Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Germination and Treatment
2.2. Chemicals
2.3. Total Se Determination
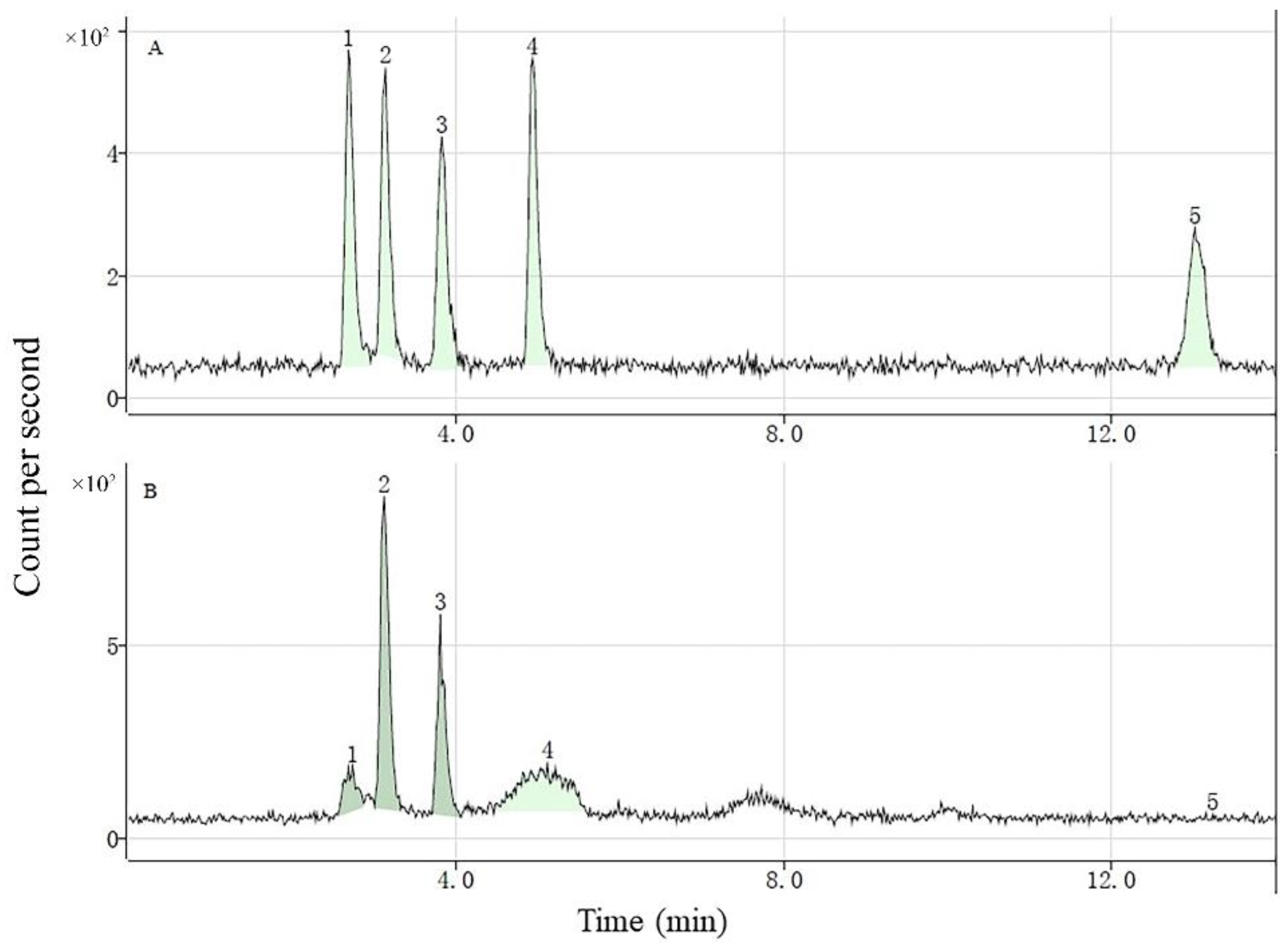
2.4. Se Species Determination
2.5. Protein and Amino Acid Determination
2.6. Total Phenolic and Total Flavonoid Content Determination
2.7. Data Analysis
3. Results and Discussion
3.1. Total Se Contents in Se-Enriched Soybean Sprouts
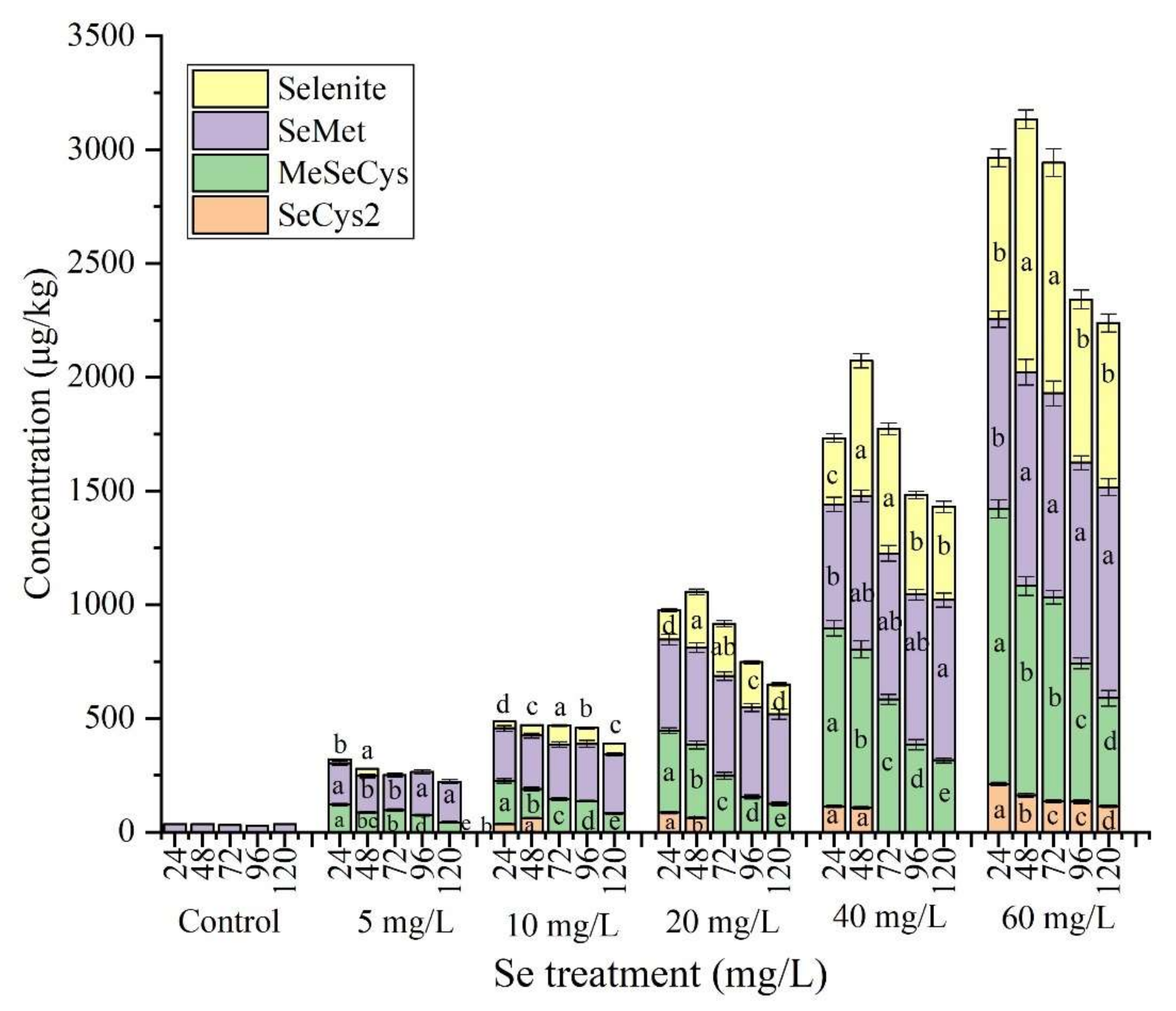
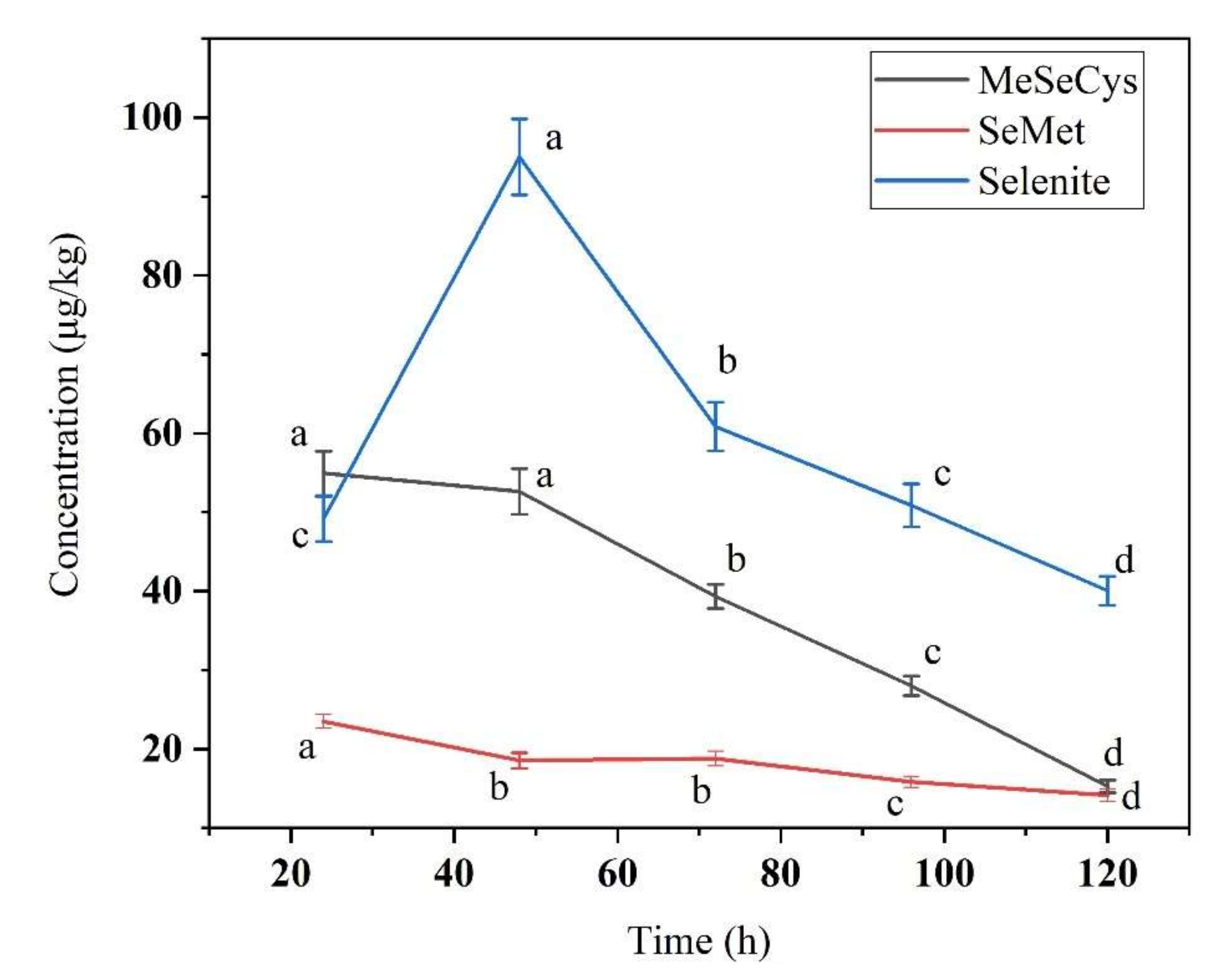
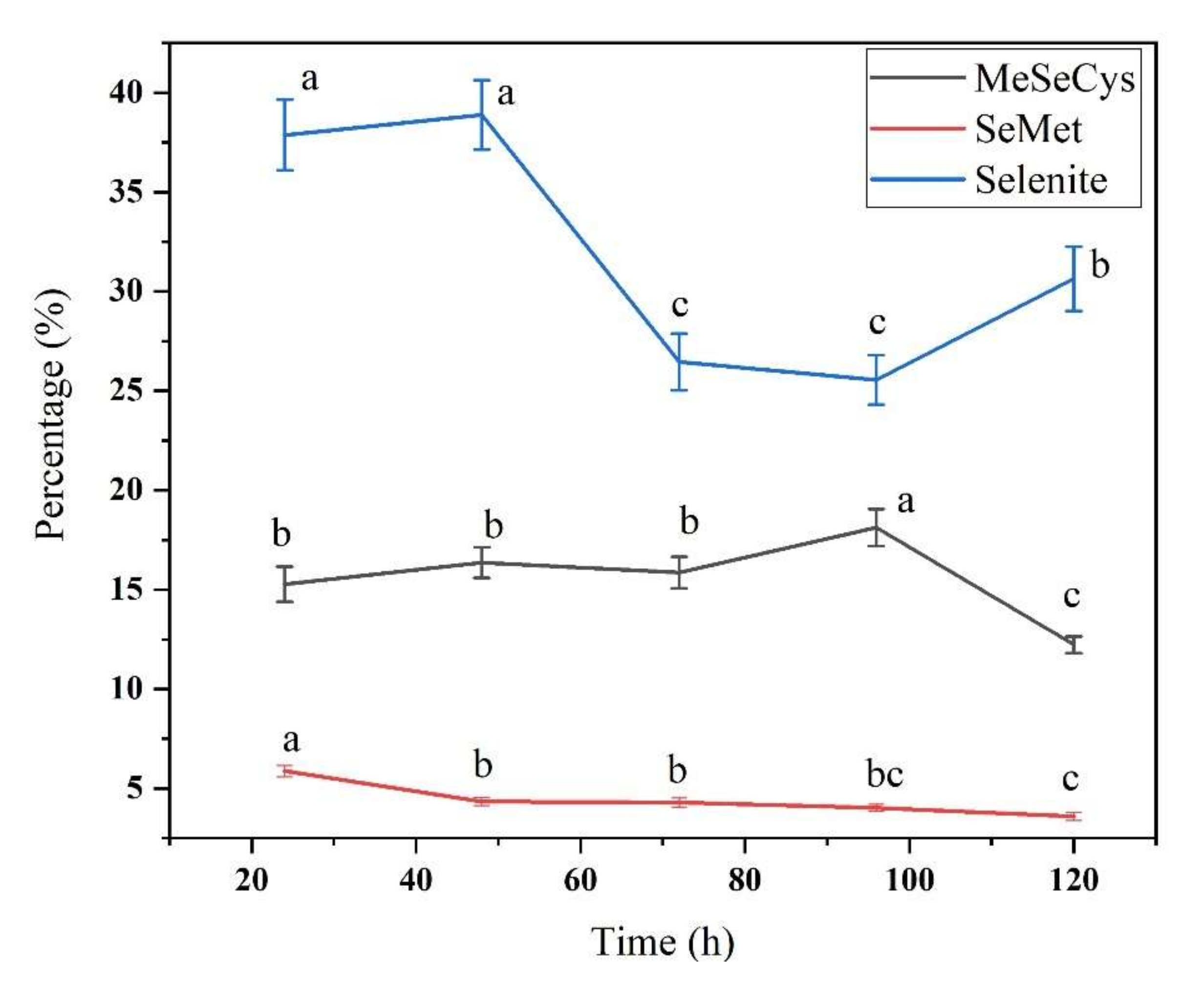
3.2. Se Species in Se-Enriched Soybean Sprouts
3.3. Free Se and Bound Se in Se-Enriched Soybean Sprouts
3.4. Protein and Amino Acid Compositions in Se-Enriched Soybean Sprouts
3.5. TPC and TFC in Se-Enriched Soybean Sprouts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khadra, M.; Planas, D.; Brodeur, P.; Amyot, M. Mercury and Selenium Distribution in Key Tissues and Early Life Stages of Yellow Perch (Perca flavescens). Environ. Pollut. 2019, 54, 112963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.F.; Zhang, Z.; Gao, Y.; Zhao, J. A Comparative Study on the Accumulation, Translocation and Transformation of Selenite, Selenate, and Senps in a Hydroponic-Plant System. Ecotoxicol. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and Selenocysteine: Roles in Cancer, Health, and Development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrzynska, K.; Sentkowska, A. Selenium in Plant Foods: Speciation Analysis, Bioavailability, and Factors Affecting Composition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing Selenium Functional Foods and Beverages: A Review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohe, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; Society German Nutrition. Revised Reference Values for Selenium Intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-Enriched Foods Are More Effective at Increasing Glutathione Peroxidase (Gpx) Activity Compared with Selenomethionine: A Meta-Analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A. Whole Grain Rice Fortification as a Solution to Micronutrient Deficiency: Technologies and Need for More Viable Alternatives. Food Chem. 2020, 326, 127049. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium Speciation Profiles in Selenite-Enriched Soybean (Glycine max) by Hplc-Icpms and Esi-Itms. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef]
