Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications—A Review
Abstract
:1. Introduction
2. Water-Soluble Vitamins
2.1. B-Complex Group
2.1.1. Vitamin B1
2.1.2. Vitamin B2
2.1.3. Vitamin B3
2.1.4. Vitamin B5
2.1.5. Vitamin B6
2.1.6. Folic Acid (Vitamin B9)
2.1.7. Vitamin B12
2.2. Vitamin C
3. Spray Drying
3.1. Spray Drying Parameters
3.2. Encapsulation of Water-Soluble Vitamins by Spray Drying
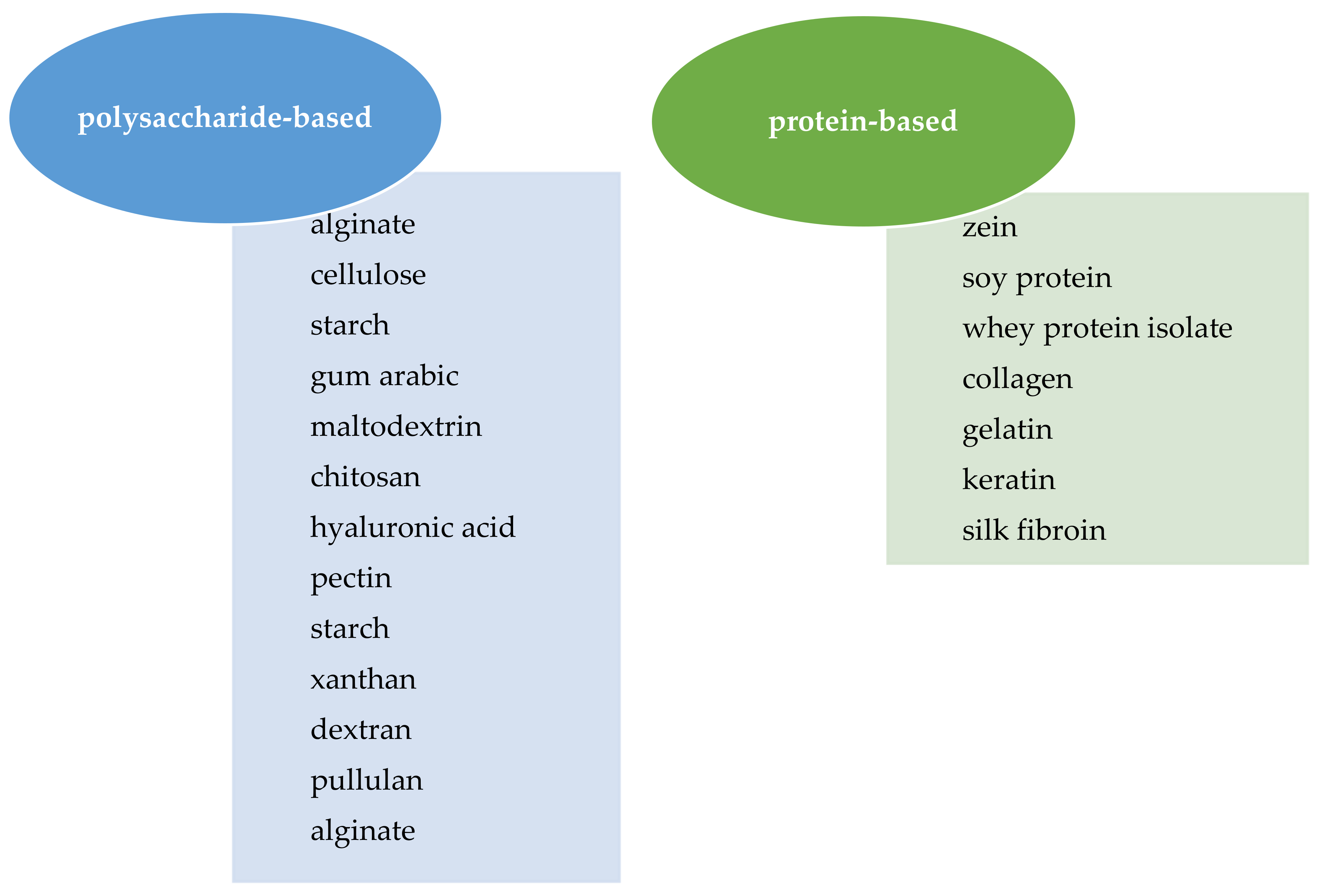
4. Electrohydrodynamic Techniques
4.1. Electrospinning Factors
4.2. Type of Electrospinning
4.3. Encapsulation of Water-Soluble Vitamins by Electrospinning and Electrospraying Techniques
5. Physico-Chemical Characterization Techniques
5.1. Particle Size Distribution
5.2. Scannning Electron Microscopy (SEM)
5.3. Transmission Electron Microscopy (TEM)
5.4. Atomic Force Microscopy (AFM)
5.5. X-ray Diffraction (XRD)
5.6. Fourier Transformed Infrared Spectroscopy-Attenuated Toral Reflectance (FTIR-ATR)
5.7. Differential Scanning Calorimeter (DSC)
5.8. Thermogravimetrical Analysis (TGA)
5.9. Functional Characteristics
6. Conclusions and Future Challenge
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.; Zong, M.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Wilson, N.; Shah, N. Microencapsulation of Vitamins. ASEAN Food J. 2007, 14, 1–14. [Google Scholar]
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-encapsulation of vitamins B12 and D3 using spray drying: Wall material optimization, product characterization, and release kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Achinna, P.; Kuna, A. Microencapsulation technology: A review. J. Res. Angrau. 2010, 38, 86–102. [Google Scholar]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef]
- Wazarkar, K.; Patil, D.; Rane, A.; Balgude, D.; Kathalewar, M.; Sabnis, A. Microencapsulation: An emerging technique in the modern coating industry. RSC Adv. 2016, 6, 106964–106979. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Design of polymer-free Vitamin-A acetate/cyclodextrin nanofibrous webs: Antioxidant and fast-dissolving properties. Food Funct. 2020, 11, 7626–7637. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Liu, Y.; Zhang, D.; Lin, J.; Mu, W.; Liu, F. Easily Tunable Membrane Thickness of Microcapsules by Using a Coordination Assembly on the Liquid-Liquid Interface. Front. Chem. 2018, 6, 387. [Google Scholar] [CrossRef]
- Santos, D.T.; Meireles, M.A.A. Encapsulation Techniques Based on Physical Nature Combination between Coating Material and Core Material. Chemistry 2010. [Google Scholar]
- Noruzi, M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016, 96, 4663–4678. [Google Scholar] [CrossRef]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Branta Lopes, D.; Speranza, P.; Alves Macedo, G. 18—A new approach for flavor and aroma encapsulation. In Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 623–661. ISBN 978-0-12-804308-0. [Google Scholar]
