Functionalities of Gelatin Modified with 2-Octenyl Succinic Anhydride and Gallic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microbial Strains
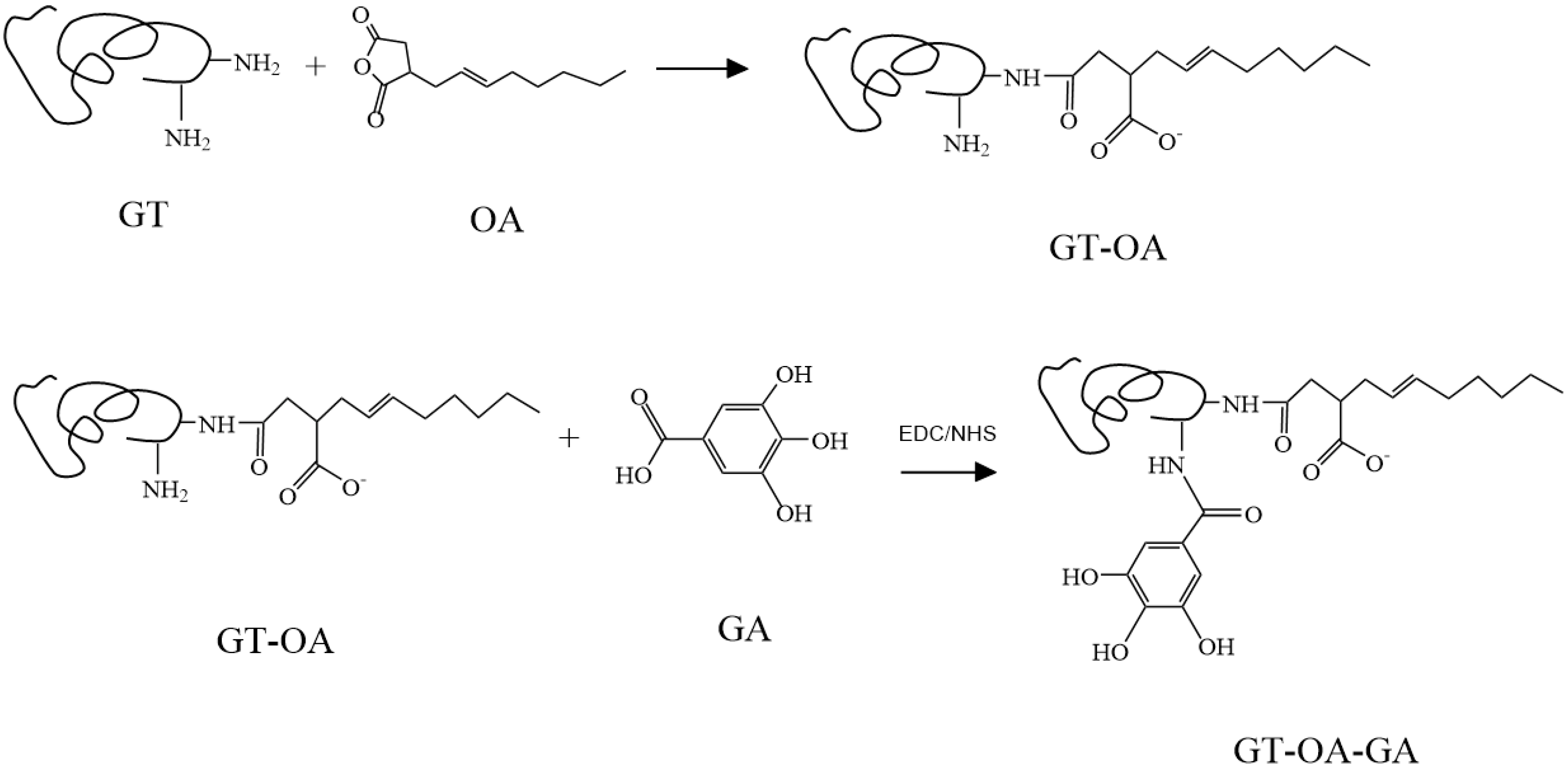
2.3. Synthesis of GT and OA Conjugate (GT-OA)
2.4. Synthesis of GT-OA-GA
2.5. NMR Analysis
2.6. Degree of Amino Group Substitution of GT
2.7. Surface Tension Measurement
2.8. Foaming Properties
2.9. pH Buffering Capacity
2.10. Antioxidant Activity
2.10.1. DPPH Radical Scavenging Activity
2.10.2. Chelating Activity of Ferrous Ions
2.10.3. Ferric Ion Reducing Power
2.11. Complexation of GT Conjugates with the Zinc Ion
2.12. Antimicrobial Activity Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of GT-OA and GT-OA-GA
3.2. NMR Spectra of GT-OA and GT-OA-GA
3.3. Characterization of GT-OA
3.3.1. Surface Activity, Zeta Potential, and Viscosity
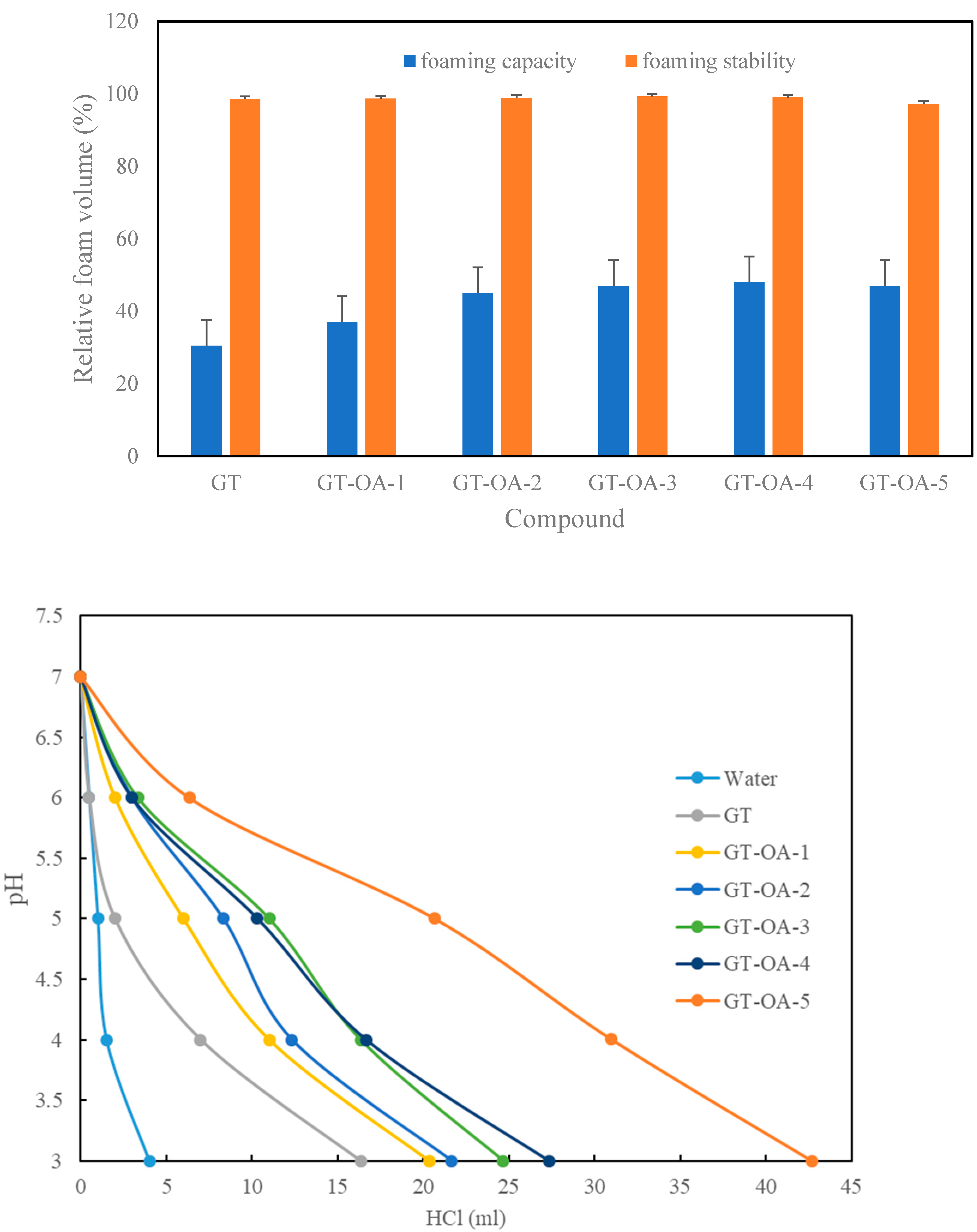
3.3.2. Foaming Properties
3.3.3. pH Buffering Capacity
3.4. Antioxidant Activity of GT Conjugates
3.4.1. DPPH Radical Scavenging Activity
3.4.2. Ferrous Ion Chelating Activity
3.4.3. Ferric Ion Reducing Antioxidant Power Assay
3.5. Complexation of GT Conjugates with the Zinc Ion and Antimicrobial Activity of the Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haug, I.J.; DragetPhillips, K.I. Gelatin. In Handbooks of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 92–115. [Google Scholar]
- Toledano, O.; Magdassi, S. Formation of surface active gelatin by covalent attachment of hydrophobic chains. J. Colloid Interface Sci. 1997, 193, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, B.; Emadzadeh, B.; Rezaeinia, H.; Russell, S.J. Improvements in gelatin cold water solubility after electrospinning and associated physicochemical, functional and rheological properties. Food Hydrocoll. 2020, 104, 105740. [Google Scholar] [CrossRef]