- Deng, B.; Tian, S.; Li, S.; Guo, M.; Liu, H.; Li, Y.; Wang, Q.; Zhao, X. A Simple, Rapid and Efficient Method for Essential Element Supplementation Based on Seed Germination. Food Chem. 2020, 325, 126827. [Google Scholar] [CrossRef] [PubMed]
- Trolove, S.N.; Tan, Y.; Morrison, S.C.; Feng, L.; Eason, J. Development of a Method for Producing Selenium-Enriched Radish Sprouts. LWT 2018, 95, 187–192. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative Study of the Bioactive Properties and Elemental Composition of Red Clover (Trifolium pratense) and Alfalfa (Medicago sativa) Sprouts during Germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se Treatment on Glucosinolate Metabolism and Health-Promoting Compounds in the Broccoli Sprouts of Three Cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Nurminsky, V.N.; Perfileva, A.I.; Kapustina, I.S.; Graskova, I.A.; Sukhov, B.G.; Trofimov, B.A. Growth-Stimulating Activity of Natural Polymer-Based Nanocomposites of Selenium during the Germination of Cultivated Plant Seeds. Dokl. Biochem. Biophys. 2020, 495, 296–299. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Pasko, P.; Galanty, A.; Tyszka-Czochara, M.; Wietecha-Posluszny, R.; Rubio, P.S.; Barton, H.; Prochownik, E.; Muszynska, B.; Sulkowska-Ziaja, K.; et al. Does Selenium Fortification of Kale and Kohlrabi Sprouts Change Significantly Their Biochemical and Cytotoxic Properties? J. Trace Elem. Med. Biol. 2020, 59, 126466. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, H.; Song, Y.; Gu, Z. Effects of Soaking and Aeration Treatment on Γ-Aminobutyric Acid Accumulation in Germinated Soybean (Glycine max L.). Eur. Food Res. Technol. 2011, 232, 787–795. [Google Scholar] [CrossRef]
- Umysova, D.; Vitova, M.; Douskova, I.; Bisova, K.; Hlavova, M.; Cizkova, M.; Machat, J.; Doucha, J.; Zachleder, V. Bioaccumulation and Toxicity of Selenium Compounds in the Green Alga Scenedesmus Quadricauda. BMC Plant Biol. 2009, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Pyrzynska, K. Nanomaterials in Speciation Analysis of Metals and Metalloids. Talanta 2020, 212, 120784. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Zhao, G.; Guo, Y. Selenium Enriched Hypsizygus Marmoreus, a Potential Food Supplement with Improved Se Bioavailability. LWT 2021, 140, 110819. [Google Scholar] [CrossRef]