- Alonso, S. Novel Preservation Techniques for Microbial Cultures BT—Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 7–33. ISBN 978-3-319-42457-6. [Google Scholar]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Srinarong, P.; de Waard, H.; Frijlink, H.W.; Hinrichs, W.L.J. Improved dissolution behavior of lipophilic drugs by solid dispersions: The production process as starting point for formulation considerations. Expert Opin. Drug Deliv. 2011, 8, 1121–1140. [Google Scholar] [CrossRef]
- Ren, Y.; Mei, L.; Zhou, L.; Guo, G. Recent Perspectives in Hot Melt Extrusion-Based Polymeric Formulations for Drug Delivery: Applications and Innovations. AAPS PharmSciTech 2019, 20, 92. [Google Scholar] [CrossRef]
- Okuro, P.K.; Junior, F.E.M.; Favaro-Trindade, C.S. Technological Challenges for Spray Chilling Encapsulation of Functional Food Ingredients. Food Technol. Biotechnol. 2013, 51, 171–182. [Google Scholar]
- Jackson, L.; Lee, K. Microencapsulation in the food industry. LWT Food Sci. Technol. 1991, 24, 289–297. [Google Scholar]
- Estevinho, B.N.; Rocha, F. Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 189–220. ISBN 9780128114490. [Google Scholar]
- Abbas, S.; Da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic Acid: Microencapsulation Techniques and Trends—A Review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Bhutto, M.A.; Wu, T.; Sun, B.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Fabrication and characterization of vitamin B5 loaded poly (l-lactide-co-caprolactone)/silk fiber aligned electrospun nanofibers for schwann cell proliferation. Colloids Surf. B Biointerfaces 2016, 144, 108–117. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients—A Review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Ismail, E.S.; Gholamhosseinpour, A.; Loizzo, M.R.; Giardinieri, A.; Pacetti, D.; Pourmohammadi, K.; Ferreira, D.S. 8—Water-soluble vitamins. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2019; pp. 241–266. ISBN 978-0-12-814174-8. [Google Scholar]
- Yaman, M.; Çatak, J.; Uğur, H.; Gürbüz, M.; Belli, İ.; Tanyıldız, S.N.; Yıldırım, H.; Cengiz, S.; Yavuz, B.B.; Kişmiroğlu, C.; et al. The bioaccessibility of water-soluble vitamins: A review. Trends Food Sci. Technol. 2021, 109, 552–563. [Google Scholar] [CrossRef]
- Takahashi, M.; Taguchi, Y.; Tanaka, M. Microencapsulation of hydrophilic solid powder as a flame retardant with epoxy resin by using interfacial reaction method. Polym. Adv. Technol. 2010, 21, 224–228. [Google Scholar] [CrossRef]
- Ceylan, Z.; Yaman, M.; Sağdıç, O.; Karabulut, E.; Yilmaz, M.T. Effect of electrospun thymol-loaded nanofiber coating on vitamin B profile of gilthead sea bream fillets (Sparus aurata). LWT 2018, 98, 162–169. [Google Scholar] [CrossRef]
- Parin, F.N. Retrospective, Perspective and Prospective of B-Complex Vitamins: Encapsulation of Vitamins and Release from Vitamin-Loaded Polymers. In B-Complex Vitamins; LeBlanc, J.G., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Medicine, I. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06554-2.
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Balakrishnan, S.B.; Thambusamy, S. Preparation of silver nanoparticles and riboflavin embedded electrospun polymer nanofibrous scaffolds for in vivo wound dressing application. Process Biochem. 2020, 88, 148–158. [Google Scholar] [CrossRef]
