Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
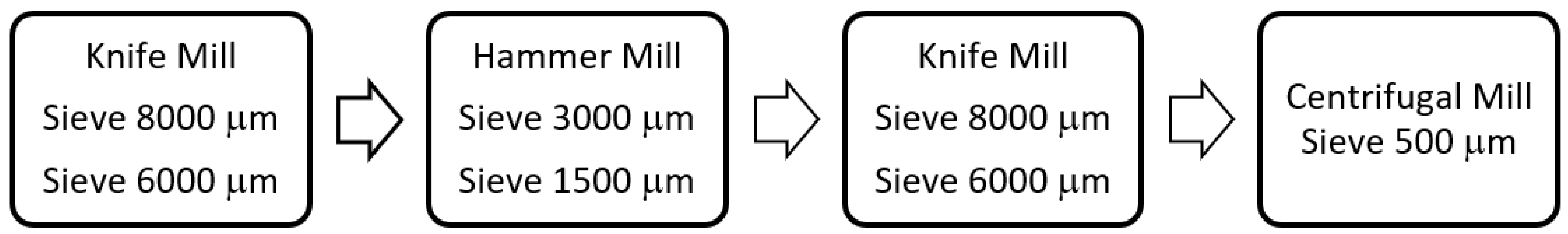
2.2. Carob Flour Preparation
2.3. Nutritional and Physicochemical Analysis
2.4. Granulometry Analysis
2.5. Simulated Gastrointestinal Tract Digestion
2.5.1. Sugar Content
2.5.2. Total Phenolic Content
2.5.3. Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nutritional, Physicochemical and Granulometry Analyses
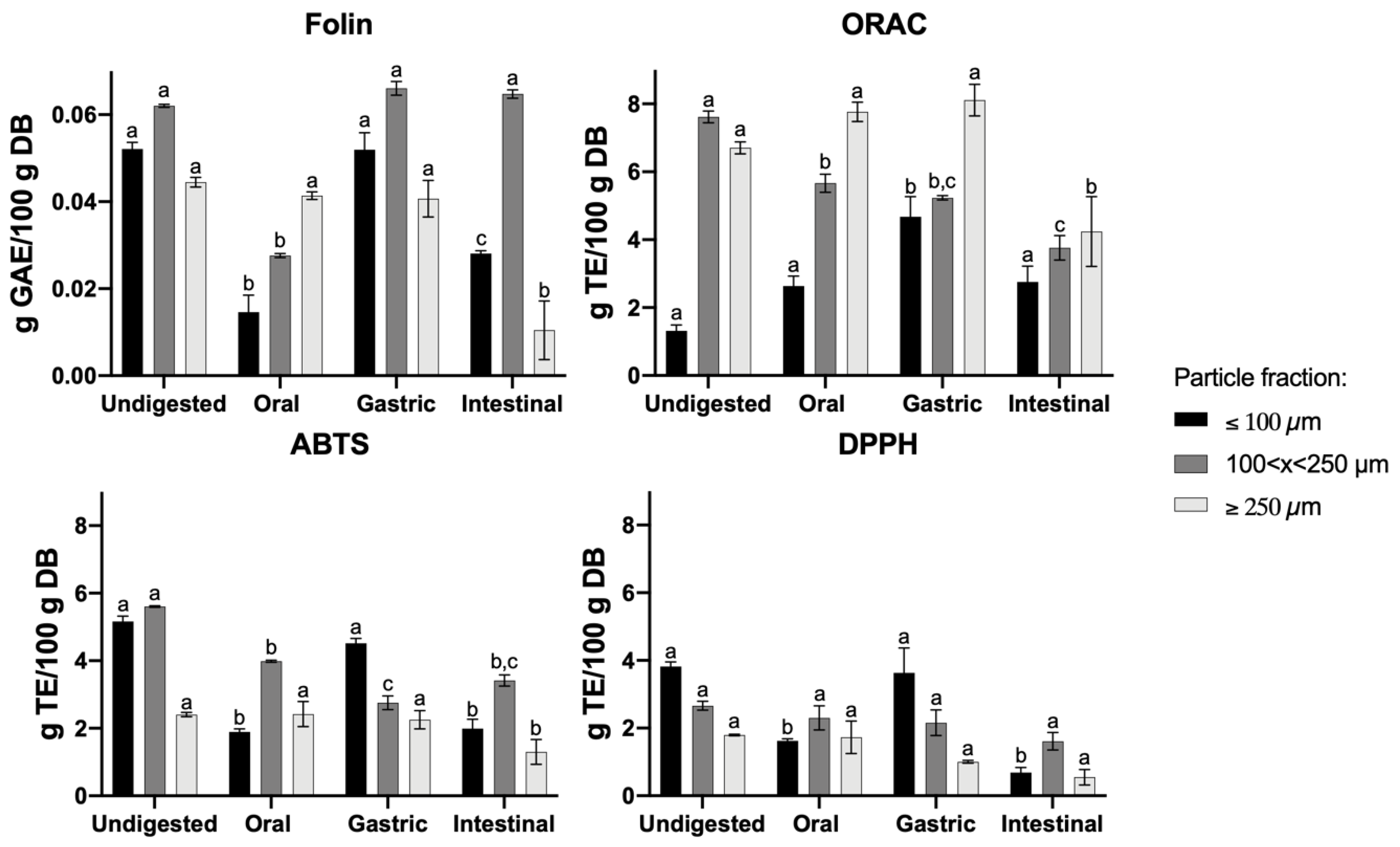
3.2. Effect of GIT Simulation on Different Particle Fractions of CF—Sugar Content
3.3. Effect of GIT Simulation on Different Particle Fractions of CF—Total Phenolic Content (TPC) and Antioxidant Activity (AA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eshghi, S.; Rostami, A.A.; Jamali, B. Carob tree: A suitable species for the future. Acta Hortic. 2018, 1190, 67–70. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Krokou, A.; Stylianou, M.; Agapiou, A. Assessing the volatile profile of carob tree (Ceratonia siliqua L.). Environ. Sci. Pollut. Res. 2019, 26, 35365–35374. [Google Scholar] [CrossRef] [PubMed]
- Puppo, M.C.; Ribotta, D.P. Functional Properties of Traditional Foods; Springer: Boston, MA, USA, 2016; pp. 107–114. [Google Scholar] [CrossRef]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef]
- Carbas, B.; Salinas, M.V.; Serrano, C.; Passarinho, J.A.; Puppo, M.C.; Ricardo, C.P.; Brites, C. Chemical composition and antioxidant activity of commercial flours from Ceratonia siliqua and Prosopis spp. J. Food Meas. Charact. 2019, 13, 305–311. [Google Scholar] [CrossRef]
- Benchikh, Y.; Louaileche, H.; George, B.; Merlin, A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crops Prod. 2014, 60, 298–303. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Fidan, H.; Petkova, N.; Sapoundzhieva, T.; Abanoz, E.I. Carbohydrate Content in Bulgarian and Turkish Carob Pods and Their Products. CBU Int. Conf. Proc. 2016, 4, 796–802. [Google Scholar] [CrossRef][Green Version]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar] [CrossRef]
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Ceratonia siliqua: Characterization, pharmaceutical products and analysis of bioactive compounds: A review. Res. J. Pharm. Technol. 2017, 10, 3585–3589. [Google Scholar] [CrossRef]
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of chemical composition of carob pod (Ceratonia siliqua L) and its morphological study. J. Mater. Environ. Sci. 2013, 4, 348–353. [Google Scholar]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob (Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silván, J.M.; Dueñas, M.; de Luis, D.A.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef]