- Cui, M.; Wu, D.; Bao, K.; Wen, Z.; Hao, Y.; Luo, L. Dynamic Changes of Phenolic Compounds during Artificial Aging of Soybean Seeds Identified by High-Performance Liquid Chromatography Coupled with Transcript Analysis. Anal. Bioanal. Chem. 2019, 411, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Woch, W.; Hawrylak-Nowak, B. Selected Antioxidant Properties of Alfalfa, Radish, and White Mustard Sprouts Biofortified with Selenium. Acta Agrobot. 2019, 72, 1768. [Google Scholar] [CrossRef]
- Guardado-Felix, D.; Serna-Saldivar, S.O.; Cuevas-Rodriguez, E.O.; Jacobo-Velazquez, D.A.; Gutierrez-Uribe, J.A. Effect of Sodium Selenite on Isoflavonoid Contents and Antioxidant Capacity of Chickpea (Cicer arietinum L.) Sprouts. Food Chem. 2017, 226, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Vélez, M.A.; Guardado-Félix, D.; Avilés-González, J.; Romo-López, I.; Serna-Saldívar, S.O. Effect of Germination with Sodium Selenite on the Isoflavones and Cellular Antioxidant Activity of Soybean (Glycine max). LWT 2018, 93, 64–70. [Google Scholar] [CrossRef]
- Guardado-Felix, D.; Serna-Saldivar, S.O.; Gutierrez-Uribe, J.A.; Chuck-Hernandez, C. Selenium in Germinated Chickpea (Cicer arietinum L.) Increases the Stability of Its Oil Fraction. Plants 2019, 8, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of Selenium Biofortification on the Bioactive Compounds and Antioxidant Activity of Wheat Microgreen Extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Zhang, K.; Wen, Q.; Ming, K.; Xiong, H.; Ning, F. Peanut Selenium Distribution, Concentration, Speciation, and Effects on Proteins after Exogenous Selenium Biofortification. Food Chem. 2021, 354, 129515. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, X.; Zhao, Q.; Han, Y.; Zhan, T.; Li, Y.; Tang, C.; Zhang, J. Development and Application of a HPLC-ICP-MS Method to Determine Selenium Speciation in Muscle of Pigs Treated with Different Selenium Supplements. Food Chem. 2020, 302, 125371. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.; Wu, H. Selenium Accumulation, Speciation, and Its Effect on Nutritive Value of Flammulina Velutipes (Golden Needle Mushroom). Food Chem. 2021, 350, 128667. [Google Scholar] [CrossRef]