- Shilpi, D.; Kushwah, V.; Agrawal, A.K.; Jain, S. Improved stability and enhanced oral bioavailability of atorvastatin loaded stearic acid modified gelatin nanoparticles. Pharm. Res. 2017, 34, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Lee, B.J. Synthetic optimization of gelatin-oleic conjugate and aqueous-based formation of self-assembled nanoparticles without cross-linkers. Macromol. Res. 2017, 25, 466–473. [Google Scholar] [CrossRef]
- Alias, N.A.B.; Omosebi, B.O.; David, W.; Huda, N. Improving the Physicochemical Properties of Commercial Bovine Gelatin using Succinylation. Asia Pac. J. Sustain. Agric. Food Energy 2017, 5, 2–6. [Google Scholar]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as emulsifiers for oil-in-water emulsions: Extraction, chemical composition, molecular structure, and molecular modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Cirillo, G.; Kraemer, K.; Fuessel, S.; Puoci, F.; Curcio, M.; Spizzirri, U.G.; Altimari, I.; Iemma, F. Biological activity of a gallic acid− gelatin conjugate. Biomacromolecules 2010, 11, 3309–3315. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Eun, J.-B.; Wierenga, P.A.; Gruppen, H. Antioxidative activity and emulsifying properties of cuttlefish skin gelatin modified by oxidised phenolic compounds. Food Chem. 2009, 117, 160–168. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Q.; Li, Z.; Fu, D.; Dong, Z. Effects of amylose content on the paste properties and emulsification of octenyl succinic starch esters. Starch-Stärke 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Shilpashree, B.; Arora, S.; Chawla, P.; Tomar, S. Effect of succinylation on physicochemical and functional properties of milk protein concentrate. Food Res. Int. 2015, 72, 223–230. [Google Scholar] [CrossRef]
- Liu, T.T.; Su, G.Z.; Yang, T.S. Functionalities of chitosan conjugated with lauric acid and l-carnitine and application of the modified chitosan in an oil-in-water emulsion. Food Chem. 2021, 359, 129851. [Google Scholar] [CrossRef]