- Petkova, N.; Petrova, I.; Ivanov, I.; Mihov, R.; Hadjikinova, R. Nutritional and antioxidant potential of carob (Ceratonia siliqua) flour and evaluation of functional properties of its polysaccharide fraction. J. Pharm. Sci. Res. 2017, 9, 2189–2195. [Google Scholar]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of functional flours from pineapple by-products on human intestinal microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Arribas, C.; Cabellos, B.; Sánchez, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. The impact of extrusion on the nutritional composition, dietary fiber and in vitro digestibility of gluten-free snacks based on rice, pea and carob flour blends. Food Funct. 2017, 8, 3654–3663. [Google Scholar] [CrossRef]
- Rico, D.; de Linaje, A.A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; de Luis, D.A.; Martin-Diana, A.B. Carob by-products and seaweeds for the development of functional bread. J. Food Process. Preserv. 2018, 42, e13700. [Google Scholar] [CrossRef]
- Pawłowska, K.; Kuligowski, M.; Jasińska-Kuligowska, I.; Kidoń, M.; Siger, A.; Rudzińska, M.; Nowak, J. Effect of Replacing Cocoa Powder by Carob Powder in the Muffins on Sensory and Physicochemical Properties. Plant Foods Hum. Nutr. 2018, 73, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Červenka, L.; Frühbauerová, M.; Velichová, H. Functional properties of muffin as affected by substituing wheat flour with carob powder. Potravin. Slovak J. Food Sci. 2019, 13, 212–217. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigos, M.C.; Balart, R.; Torres-Giner, S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Methods 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Čepo, D.V.; Mornar, A.; Nigović, B.; Kremer, D.; Radanović, D.; Dragojević, I.V. Optimization of roasting conditions as an useful approach for increasing antioxidant activity of carob powder. LWT-Food Sci. Technol. 2014, 58, 578–586. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The influence of in vitro gastrointestinal digestion on the chemical composition and antioxidant and enzyme inhibitory capacities of carob liqueurs obtained with different elaboration techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- ISO. International Organization for Standardization. 2009. Available online: https://www.iso.org/ (accessed on 3 September 2020).
- ISO. International Organization for Standardization. 2013. Available online: https://www.iso.org/ (accessed on 3 September 2020).