- Deng, X.; Liao, J.; Zhao, Z.; Qin, Y.; Liu, X. Distribution and Speciation of Selenium in Soybean Proteins and Its Effect on Protein Structure and Functionality. Food Chem. 2021, 370, 130982. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; McCallum, J.L.; Wang, S.; Liu, R.; Zhu, H.; Tsao, R. Evaluation of Antioxidant Activities and Chemical Characterisation of Staghorn Sumac Fruit (Rhus hirta L.). Food Chem. 2013, 138, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, L.; Li, H.; Li, Y.; Wei, B. Dietary Selenium Intake Based on the Chinese Food Pagoda: The Influence of Dietary Patterns on Selenium Intake. Nutr. J. 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and Partitioning of Selenium in Basil (Ocimum basilicum L.) Plants Grown in Hydroponics. Sci. Hortic. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2016, 7, 2074. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Martin, G.; Sanz-Landaluze, J.; Leon-Gonzalez, M.E.; Madrid, Y. In-Vivo Solid Phase Microextraction for Quantitative Analysis of Volatile Organoselenium Compounds in Plants. Anal. Chim. Acta 2019, 1081, 72–80. [Google Scholar] [CrossRef]
- Mylenko, M.; Vu, D.L.; Kuta, J.; Ranglova, K.; Kubac, D.; Lakatos, G.; Grivalsky, T.; Caporgno, M.P.; da Camara Manoel, J.A.; Kopecky, J.; et al. Selenium Incorporation to Amino Acids in Chlorella Cultures Grown in Phototrophic and Heterotrophic Regimes. J. Agric. Food Chem. 2020, 68, 1654–1665. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, Y.; Suzuki, N.; Guo, X.; Xiong, H.; Ogra, Y. Speciation of Selenium in Brown Rice Fertilized with Selenite and Effects of Selenium Fertilization on Rice Proteins. Int. J. Mol. Sci. 2018, 19, 3494. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium Accumulation and Metabolism in Algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bierla, K.; Jimenez-Lamana, J.; Kot, A.M.; Alcantara-Duran, J.; Piwowarek, K.; Blazejak, S.; Szpunar, J. Metabolic Response of the Yeast Candida Utilis during Enrichment in Selenium. Int. J. Mol. Sci. 2020, 21, 5287. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Velez, M.A.; Aviles-Gonzalez, J.; Serna-Saldivar, S.O.; Temblador-Perez, M.C. Optimization of Wheat Sprouting for Production of Selenium Enriched Kernels Using Response Surface Methodology and Desirability Function. LWT 2016, 65, 1080–1086. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S.; Bzducha-Wrobel, A.; Kot, A.M. Effect of Selenium on Growth and Antioxidative System of Yeast Cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Nam, P.K.; Ma, Y. Comprehensive Profiling of Isoflavones, Phytosterols, Tocopherols, Minerals, Crude Protein, Lipid, and Sugar during Soybean (Glycine max) Germination. J. Agric. Food Chem. 2010, 58, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Du, B.; He, L.; Zheng, A.; Pan, S.; Tang, X. Foliar Application of Sodium Selenate Induces Regulation in Yield Formation, Grain Quality Characters and 2-Acetyl-1-Pyrroline Biosynthesis in Fragrant Rice. BMC Plant Biol. 2019, 19, 502. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Wu, H.; Zhang, M. Selenium Accumulation in Protein Fractions of Tenebrio Molitor Larvae and the Antioxidant and Immunoregulatory Activity of Protein Hydrolysates. Food Chem. 2021, 334, 127475. [Google Scholar] [CrossRef]
- Wrobel, K.; Guerrero Esperanza, M.; Yanez Barrientos, E.; Corrales Escobosa, A.R.; Wrobel, K. Different Approaches in Metabolomic Analysis of Plants Exposed to Selenium: A Comprehensive Review. Acta Physiol. Plant. 2020, 42, 1–20. [Google Scholar] [CrossRef]
- Sun, W.X.; Zhang, R.J.; Fan, J.; He, Y.; Mao, X.H. Comprehensive Transformative Profiling of Nutritional and Functional Constituents during Germination of Soybean Sprouts. J. Food Meas. Charact. 2018, 12, 1295–1302. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the Effects of Selenium Treatment on the Nutritional Quality of Tomato Fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Q.; Chen, H.; Xiong, H. Distribution and Effects of Natural Selenium in Soybean Proteins and Its Protective Role in Soybean Beta-Conglycinin (7s Globulins) under Aaph-Induced Oxidative Stress. Food. Chem. 2019, 272, 201–209. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Hui, G.; Zhang, J.; Li, H.; Wu, W.; Wei, X.; Guo, Y. Selenium Biofortification and Its Effect on Multi-Element Change in Auricularia Auricular. Food Chem. 2019, 295, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liang, S.; Zhu, H. Comparative Proteomics Analysis of the Effect of Selenium Treatment on the Quality of Foxtail Millet. LWT 2020, 131, 109691. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Wang, D.; Fang, F.; Lai, J.; Wu, T.; Tsao, R. Isoflavone, Γ-Aminobutyric Acid Contents and Antioxidant Activities Are Significantly Increased during Germination of Three Chinese Soybean Cultivars. J. Funct. Foods 2015, 14, 596–604. [Google Scholar] [CrossRef]