- Yang, T.S.; Liu, T.T.; Lin, I.H. Functionalities of chitosan conjugated with stearic acid and gallic acid and application of the modified chitosan in stabilizing labile aroma compounds in an oil-in-water emulsion. Food Chem. 2017, 228, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef]
- Yang, T.-S.; Liou, M.-L.; Hu, T.-F.; Peng, C.-W.; Liu, T.-T. Antimicrobial activity of the essential oil of Litsea cubeba on cariogenic bacteria. J. Essent. Oil Res. 2013, 25, 120–128. [Google Scholar] [CrossRef]
- Hui, R.; Qi-He, C.; Ming-liang, F.; Qiong, X.; Guo-qing, H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Lawal, O.S. Foaming, gelation and electrophoretic characteristics of mucuna bean (Mucuna pruriens) protein concentrates. Food Chem. 2003, 83, 237–246. [Google Scholar] [CrossRef]
- Townsend, A.A.; Nakai, S. Relationships between hydrophobicity and foaming characteristics of food proteins. J. Food Sci. 1983, 48, 588–594. [Google Scholar] [CrossRef]
- Lawal, O.S. Functionality of native and succinylated Lablab bean (Lablab purpureus) protein concentrate. Food Hydrocoll. 2005, 19, 63–72. [Google Scholar] [CrossRef]
- Narsimhan, G.; Xiang, N. Role of proteins on formation, drainage, and stability of liquid food foams. Annu. Rev. Food Sci. Technol. 2018, 9, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Dontha, S.; Kamurthy, H.; Manthriparagada, B. Phytochemical screening and evaluation of in-vitro antioxidant activity of extracts of Ixora javanica DC flowers. Am. Chem. Sci. J. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅ OH/⋅ O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]

| Sample | GT (g) | OA (g) | Amino-Substitution (%) | Surface Tension Reduction (%) 1 | Zeta Potential (mv) | Viscosity (cp) | Yield (%) |
|---|---|---|---|---|---|---|---|
| GT | 1 | 0 | - | 22.81 ± 0.31 e,2 | 0.28 ± 0.72 a | 4.67 ± 0.11 a | 77.82 ± 0.61 a |
| GT-OA-1 | 1 | 0.5 | 70.88 ± 0.18 e | 24.53 ± 0.65 d | −13.63 ± 0.21 b | 3.98 ± 0.05 b | 65.02 ± 0.74 b |
| GT-OA-2 | 1 | 1 | 76.41 ± 0.44 d | 25.05 ± 1.63 d | −14.43 ± 0.25 c | 3.66 ± 0.23 c | 50.29 ± 1.01 c |