- Norma NP 518; Norma Portuguesa: Monte da Caparica, Portugal, 1986.
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Malpiedi, L.P.; Nerli, B.B. Micellar systems of aliphatic alcohol ethoxylates as a sustainable alternative to extract soybean isoflavones. Food Chem. 2018, 264, 135–141. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Evaluation of antioxidant activity and total phenols index in persimmon vinegars produced by different processes. LWT-Food Sci. Technol. 2011, 44, 1591–1596. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Bengoechea, C.; Romero, A.; Villanueva, A.; Moreno, G.; Alaiz, M.; Millán, F.; Guerrero, A.; Puppo, M.C. Composition and structure of carob (Ceratonia siliqua L.) germ proteins. Food Chem. 2008, 107, 675–683. [Google Scholar] [CrossRef]
- Dakia, P.A.; Wathelet, B.; Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007, 102, 1368–1374. [Google Scholar] [CrossRef]
- Bouzouita, N.; Khaldi, A.; Zgoulli, S.; Chebil, L.; Chekki, R.; Chaabouni, M.M.; Thonart, P. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007, 101, 1508–1515. [Google Scholar] [CrossRef]
- Dakia, P.A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Fidan, H.; Mihaylova, D.; Petkova, N.; Sapoundzhieva, T.; Slavov, A.; Krastev, L. Determination of chemical composition, antibacterial and antioxidant properties of products obtained from carob and honey locust. Turk. J. Biochem. 2019, 44, 316–322. [Google Scholar] [CrossRef]
- Gunel, Z.; Torun, M.; Sahin-Nadeem, H. Sugar, d-pinitol, volatile composition, and antioxidant activity of carob powder roasted by microwave, hot air, and combined microwave/hot air. J. Food Process. Preserv. 2020, 44, e14371. [Google Scholar] [CrossRef]
- Cantalejo, M.J. Effects of Roasting Temperature on the Aroma Components of Carob (Ceratonia siliqua L.). J. Agric. Food Chem. 1997, 45, 1345–1350. [Google Scholar] [CrossRef]
- Biner, B.; Gubbuk, H.; Karhan, M.; Aksu, M.; Pekmezci, M. Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. Food Chem. 2007, 100, 1453–1455. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia Siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Turhan, I. Relationship between sugar profile and d-pinitol content of pods of wild and cultivated types of carob bean (Ceratonia siliqua L.). Int. J. Food Prop. 2014, 17, 363–370. [Google Scholar] [CrossRef]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J.; Ellis, P.R. The role of sugars and sweeteners in food, diet and health: Alternatives for the future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Frühbauerová, M.; Červenka, L.; Hájek, T.; Pouzar, M.; Palarčík, J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem. 2021, 377, 131968. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Chen, F.; Xie, Y.Q.; Han, M.D.; Luo, C.X.; Zhao, Y.Y.; Gao, Y.Q. Nutraceutical potential and antioxidant benefits of selected fruit seeds subjected to an in vitro digestion. J. Funct. Foods 2016, 20, 317–331. [Google Scholar] [CrossRef]
- Zhu, B.J.; Zayed, M.Z.; Zhu, H.X.; Zhao, J.; Li, S.P. Functional polysaccharides of carob fruit: A review. Chin. Med. 2019, 14, 40. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Baker, I.; Chohan, M.; Opara, E.I. Impact of Cooking and Digestion, In Vitro, on the Antioxidant Capacity and Anti-Inflammatory Activity of Cinnamon, Clove and Nutmeg. Plant Foods Hum. Nutr. 2013, 68, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]

| Proximate Composition (g/100 g Dry Basis) | Carob Flour |
|---|---|
| Moisture | 6.35 ± 1.24 |
| Ash | 2.41 ± 0.02 |
| Carbohydrate | 86.70 ± 1.13 |
| Protein | 4.31 ± 0.06 |
| Lipids | 0.23 ± 0.12 |
| Energy (kcal/100 g) | 366.14 ± 5.60 |
| Total Dietary Fiber | 14.64 ± 1.81 |
| Insoluble Dietary Fiber | 14.12 ± 1.48 |
| Soluble Dietary Fiber | 0.52 ± 0.52 |
| Total Starch | 0.96 ± 0.11 |
| Particle Size (µm) | Fraction Weight (%) |
|---|---|
| ≤100 | 70.30 ± 0.31 |
| 100 < x < 250 | 20.18 ± 0.76 |
| ≥250 | 9.52 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, A.M.; Brassesco, M.E.; Quintino, A.C.; Vieira, M.C.; Brandão, T.R.S.; Silva, C.L.M.; Azevedo, M.; Pintado, M. Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion. Foods 2022, 11, 1272. https://doi.org/10.3390/foods11091272
Vilas-Boas AM, Brassesco ME, Quintino AC, Vieira MC, Brandão TRS, Silva CLM, Azevedo M, Pintado M. Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion. Foods. 2022; 11(9):1272. https://doi.org/10.3390/foods11091272
Chicago/Turabian StyleVilas-Boas, Ana M., María E. Brassesco, Andreia C. Quintino, Margarida C. Vieira, Teresa R. S. Brandão, Cristina L. M. Silva, Miguel Azevedo, and Manuela Pintado. 2022. "Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion" Foods 11, no. 9: 1272. https://doi.org/10.3390/foods11091272
APA StyleVilas-Boas, A. M., Brassesco, M. E., Quintino, A. C., Vieira, M. C., Brandão, T. R. S., Silva, C. L. M., Azevedo, M., & Pintado, M. (2022). Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion. Foods, 11(9), 1272. https://doi.org/10.3390/foods11091272