- Cabezudo, I.; Meini, M.R.; Di Ponte, C.C.; Melnichuk, N.; Boschetti, C.E.; Romanini, D. Soybean (Glycine max) Hull Valorization through the Extraction of Polyphenols by Green Alternative Methods. Food Chem. 2021, 338, 128131. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se Yield and Nutritional Traits with Focus on Phenolic Acid Profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.S.; Daum, D.; Rohn, S. Influence of a Selenium Biofortification on Antioxidant Properties and Phenolic Compounds of Apples (Malus domestica). Antioxidants 2020, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Sandoval, S.N.; Guardado-Félix, D.; Gutiérrez-Uribe, J.A. Deglycosylation of Isoflavones in Selenized Germinated Chickpea Flours Due to Convection Drying. LWT 2022, 15, 112417. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Wang, P.; Yang, R.; Zhou, T.; Gu, Z. Uv-B Mediates Isoflavone Accumulation and Oxidative-Antioxidant System Responses in Germinating Soybean. Food Chem. 2019, 275, 628–636. [Google Scholar] [CrossRef]

| Se Treatment Solution (mg/L) | Total Se Content (μg/kg Dried Weight) | |||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | 33.03 ± 1.44 | 35.93 ± 1.75 | 33.44 ± 2.81 | 33.44 ± 1.31 | 32.74 ± 2.39 | 36.12 ± 1.43 |
| 5 | 371.1 ± 15.00 a | 302.8 ± 6.63 b | 302.8 ± 17.41 b | 309.1 ± 21.38 b | 284.5 ± 1.18 b | 242.5 ± 16.88 c |
| 10 | 630.6 ± 21.70 a | 520.2 ± 26.92 bc | 583.0 ± 38.81 ab | 613.8 ± 41.18 a | 513.5 ± 29.69 bc | 446.1 ± 17.73 c |
| 20 | 1102 ± 30.70 a | 1006 ± 43.82 ab | 1064 ± 84.71 ab | 960.5 ± 24.44 b | 806.8 ± 41.42 c | 713.7 ± 34.23 c |
| 40 | 2545 ± 200.4 ab | 2217 ± 93.92 bc | 2557 ± 81.17 a | 2252 ± 100.6 abc | 2108 ± 124.3 cd | 1880 ± 144.4 d |
| 60 | 4099 ± 210.6 a | 3188 ± 152.2 cd | 3426 ± 36.43 bc | 3539 ± 125.8 b | 2985 ± 135.2 d | 2882 ± 86.97 d |
| Statistical analysis | Se treatment solution (S) *** | Germination hours (G) *** | S × G *** | |||
| Se Treatment Solution (mg/L) | Protein Content (g/100 g Dried Weight) | |||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | 35.32 ± 0.83 c | 36.16 ± 0.26 bc | 36.69 ± 0.97 abc | 37.03 ± 0.80 abc | 38.15 ± 0.72 ab | 38.68 ± 0.91 a |
| 5 | 35.24 ± 0.51 d | 35.69 ± 0.39 cd | 36.34 ± 0.90 cd | 36.91 ± 0.61 bc | 37.96 ± 0.23 ab | 38.40 ± 0.37 a |
