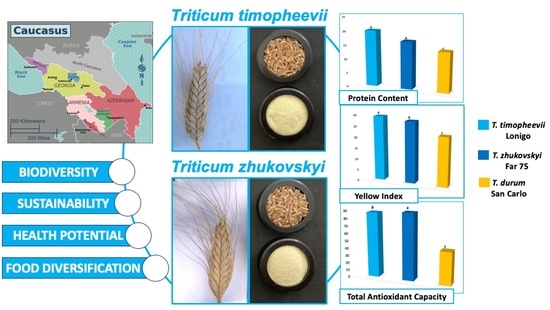
Ancient Caucasian Wheats: A Contribution for Sustainable Diets and Food Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Grain Physical Analyses
2.3. Chemical Characterization
2.4. Rheological and Technological Tests
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Kernel Traits
3.2. Chemical and Nutritional Traits
3.3. Technological and Rheological Traits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nation (FAO). Available online: https://www.fao.org/3/u8480e/u8480e07.htm (accessed on 2 February 2022).
- Arzani, A.; Ashraf, M. Cultivated Ancient Wheats (Triticum spp.): A Potential Source of Health-Beneficial Food Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2017, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A.; Moscaritolo, S. Chemical composition and pasting properties of einkorn (Triticum monococcum L. subsp. monococcum) whole meal flour. J. Cereal Sci. 2008, 47, 599–609. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hawkesford, M.J.; Piironen, V.; Lampi, A.M.; Gebruers, K.; Boros, D.; Andersson, A.A.M.; Aman, P.; Rakszegi, M.; Bedo, Z.; et al. Natural Variation in Grain Composition of Wheat and Related Cereals. J. Agric. Food Chem. 2013, 61, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Molberg, O.; Uhlen, A.K.; Jensen, T.; Flaete, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; van Veelen, P.; Drijfhout, J.W.; Jonker, H.; van Soest, L.; Smulders, M.J.; Bosch, D.; Gilissen, L.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806. [Google Scholar] [CrossRef]
- Picascia, A.; Camarca, M.; Malamisura, R.; Mandile, M.; Galatola, M.; Cielo, D.; Gazza, L.; Mamone, G.; Auricchio, S.; Troncone, R.; et al. In celiac disease patients the in vivo challenge with the diploid Triticum monococcum elicits a reduced immune response compared to hexaploid wheat. Mol. Nutr. Food Res. 2020, 64, 1901032. [Google Scholar] [CrossRef]
- Mori, N.; Kondo, Y.; Ishii, T.; Kawahara, T.; Valkoun, J.; Nakamura, C. Genetic diversity and origin of timopheevi wheat inferred by chloroplast DNA fingerprinting. Breed. Sci. 2009, 59, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Dvořák, J.; Terlizzi, P.D.; Zhang, H.B.; Resta, P. The evolution of polyploid wheats: Identification of the A genome donor species. Genome 1993, 36, 21–31. [Google Scholar] [CrossRef]
- Matsuoka, Y. Evolution of Polyploid Triticum Wheats under Cultivation: The Role of Domestication, Natural Hybridization and Allopolyploid Speciation in their Diversification. Plant Cell Physiol. 2011, 52, 750–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zair, W.; Magos Brehm, J. Triticum timopheevii. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Vincent, H.; Wiersema, J.; Kell, S.; Fielder, H.; Dobbie, S.; Castaneda-Alvarez, N.P.; Guarino, L.; Eastwood, R.; Leόn, B.; Maxted, N. A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 2013, 167, 265–275. [Google Scholar] [CrossRef]
- Devi, U.; Grewal, S.; Yang, C.Y.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.; King, I.P.; King, J. Development and characterisation of interspecific hybrid lines with genome-wide introgressions from Triticum timopheevii in a hexaploid wheat background. BMC Plant Biol. 2019, 19, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorjadze, M.; Berishvili, T.; Shatberashvili, E. The ancient wheats of Georgia and their traditional use in the southern part of the country. Emir. J. Food Agric. 2014, 26, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Brown-Guedira, G.L.; Gill, B.S.; Bockus, W.W.; Cox, T.S.; Hatchett, J.H.; Leath, S.; Peterson, C.J.; Thomas, J.B.; Zwer, P.K. Evaluation of a collection of wild Timopheevii wheat for resistance to disease and arthropod pests. Plant Dis. 1996, 80, 928–933. [Google Scholar] [CrossRef]
- Järve, K.; Jakobson, I.; Enno, T. Tetraploid wheat species Triticum timopheevii and Triticum militinae in common wheat improvement. Acta Agron. Hung. 2002, 50, 463–477. [Google Scholar] [CrossRef]
- Intagible Cultural Heritage UNESCO. Available online: https://ich.unesco.org/doc/src/47213-EN.doc (accessed on 15 February 2022).
