L.; Cruz-Orea, A.; Lomelí Mejia, P.; Gutierrez-Fuentes, R. Monitoring the Thermal Parameters of Different Edible Oils by Using Thermal Lens Spectrometry. Int. J. Thermophys. 2009, 30, 1396–1399. [Google Scholar] [CrossRef]
- Lara-Hernández, G.; Suaste-Gómez, E.; Cruz-Orea, A.; Mendoza-Alvarez, J.G.; Sánchez-Sinéncio, F.; Valcárcel, J.P.; García-Quiroz, A. Thermal Characterization of Edible Oils by Using Photopyroelectric Technique. Int. J. Thermophys. 2013, 34, 962–971. [Google Scholar] [CrossRef]
- Gustafsson, S.E.; Karawacki, E.; Khan, M.N. Transient Hot-Strip Method for Simultaneously Measuring Thermal Conductivity and Thermal Diffusivity of Solids and Fluids. J. Phys. D Appl. Phys. 1979, 12, 1411–1421. [Google Scholar] [CrossRef]
- Sergeev, O.A.; Shashkov, A.G.; Umanskii, A.S. Thermophysical Properties of Quartz Glass. J. Eng. Phys. 1982, 43, 1375–1383. [Google Scholar] [CrossRef]
- Turgut, A.; Tavman, I.; Tavman, S. Measurement of Thermal Conductivity of Edible Oils Using Transient Hot Wire Method. Int. J. Food Prop. 2009, 12, 741–747. [Google Scholar] [CrossRef]
- Rojas, E.E.G.; Coimbra, J.S.R.; Telis-Romero, J. Thermophysical Properties of Cotton, Canola, Sunflower and Soybean Oils as a Function of Temperature. Int. J. Food Prop. 2013, 16, 1620–1629. [Google Scholar] [CrossRef] [Green Version]
- Cahill, D.G. Thermal Conductivity Measurement from 30 to 750 K: The 3ω Method. Rev. Sci. Instrum. 1990, 61, 802. [Google Scholar] [CrossRef]
- Cahill, D.G. Erratum: “Thermal Conductivity Measurement from 30 to 750 K: The 3ω Method” [Rev. Sci. Instrum. 61, 802 (1990)]. Rev. Sci. Instrum. 2002, 73, 3701. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Laguna, M.R.; Castro-Alvarez, A.; Sledzinska, M.; Maire, J.; Costanzo, F.; Ensing, B.; Pruneda, M.; Ordejón, P.; Sotomayor Torres, C.M.; Gómez-Romero, P.; et al. Mechanisms behind the Enhancement of Thermal Properties of Graphene Nanofluids. Nanoscale 2018, 10, 15402–15409. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Angel, E.; Reuter, N.; Komar, P.; Heinz, S.; Kolb, U.; Kleebe, H.-J.; Jakob, G. Subamorphous Thermal Conductivity of Crystalline Half-Heusler Superlattices. Nanoscale Microscale Thermophys. Eng. 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in Extra-Virgin Olive Oil: Authenticity and Quality. In Products from Olive Tree; InTech: London, UK, 2016. [Google Scholar]
- Peterson, R.B.; Oja, V.; Laisk, A. Chlorophyll Fluorescence at 680 and 730 Nm and Leaf Photosynthesis. Photosynth. Res. 2001, 70, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.Y.; Zhang, X.; Ren, H.R.; Liu, L.; Zhao, Y.M.; Wang, Z.; Wu, Z.L.; Liu, L.M.; Xu, H.J. A Rapid Method to Authenticate Vegetable Oils through Surface-Enhanced Raman Scattering. Sci. Rep. 2016, 6, 23405. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Visible Raman Spectroscopy for the Discrimination of Olive Oils from Different Vegetable Oils and the Detection of Adulteration. J. Raman Spectrosc. 2009, 40, 1284–1289. [Google Scholar] [CrossRef]