| 10 | 35.63 ± 0.21 b | 35.77 ± 0.09 b | 36.29 ± 0.58 b | 37.00 ± 0.58 ab | 37.94 ± 0.84 a | 38.01 ± 0.65 a |
| 20 | 35.78 ± 0.40 c | 35.69 ± 0.10 c | 36.25 ± 0.36 bc | 36.79 ± 0.35 b | 38.18 ± 0.11 a | 38.46 ± 0.08 a |
| 40 | 35.52 ± 0.10 c | 34.97 ± 0.24 bc | 36.21 ± 0.02 b | 36.48 ± 0.92 b | 38.19 ± 0.12 a | 38.29 ± 0.20 a |
| 60 | 35.38 ± 0.51 c | 35.41 ± 0.17 c | 35.95 ± 0.07 bc | 36.82 ± 0.36 b | 37.90 ± 0.59 a | 38.14 ± 0.52 a |
| Statistical analysis | Se treatment solution (S) ns | Germination hours (G) *** | S × G ns | |||
| Amino Acids | Se Treatment Solution (mg/L) | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 40 | 60 | |
| Aspartic (Asp) | 5.82 ± 0.70 a | 6.85 ± 0.50 a | 6.15 ± 1.12 a | 6.23 ± 0.03 a | 6.21 ± 0.02 a | 4.26 ± 0.00 b |
| Threonine (Thr) | 1.50 ± 0.05 ab | 1.47 ± 0.03 ab | 1.60 ± 0.07 a | 1.57 ± 0.04 ab | 1.56 ± 0.11 ab | 1.44 ± 0.00 b |
| Serine (Ser) | 2.02 ± 0.07 | 2.00 ± 0.03 | 2.15 ± 0.11 | 2.11 ± 0.03 | 2.10 ± 0.15 | 1.95 ± 0.01 |
| Glutamate (Glu) | 6.47 ± 0.08 ab | 6.19 ± 0.32 b | 7.01 ± 0.27 a | 6.80 ± 0.05 ab | 6.76 ± 0.50 ab | 6.62 ± 0.09 ab |
| Proline (Pro) | 1.83 ± 0.05 | 1.86 ± 0.00 | 2.03 ± 0.16 | 1.92 ± 0.01 | 1.93 ± 0.21 | 1.86 ± 0.02 |
| Glycine (Gly) | 1.54 ± 0.05 | 1.51 ± 0.03 | 1.64 ± 0.09 | 1.61 ± 0.01 | 1.62 ± 0.13 | 1.57 ± 0.01 |
| Alanine (Ala) | 1.78 ± 0.07 | 1.77 ± 0.02 | 1.86 ± 0.13 | 1.84 ± 0.03 | 1.86 ± 0.13 | 1.67 ± 0.02 |
| Cysteine (Cys) | 0.39 ± 0.02 | 0.38 ± 0.02 | 0.37 ± 0.01 | 0.38 ± 0.02 | 0.39 ± 0.01 | 0.35 ± 0.04 |
| Valine (Val) | 1.89 ± 0.07 | 1.90 ± 0.00 | 2.00 ± 0.17 | 1.99 ± 0.03 | 1.98 ± 0.16 | 1.90 ± 0.01 |
| Methionine (Met) | 0.39 ± 0.01 a | 0.34 ± 0.01 b | 0.33 ± 0.03 b | 0.33 ± 0.01 b | 0.31 ± 0.03 b | 0.32 ± 0.01 b |
| Isoleucine (Ile) | 1.75 ± 0.03 | 1.77 ± 0.03 | 1.88 ± 0.12 | 1.84 ± 0.05 | 1.85 ± 0.16 | 1.80 ± 0.04 |
| Leucine (Leu) | 2.87 ± 0.08 | 2.87 ± 0.05 | 3.06 ± 0.19 | 3.01 ± 0.03 | 3.02 ± 0.21 | 3.08 ± 0.03 |
| Tyrosine (Tyr) | 1.25 ± 0.01 | 1.27 ± 0.01 | 1.33 ± 0.12 | 1.32 ± 0.04 | 1.35 ± 0.10 | 1.22 ± 0.01 |
| Phenylalanine (Phe) | 1.99 ± 0.04 | 2.05 ± 0.01 | 2.15 ± 0.13 | 2.08 ± 0.05 | 2.12 ± 0.15 | 2.06 ± 0.03 |
| Lysine (Lys) | 2.52 ± 0.08 a | 2.43 ± 0.03 a | 2.67 ± 0.15 a | 2.51 ± 0.13 a | 2.49 ± 0.22 a | 1.96 ± 0.01 b |
| Histidine (His) | 1.04 ± 0.05 a | 1.08 ± 0.02 a | 1.09 ± 0.10 a | 1.09 ± 0.04 a | 1.09 ± 0.09 a | 0.76 ± 0.01 b |