- Relina, L.I.; Boguslavskyi, R.L.; Vecherska, L.A.; Didenko, S.Y.; Golik, O.V.; Sheliakina, T.A.; Pozdniakov, V.V. Grain quality of tetraploid wheat Triticum timopheevii (zhuk.) zhuk. Plant Breed. Seed Prod. 2018, 114, 106–119. [Google Scholar] [CrossRef]
- Engert, N.; Honermeier, B. Characterization of grain quality and phenolic acids in ancient wheat species (Triticum sp). J. Appl. Bot. Food Qual. 2012, 84, 33. [Google Scholar]
- Zamaratskaia, G.; Gerhardt, K.; Wendin, K. Biochemical characteristics and potential applications of ancient cereals-An underexploited opportunity for sustainable production and consumption. Trends Food Sci. Technol. 2021, 107, 114–123. [Google Scholar] [CrossRef]
- The EU Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Available online: https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 2 February 2022).
- Quaranta, F.; Amoriello, T.; Aureli, G.; Belocchi, A.; D’Egidio, M.G.; Fornara, M.; Melloni, S.; Desiderio, E. Grain yield, quality and deoxynivalenol (DON) contamination of durum wheat (Triticum durum Desf.): Results of national networks in organic and conventional cropping systems. Ital. J. Agric. 2010, 4, 353–366. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO 2010). Cereals and Pulses-Determination of the Mass of 1000 Grains; Method 520:2010; ISO: Geneva, Switzerland, 2010; p. 10. [Google Scholar]
- International Organization for Standardization (ISO 2009). Determination of Bulk Density, Called Mass per Hectolitre-Part 1: Reference Method; Method 7971-1:2009; ISO: Geneva, Switzerland, 2009; p. 8. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 105/2); ICC: Vienna, Austria, 2003. [Google Scholar]
- McCleary, B.V.; Gibson, T.S.; Lugford, D.C. Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: Collaborative study. J. AOAC Int. 1997, 80, 571–579. [Google Scholar] [CrossRef] [Green Version]
- McCleary, B.V.; McNally, M.; Rossiter, P. Measurement of resistant starch by enzymatic digestion and selected plant materials: Collaborative study. J. AOAC Int. 2002, 5, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists. Official Methods of Analysis 991, 16th ed.; Cunniff, P., Ed.; AOAC: Gaithersburg, MD, USA, 1995; p. 42. [Google Scholar]
- Ciccoritti, R.; Taddei, F.; Nicoletti, I.; Gazza, L.; Corradini, D.; D’Egidio, M.G.; Martini, D. Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential. Food Chem. 2017, 225, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Amato, M.; Phillips, T.D.; Angelino, D.; Morreale, F.; Fares, C. Gluten-free pasta incorporating chia (Salvia hispanica L.) as thickening agent: An approach to naturally improve the nutritional profile and the in vitro carbohydrate digestibility. Food Chem. 2017, 221, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 158); ICC: Vienna, Austria, 1995. [Google Scholar]
- Dexter, J.E.; Marchylo, B.A. Recent Trends in Durum Wheat Milling and Pasta Processing: Impact on Durum Wheat Quality Requirements. In Proceedings of the International Workshop on Durum Wheat, Semolina and Pasta Quality: Recent Achievements and New Trends, Montpellier, France, 27 November 2000; Abeccassis, J., Autran, J.C., Feillet, P., Eds.; Institut National de la Recherche: Montpellier, France, 2001; pp. 139–164. [Google Scholar]
- Belcar, J.; Sobczyk, A.; Sobolewska, M.; Stankowski, S.; Gorzelany, J. Characteristics of Technological Properties of Grain and Flour from Ancient Varieties of Wheat (Einkorn, Emmer and Spelt). Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 269–278. [Google Scholar] [CrossRef]
- Mikó, P.; Megyeri, M.; Molnár-Láng, M.; Kovács, G. Characterization of Triticum timopheevii Zhuk. gene bank accessions for the development of synthetic amphiploid wheat lines. Acta Agron. Hung. 2013, 61, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fu, B.X. Inter-relationships between test weight, thousand kernel weight, kernel size distribution and their effects on durum wheat milling, semolina composition and pasta processing quality. Foods 2020, 9, 1308. [Google Scholar] [CrossRef]
- Canadian Grain Commission. Wheat: Export Grade Determinants Tables for Canada Western Amber Durum (CWAD) Wheat. 2020. Available online: https://www.grainscanada.gc.ca/en/grain-quality/official-graingrading-guide/04-wheat/export-grade-determinants/cwad-en.html (accessed on 12 February 2022).