| GT-OA-3 | 1 | 1.5 | 80.10 ± 0.42 c | 28.46 ± 0.79 c | −17.47 ± 0.15 d | 3.12 ± 0.24 d | 40.89 ± 0.53 d |
| GT-OA-4 | 1 | 2 | 84.29 ± 0.43 b | 30.18 ± 0.76 b | −19.03 ± 0.12e | 2.94 ± 0.18 de | 32.90 ± 0.72 e |
| GT-OA-5 | 1 | 4 | 90.02 ± 0.68 a | 32.29 ± 0.93 a | −20.93 ± 0.32 f | 2.70 ± 0.05 e | 20.16 ± 0.63 f |
| Sample | GT-OA (g) | GA (mmol) | EDC (mmol) | NHS (mmol) | Assays (IC50) | |||
|---|---|---|---|---|---|---|---|---|
| DPPH (mg/mL) | Fe2+-Chelating Power (μg/mL) | Fe3+-Reducing Power (Ascorbic Acid, μg/mL) 1 | Fe3+-Reducing Power (Gallic Acid, μg/mL) 1 | |||||
| GT | - | - | - | - | - | >25,000 | - | - |
| GA | - | - | - | - | 0.0062 ± 0.0001 | >25,000 | - | - |
| GT-OA | - | - | - | - | >10 | >25,000 | - | - |
| GT-OA-GA | 0.25 | 2.4 | 2.4 | 2.4 | 1.79 ± 0.02 b,2 | 225.41 ± 5.33 b | 96.71 ± 2.71 a | 52.32 ± 1.46 a |
| GT-OA-GA-Zn | 0.25 | 2.4 | 2.4 | 2.4 | 2.34 ± 0.06 a | 241.23 ± 7.32 a | 92.43 ± 8.37 a | 50.02 ± 4.52 a |
| Bacterium | Minimal Bactericidal Concentration (µg/mL) | |||||
|---|---|---|---|---|---|---|
| GT | GT-OA | GT-OA-GA | GT-OA-Zn | GT-OA-GA-Zn | Zn (NO3)2 | |
| S. aureus | - | - | - | 5000 | 5000 | 75 |
| E. coli | - | - | - | 1250 | 2500 | 18.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-T.; Zhuang, X.-Y.; Yang, T.-S. Functionalities of Gelatin Modified with 2-Octenyl Succinic Anhydride and Gallic Acid. Foods 2022, 11, 1241. https://doi.org/10.3390/foods11091241
Liu T-T, Zhuang X-Y, Yang T-S. Functionalities of Gelatin Modified with 2-Octenyl Succinic Anhydride and Gallic Acid. Foods. 2022; 11(9):1241. https://doi.org/10.3390/foods11091241
Chicago/Turabian StyleLiu, Tai-Ti, Xin-Yi Zhuang, and Tsung-Shi Yang. 2022. "Functionalities of Gelatin Modified with 2-Octenyl Succinic Anhydride and Gallic Acid" Foods 11, no. 9: 1241. https://doi.org/10.3390/foods11091241
APA StyleLiu, T.-T., Zhuang, X.-Y., & Yang, T.-S. (2022). Functionalities of Gelatin Modified with 2-Octenyl Succinic Anhydride and Gallic Acid. Foods, 11(9), 1241. https://doi.org/10.3390/foods11091241