- Qi, Y.; Lohman, J.; Bratlie, K.M.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Yoon, K.-J.; Barrett, T.A.; Wang, Q. Vitamin C and B(3) as new biomaterials to alter intestinal stem cells. J. Biomed. Mater. Res. A 2019, 107, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. PELLAGRA. In Encyclopedia of Food Sciences and Nutrition Caballero, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4456–4460. ISBN 978-0-12-227055-0. [Google Scholar]
- Rivera-Calimlim, L.; Hartley, D.; Osterhout, D. Effects of ethanol and pantothenic acid on brain acetylcholine synthesis. Br. J. Pharmacol. 1988, 95, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.H.J.M.; Hageman, G.J.; Bast, A.; Haenen, G.R.M.M. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol. In Vitr. 2017, 44, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.S.; Anandan, R.; Navitha, M.; Asha, K.K.; Kumar, K.A.; Mathew, S.; Ravishankar, C.N. Development of thiamine and pyridoxine loaded ferulic acid-grafted chitosan microspheres for dietary supplementation. J. Food Sci. Technol. 2016, 53, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koseoglu, S.Z.A. Determination and evaluation of the pyridoxal, pyridoxine, and pyridoxamine forms of vitamin B6 in plant-based foods in terms of healthy vegetarian nutrition: Vitamin B6 profile in plant baded foods. Prog. Nutr. 2020, 22, e2020015. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Crizel, R.L.; da Silva, F.T.; Fontes, M.R.V.; da Rosa Zavareze, E.; Dias, A.R.G. Starch nanofibers as vehicles for folic acid supplementation: Thermal treatment, UVA irradiation and in vitro simulation of digestion. J. Sci. Food Agric. 2020, 101, 1935–1943. [Google Scholar] [CrossRef]
- Do Evangelho, J.A.; Crizel, R.L.; Chaves, F.C.; Prietto, L.; Pinto, V.Z.; de Miranda, M.Z.; Dias, A.R.G.; Zavareze, E.R. Thermal and irradiation resistance of folic acid encapsulated in zein ultrafine fibers or nanocapsules produced by electrospinning and electrospraying. Food Res. Int. 2019, 124, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Qureshi, S.; Maqsood, S.; Gani, A.; Masoodi, F.A. Micro-encapsulation of folic acid using horse chestnut starch and β-cyclodextrin: Microcapsule characterization, release behavior & antioxidant potential during GI tract conditions. Food Hydrocoll. 2017, 66, 154–160. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Kinetic models applied to soluble vitamins delivery systems prepared by spray drying. Dry. Technol. 2017, 35, 1249–1257. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.; Blaga, A.; Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. 2016, 289, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S.M. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Tan, E.; Loh, X.J. Encapsulation of Vitamin C with its Protection from Oxidation by Poly(Vinyl Alcohol). J. Mol. Eng. Mater. 2017, 05, 1750013. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Encapsulation of bioactive ingredients by spray drying. In Spray Drying Techniques for Food Ingredient Encapsulation; Wiley Online Books; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 156–179. ISBN 9781118863985. [Google Scholar]