- Kulathunga, J.; Reuhs, B.L.; Zwinger, S.; Simsek, S. Comparative study on kernel quality and chemical composition of ancient and modern wheat species: Einkorn, emmer, spelt and hard red spring wheat. Foods 2021, 10, 761. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Hareland, G.A.; Chao, S.; Anderson, J.A. Genetic mapping and QTL analysis of flour color and milling yield related traits using recombinant inbred lines in hard red spring wheat. Crop Sci. 2011, 51, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Gazza, L.; Conti, S.; Taddei, F.; Pogna, N.E. Molecular characterization of puroindolines and their encoding genes in Aegilops ventricosa. Mol. Breed. 2006, 17, 191–200. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Europ. J. Agric. 2017, 87, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Rachon, L.; Bobryk-Mamczarz, A.; Kiełtyka-Dadasiewicz, A. Hulled Wheat Productivity and Quality in Modern Agriculture Against Conventional Wheat Species. Agriculture 2020, 10, 275. [Google Scholar] [CrossRef]
- Hucl, P.; Chibbar, R.N. Variation for starch concentration in spring wheat and its repeatability relative to protein concentration. Cereal Chem. 1996, 73, 756–758. [Google Scholar]
- Prasadi, V.P.N.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Serban, L.R.; Păucean, A.; Man, S.M.; Chis, M.S.; Mureşan, V. Ancient Wheat Species: Biochemical Profile and Impact on Sourdough Bread Characteristics—A Review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017, 64, 587–597. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C.; Piscozzi, R. Carotenoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 2006, 44, 182–193. [Google Scholar] [CrossRef]
- Peña, R.J. Durum wheat for pasta and bread-making. Comparison of methods used in breeding to determine gluten quality-related parameters. In Durum Wheat Improvement in the Mediterranean Region: New Challenges; Serie A: Séminaires Méditerranéennes; CIHEAM: Zaragoza, Spain, 2000; pp. 423–430. [Google Scholar]
- AbuHammad, W.A.; Elias, E.M.; Manthey, F.A.; Alamri, M.S.; Mergoum, M.A. Comparison of methods for assessing dough and gluten strength of durum wheat and their relationship to pasta cooking quality. Int. J. Food Sci. Technol. 2012, 47, 2561–2573. [Google Scholar] [CrossRef]
- UNI Italian Organization for Standardization. Durum Wheat Products for Pasta-Making—Definition, Characteristics and Quality Grades; NumberUNI10709; UNI: Milano, Italy, 1998. [Google Scholar]
- UNI Italian Organization for Standardization. Durum Wheat Products for Pasta-Making—Definition, Characteristics and Quality Grades; NumberUNI10940; UNI: Milano, Italy, 2001. [Google Scholar]
- Quaglia, G.B. Other durum wheat products. In Durum Wheat: Chemistry and Technology; Fabriani, G., Lintas, C., Eds.; AACC: St Paul, MN, USA, 1988; pp. 263–274. [Google Scholar]