| Arginine (Arg) | 2.69 ± 0.10 a | 2.69 ± 0.05 a | 2.90 ± 0.18 a | 2.80 ± 0.04 a | 2.80 ± 0.26 a | 2.16 ± 0.01 b |
| Total amino acids (TAA) | 37.74 ± 1.49 ab | 38.43 ± 0.05 ab | 40.20 ± 3.14 a | 39.41 ± 0.64 ab | 39.42 ± 2.63 ab | 34.98 ± 0.23 b |
| Total essential amino acids (TEAA) | 12.91 ± 0.33 ab | 12.84 ± 0.08 ab | 13.68 ± 0.85 a | 13.32 ± 0.34 ab | 13.32 ± 1.03 ab | 12.56 ± 0.10 b |
| Se Treatment (mg/L) | 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | |
|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g DW) | Control | 0.11 ± 0.014 e | 0.11 ± 0.014 e | 0.15 ± 0.014 d | 0.18 ± 0.007 c | 0.31 ± 0.000 b | 0.45 ± 0.014 a |
| 20 | 0.11 ± 0.014 e | 0.12 ± 0.007 e | 0.17 ± 0.014 d | 0.22 ± 0.007 c | 0.42 ± 0.028 b | 0.53 ± 0.000 a | |
| 60 | 0.11 ± 0.014 e | 0.12 ± 0.007 e | 0.16 ± 0.007 d | 0.24 ± 0.007 c | 0.46 ± 0.007 b | 0.60 ± 0.007 a | |
| Statistical analysis | Se treatment (S) *** | Germination hours (G) *** | S × G *** | ||||
| TFC (mg CE/g DW) | Control | 7.01 ± 0.04 e | 8.95 ± 0.14 d | 8.63 ± 0.18 d | 9.39 ± 0.13 c | 10.28 ± 0.11 b | 11.32 ± 0.20 a |
| 20 | 7.01 ± 0.04 e | 9.29 ± 0.05 d | 9.03 ± 0.14 d | 9.85 ± 0.20 c | 11.75 ± 0.07 b | 12.90 ± 0.16 a | |
| 60 | 7.01 ± 0.04 e | 9.21 ± 0.27 d | 9.12 ± 0.15 d | 9.70 ± 0.07 c | 11.00 ± 0.17 b | 12.09 ± 0.10 a | |
| Statistical analysis | Se treatment (S) *** | Germination hours (G) *** | S × G *** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Lei, N.; Xiong, Y.; Liu, Y.; Tong, L.; Wang, F.; Fan, B.; Maesen, P.; Blecker, C. Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality. Foods 2022, 11, 1200. https://doi.org/10.3390/foods11091200
Huang Y, Lei N, Xiong Y, Liu Y, Tong L, Wang F, Fan B, Maesen P, Blecker C. Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality. Foods. 2022; 11(9):1200. https://doi.org/10.3390/foods11091200
Chicago/Turabian StyleHuang, Yatao, Ningyu Lei, Yangyang Xiong, Yanfang Liu, Litao Tong, Fengzhong Wang, Bei Fan, Philippe Maesen, and Christophe Blecker. 2022. "Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality" Foods 11, no. 9: 1200. https://doi.org/10.3390/foods11091200
APA StyleHuang, Y., Lei, N., Xiong, Y., Liu, Y., Tong, L., Wang, F., Fan, B., Maesen, P., & Blecker, C. (2022). Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality. Foods, 11(9), 1200. https://doi.org/10.3390/foods11091200