- Cortés, R.M.; Hernández, G.; Estrada, M.E.M. Optimization of the spray drying process for obtaining cape gooseberry powder: An innovative and promising functional food. Vitae 2017, 24, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Hawlader MN, A.; Zhu, H.J. Microencapsulation of ascorbic acid: Effect of process variables on product characteristics. J. Microencapsul. 2001, 18, 199–209. [Google Scholar] [CrossRef]
- Trindade, M.A.; Grosso, C.R.F. The stability of ascorbic acid microencapsulated in granules of rice starch and in gum arabic. J. Microencapsul. 2000, 17, 169–176. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Both, E.M.; Boom, R.M.; Schutyser, M.A.I. Particle morphology and powder properties during spray drying of maltodextrin and whey protein mixtures. Powder Technol. 2020, 363, 519–524. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Pineapple Peel Extract by Spray Drying Using Maltodextrin, Inulin, and Arabic Gum as Wall Matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Nogueiro Estevinho, B.; Lazar, R.; Blaga, A.; Rocha, F. Preliminary evaluation and studies on the preparation, characterization and in vitro release studies of different biopolymer microparticles for controlled release of folic acid. Powder Technol. 2020, 369, 279–288. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.C.; Rocha, F. Study of different encapsulating agents for the Microencapsulation of Vitamin B12. Environ. Eng. Manag. J. 2018, 17, 855–864. [Google Scholar] [CrossRef]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Study of microencapsulation and controlled release of modified chitosan microparticles containing vitamin B12. Powder Technol. 2017, 318, 162–169. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Van Pho, T.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-Ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [Green Version]
- Marcela, F.; Lucía, C.; Esther, F.; Elena, M. Microencapsulation of L-Ascorbic Acid by Spray Drying Using Sodium Alginate as Wall Material. J. Encapsulation Adsorpt. Sci. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.G.; Liu, C.; Park, H.J. Characteristics of vitamin C encapsulated tripolyphosphate-chitosan microspheres as affected by chitosan molecular weight. J. Microencapsul. 2006, 23, 79–90. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Ramos, P.E.; Cerqueira, M.F.; Teixeira, J.A.; Vicente, A.A.; Pastrana, L.M.; Pereira, R.N.; Cerqueira, M.A. Electrosprayed whey protein-based nanocapsules for β-carotene encapsulation. Food Chem. 2020, 314, 126157. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.S.; Moghe, A.K. 2—Nanofiber structures for medical biotextiles. In Biotextiles as Medical Implants; King, M.W., Gupta, B.S., Guidoin, R., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2013; pp. 48–90. ISBN 978-1-84569-439-5. [Google Scholar]