- Bobryk-Mamczarz, A.; Kiełtyka-Dadasiewicz, A.; Rachoń, L. Usefulness of Hulled Wheats Grown in Polish Environment for Wholegrain Pasta-Making. Foods 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]

| Thousand Kernel Weight (g) | Test Weight (kg/hL) | Hardness Index | Kernel Dimensions | |||
|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Thickness (mm) | ||||
| T. timopheevii accession Lonigo | 28.0 ± 0.4 b | 72.2 ± 0.1 b | 83 ± 15 a | 8.7 ± 0.6 b | 2.3 ± 0.1 c | 2.4 ± 0.2 b |
| T. zhukovskyi accession Far 75 | 27.7 ± 0.4 b | 72.0 ± 0.5 b | 85 ± 17 a | 8.9 ± 0.8 a | 2.7 ± 0.2 b | 2.5 ± 0.1 b |
| T. durum cv San Carlo | 56.2 ± 0.3 a | 84.3 ± 0.3 a | 84 ± 12 a | 8.1 ± 0.7 c | 3.7 ± 0.4 a | 3.5 ± 0.5 a |
| Protein (%) | Total Starch (%) | TDF (%) | Ash (%) | TAC (mmol TEAC/kg) | TSPC (mg FAE/g) | |
|---|---|---|---|---|---|---|
| T. timopheevii accession Lonigo | 20.1 ± 0.8 a | 62.2 ± 0.19 b | 9.3 ± 0.2 c | 2.13 ± 0.01 a | 87.4 ± 0.5 b | 0.94 ± 0.05 b |
| T. zhukovskyi accession Far 75 | 16.92 ± 0.03 b | 62.0 ± 0.3 b | 9.6 ± 0.2 b | 1.96 ± 0.02 b | 89.7 ± 0.3 a | 0.997 ± 0.005 b |
| T. durum cv San Carlo | 14.3 ± 0.5 c | 65.0 ± 0.8 a | 12.3 ± 0.3 a | 1.65 ± 0.01 c | 44.1 ± 0.3 c | 1.19 ± 0.04 a |
| SDS Sedimentation Volume (mL) | Gluten Index (%) | Dry Gluten Content (%) | Alveograph Parameters | Falling Number (s) | Color | ||||
|---|---|---|---|---|---|---|---|---|---|
| W | P/L | Yellow Index (b*) | Brown Index (100-L*) | Red Index (a*) | |||||
| T. timopheevii accession Lonigo | 34.5 ± 0.7 b | 34 ± 1 b | 17.13 ± 0.07 a | 29 ± 15 b | 1.2 ± 0.7 ab | 467 ± 1 b | 29.2 ± 0.2 a | 15.4 ± 0.2 a | −2.69 ± 0.09 a |
| T. zhukovskyi accession Far 75 | 22.5 ± 0.7 c | 1.3 ± 0.6 c | 15.3 ± 0.2 b | 9 ± 8 b | 0.8 ± 0.1 b | 476 ± 8 a | 27.7 ± 0.2 b | 15.6 ± 0.2 a | −2.23 ± 0.09 b |
| T. durum cv San Carlo | 37.5 ± 0.7 a | 93 ± 1 a | 10.5 ± 0.1 c | 227 ± 21 a | 1.8 ± 0.1 a | 483 ± 2 a | 22.1 ± 0.2 c | 14.9 ± 0.5 b | −2.3 ± 0.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocente, F.; Galassi, E.; Taddei, F.; Natale, C.; Gazza, L. Ancient Caucasian Wheats: A Contribution for Sustainable Diets and Food Diversity. Foods 2022, 11, 1209. https://doi.org/10.3390/foods11091209
Nocente F, Galassi E, Taddei F, Natale C, Gazza L. Ancient Caucasian Wheats: A Contribution for Sustainable Diets and Food Diversity. Foods. 2022; 11(9):1209. https://doi.org/10.3390/foods11091209
Chicago/Turabian StyleNocente, Francesca, Elena Galassi, Federica Taddei, Chiara Natale, and Laura Gazza. 2022. "Ancient Caucasian Wheats: A Contribution for Sustainable Diets and Food Diversity" Foods 11, no. 9: 1209. https://doi.org/10.3390/foods11091209
APA StyleNocente, F., Galassi, E., Taddei, F., Natale, C., & Gazza, L. (2022). Ancient Caucasian Wheats: A Contribution for Sustainable Diets and Food Diversity. Foods, 11(9), 1209. https://doi.org/10.3390/foods11091209