- Bernstein, P.S. New Insights into the Role of the Macular Carotenoids in Age-Related Macular Degeneration. Resonance Raman Studies. Pure Appl. Chem. 2002, 74, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman Spectra of Carotenoids in Natural Products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Carotenoids and Triacylglycerols Interactions during Thermal Oxidation of Refined Olive Oil. Food Chem. 2011, 127, 1584–1593. [Google Scholar] [CrossRef]
- Concha-Herrera, V.; Lerma-GarcÍa, M.J.; Herrero-MartiÍnez, J.M.; SimoÓ-Alfonso, E.F. Prediction of the Genetic Variety of Extra Virgin Olive Oils Produced at La Comunitat Valenciana, Spain, by Fourier Transform Infrared Spectroscopy. J. Agric. Food Chem. 2009, 57, 9985–9989. [Google Scholar] [CrossRef] [PubMed]
- Vanstone, N.; Moore, A.; Martos, P.; Neethirajan, S. Detection of the Adulteration of Extra Virgin Olive Oil by Near-Infrared Spectroscopy and Chemometric Techniques. Food Qual. Saf. 2018, 2, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Hou, H.-Y.; Huyen, N.T.; Yang, I.-S.; Chen, X.-B. Raman Spectroscopy and 2DCOS Analysis of Unsaturated Fatty Acid in Edible Vegetable Oils. Appl. Sci. 2019, 9, 2807. [Google Scholar] [CrossRef] [Green Version]
- Lasch, P.; Noda, I. Two-Dimensional Correlation Spectroscopy (2D-COS) for Analysis of Spatially Resolved Vibrational Spectra. Appl. Spectrosc. 2019, 73, 359–379. [Google Scholar] [CrossRef] [PubMed]
- FAO. Codex Standard for Fats and Oils from Vegetable Sources; FAO: Rome, Italy, 1999. [Google Scholar]

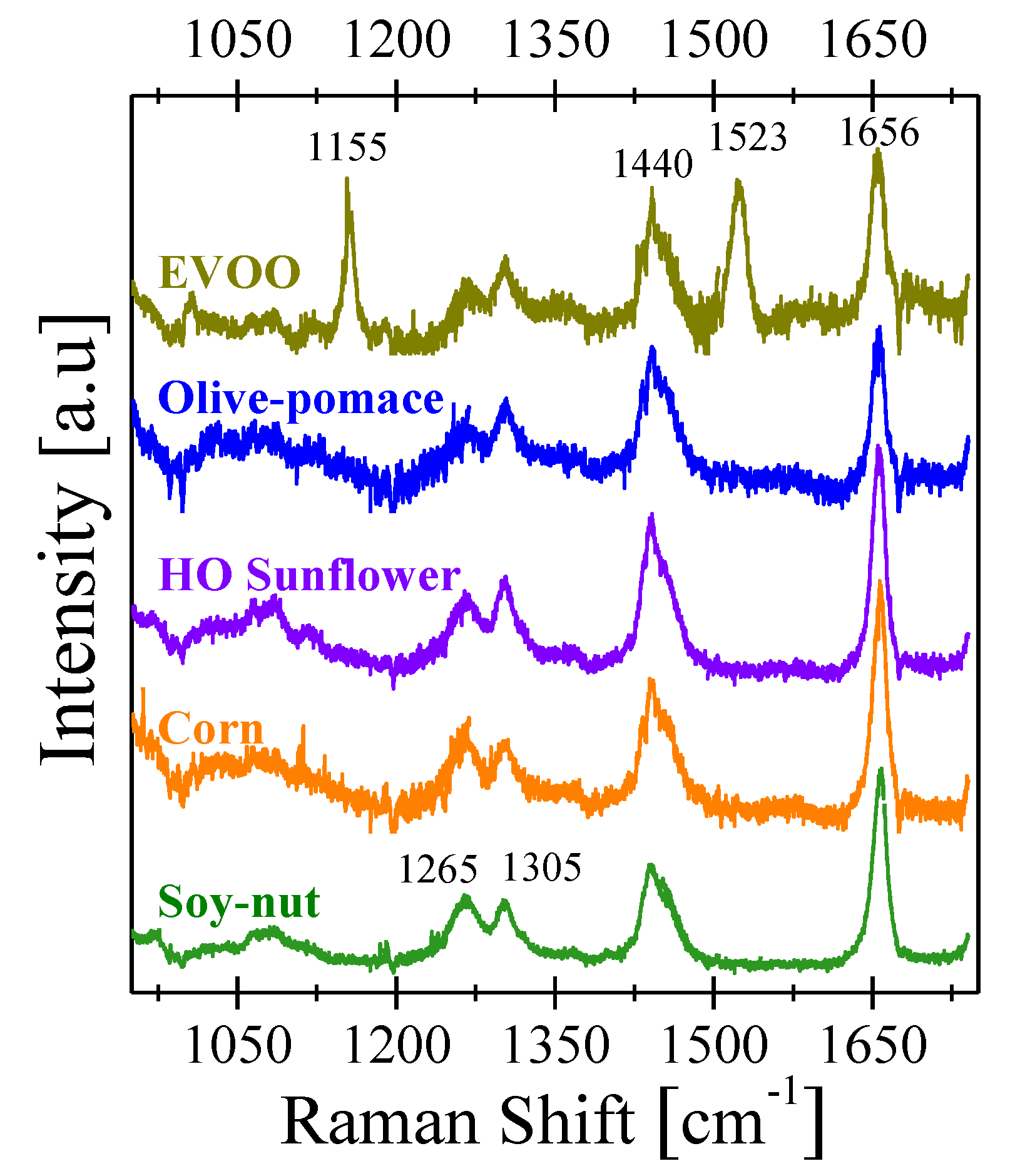

| Frequency [cm−1] | Vibrational Mode |
|---|---|
| 1155 | C–C stretching (carotenoid) |
| 1265 | =C–H bending scissoring |
| 1305 | C–H bending twisting |
| 1440 | C–H bending scissoring |
| 1523 | C=C stretching (carotenoid) |
| 1656 | C=C stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez-Angel, E.; Puertas, B.; Kreuzer, M.; Soliva Fortuny, R.; Ng, R.C.; Castro-Alvarez, A.; Sotomayor Torres, C.M. Spectroscopic and Thermal Characterization of Extra Virgin Olive Oil Adulterated with Edible Oils. Foods 2022, 11, 1304. https://doi.org/10.3390/foods11091304
Chavez-Angel E, Puertas B, Kreuzer M, Soliva Fortuny R, Ng RC, Castro-Alvarez A, Sotomayor Torres CM. Spectroscopic and Thermal Characterization of Extra Virgin Olive Oil Adulterated with Edible Oils. Foods. 2022; 11(9):1304. https://doi.org/10.3390/foods11091304
Chicago/Turabian StyleChavez-Angel, Emigdio, Blanca Puertas, Martin Kreuzer, Robert Soliva Fortuny, Ryan C. Ng, Alejandro Castro-Alvarez, and Clivia M. Sotomayor Torres. 2022. "Spectroscopic and Thermal Characterization of Extra Virgin Olive Oil Adulterated with Edible Oils" Foods 11, no. 9: 1304. https://doi.org/10.3390/foods11091304
APA StyleChavez-Angel, E., Puertas, B., Kreuzer, M., Soliva Fortuny, R., Ng, R. C., Castro-Alvarez, A., & Sotomayor Torres, C. M. (2022). Spectroscopic and Thermal Characterization of Extra Virgin Olive Oil Adulterated with Edible Oils. Foods, 11(9), 1304. https://doi.org/10.3390/foods11091304