- Lim, L.T. Encapsulation of bioactive compounds using electrospinning and electrospraying technologies. In Nanotechnology and Functional Foods: Effective Delivery of Bioactive Ingredients; Sabliov, C.M., Chen, H., Yada, R.Y., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 297–317. ISBN 9781118462201. [Google Scholar]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Photoprotection of folic acid upon encapsulation in food-grade amaranth (Amaranthus hypochondriacus L.) protein isolate—Pullulan electrospun fibers. LWT Food Sci. Technol. 2015, 62, 970–975. [Google Scholar] [CrossRef] [Green Version]




| Chemical Processes | Physical Processes |
|---|---|
| Coacervation | Spray drying |
| Interfacial polymerization Molecular Inclusion | Spray chilling Freeze drying |
| Co-crystallization | Melt extrusion Electrospinning Fluidized bed coating Solvent evaporation |
| Encapsulation Technique | Advantages | Disadvantages |
|---|---|---|
| Complex coacervation [13,14] | Encapsulation of thermo-sensitive compounds Process at room temperature low cost | Use of toxic chemical solvents Residual solvent in the produced structures Medium/high cost-in-use |
| Electrohydrodynamic techniques [15,16] | Simple and versatile techniques efficient encapsulation Enhancement of biocompounds stability encapsulation of thermo-sensitive compounds Room temperature process | Difficult to scale up |
| Fluidized bed coating [17] | Low cost-in-use Homogeneity of a sample based on size. | Slow process High process cost Thermo-sensitive compounds degradation |
| Freeze-drying [18] | Long-term storage Large-scale production | Complex process Residual solvents in products High cost |
| Ionic gelation [19] | Mild conditions during the process Low cost | Low hydrophilic compounds encapsulation efficiency |
| Liposomes [14,20] | Encapsulation of aqueous/lipid soluble compounds Efficient controlled delivery | Laboratory scale Short half-life High cost |
| Melt extrusion [21,22] | Solvent free Continuous process Easy to scale up | Not recommended for thermolabile compounds High temperature and energy |
| Solvent evaporation [13] | Simple procedure Low cost-in-use | Low encapsulation efficiency |
| Spray chilling [23] | Low temperature in the process Low cost Easy to scale up | Changes in compounds activity due to fast cooling rates Low encapsulation efficiency low shelf life |
| Spray drying [13] | Simple procedure Short time of production Good encapsulation efficiency Good product stability Scale-up to commercial manufacture Continuous production Low cost-in-use Uniform spherical structures | High energy consumption Process at high temperature |
| Vitamin | Encapsulation Agent | Processing Parameters | Structures Average Size (µm) | Product Yield (%) | Encapsulation Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Vitamin B1 | Gum arabic Carrageenan Chitosan Maltodextrin Modified chitosan Modified starch Pectin Sodium alginate Xanthan | 4 mL/min Tinlet = 120 °C Toutlet = 50–67 °C | 0.11–1.32 | 17–52 | 66–100 | [35] |
| Chitosan and ferulic acid | 10 mL/min Tinlet = 140 °C Toutlet = 77 °C | 4.5–4.8 | 63.58–65.12 | 91 ± 2.31 | [41] | |
| Vitamin B6 | Chitosan and ferulic acid | 10 mL/min Tinlet = 140 °C Toutlet = 77 °C | 4.5–4.8 | 63.58–65.12 | 83 ± 3.17 | [41] |
| Folic acid | Gum arabic Modified chitosan Modified starch Pectin Sodium alginate | 4 mL/min, Tinlet = 120 °C Toutlet = 58 °C | 0.1–3.0 | 13.1–49.8 | 100% (except for modified starch) | [60] |
| Cape gooseberry and maltodextrin | 1.5 L/h Tinlet = 194.2 °C Toutlet = 87.7 °C | - | - | 90.9 ± 1.8 | [53] | |
| Starch β-cyclodextrin | ~140 L/h Tinlet = 130 °C Toutlet = 80 °C | 28.26–227.34 30.09–145.93 | 50.29 53.15 | 57.29 76.10 | [45] | |
| Whey protein concentrate Starch | 140 L/h Tinlet = 90 °C Toutlet = 45 °C | 0.2–4.5 | - | 83.9 ± 7.8 52.5 ± 7.6 | [57] | |
| Vitamin B12 | Gum acacia Modified starch Maltodextrin | Tinlet = 140 °C Toutlet = 60 °C | 0.279–1.277 | - | 57.64–72.03 | [4] |
| Cyanobacterial extracellular polysaccharide, gum arabic | 4 ml/min, Tinlet = 120 °C Toutlet = 65 °C | 6–9 | 18.8 | - | [61] | |
| Sodium alginate Carrageenam Maltodextrin Pectin Gum arabic Modified starch Xanthan | 4 ml/min Tinlet = 120 °C Toutlet = 56–67 °C | 0.93–2.74 | 27–50 | - | [62] | |
| Zein | 4 mL/min; Tin = 90 °C; Tout = 50 °C | 2.23 | 83.1 | 82.3 | [63] | |
| Modified chitosan | 4 ml/min Tinlet = 120 °C Toutlet = 53–58 °C | 3–8 | 56.0–58.0 | - | [64] | |
| Vitamin B12 Vitamin C | Chitosan, modified chitosan Sodium alginate | 4 L/h Tinlet = 120 °C Toutlet ~ 65 °C | 3 | 41.8–55.6 43.6–45.4 | - | [48] |
| Vitamin C | Casein gel | 0.54 L/h Tinlet = 180 °C Toutlet ~ 80 °C | 5.8 ± 3.1 | - | 44.5 ± 1.2 | [65] |
| Sodium alginate Gum arabic | 2–7 mL/min Tinlet = 140 °C Toutlet = 86 °C | 9.1 6.0 | 74 51 | 90 | [66] | |
| Cape gooseberry and maltodextrin | 1.5 L/h Tinlet = 194.2 °C Toutlet = 87.7 °C | - | - | 69.7 ± 0.7 | [53] | |
| Sodium alginate | Tinlet = 110 °C Toutlet = 65 °C | 30 | 93.48 | [67] | ||
| TPP-chitosan | 3 L/h Tinlet = 175 °C Toutlet = 87.7 °C | 8.0–9.0 | 61.1–62.8 | 45.05–58.30 | [68] |
| Vitamin | Encapsulation Agent | Processing Parameters | Encapsulation Efficiency (%) | Structures Average Size (µm) | Reference |
|---|---|---|---|---|---|
| Folic acid | Zein | 1 mL/h, 16 cm,16 kV 0.6mL/h, 10 cm, 16 kV | 92.9 98.6 | 0.70 (fibres) 0.27 (capsules) | [44] |
| Starch | 0.6 mL/h, 20 cm, 25 kV | 73–95 | [43] | ||
| Whey protein concentrate Starch | 0.15 mL/h, 9–11 cm, 10 kV | 80.8 ± 12.9 44.0 ± 5.5 | 0.2–4.5 | [57] | |
| Amaranth:pullulan | 0.4 mL/h, 10 cm, 22 kV | 95.6 ± 0.2 | 0.31–0.59 | [73] | |
| Vitamin B12 | Zein | 0.2 mL/h, 7 cm, 20 kV | 100 91 | 0.31–0.5 (fibres) 1.25 to 4.38 (microspheres) | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, S.C.; Estevinho, B.N.; Rocha, F. Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications—A Review. Foods 2022, 11, 1271. https://doi.org/10.3390/foods11091271
Coelho SC, Estevinho BN, Rocha F. Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications—A Review. Foods. 2022; 11(9):1271. https://doi.org/10.3390/foods11091271
Chicago/Turabian StyleCoelho, Sílvia Castro, Berta Nogueiro Estevinho, and Fernando Rocha. 2022. "Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications—A Review" Foods 11, no. 9: 1271. https://doi.org/10.3390/foods11091271
APA StyleCoelho, S. C., Estevinho, B. N., & Rocha, F. (2022). Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications—A Review. Foods, 11(9), 1271. https://doi.org/10.3390/foods11091271








