Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Emulsion Preparation
2.3. Emulsion Properties
2.3.1. Microstructure Observation
2.3.2. Particle Size Distribution
2.3.3. Interfacial Protein Concentration
2.3.4. Apparent Viscosity
2.4. Whipped Cream Preparation
2.5. Whipped Cream Properties
2.5.1. Low-Field Pulsed NMR (LF-NMR)
2.5.2. Partial Coalescence of Fat
2.5.3. The Optimal Whipping Time (top)
2.5.4. Overrun
2.5.5. Firmness
2.5.6. Microstructure Observation
2.5.7. Serum Loss
2.5.8. Cream Performance after Storage
2.6. Statistical Analysis
3. Results and Discussion
3.1. Emulsion Properties
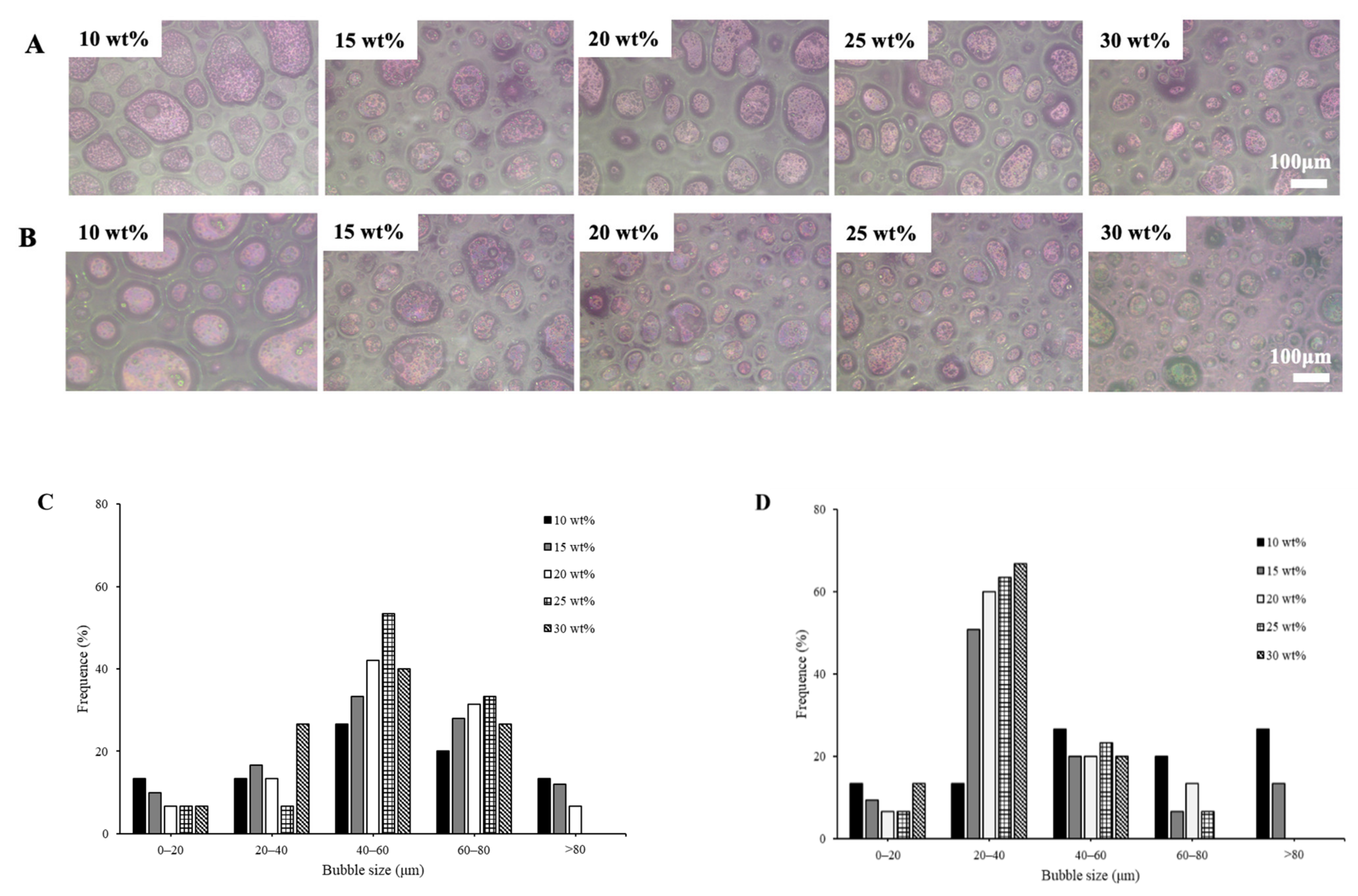
3.1.1. Particle Size Distribution and Microstructure of Emulsion
3.1.2. Interfacial Protein Concentration of Emulsion
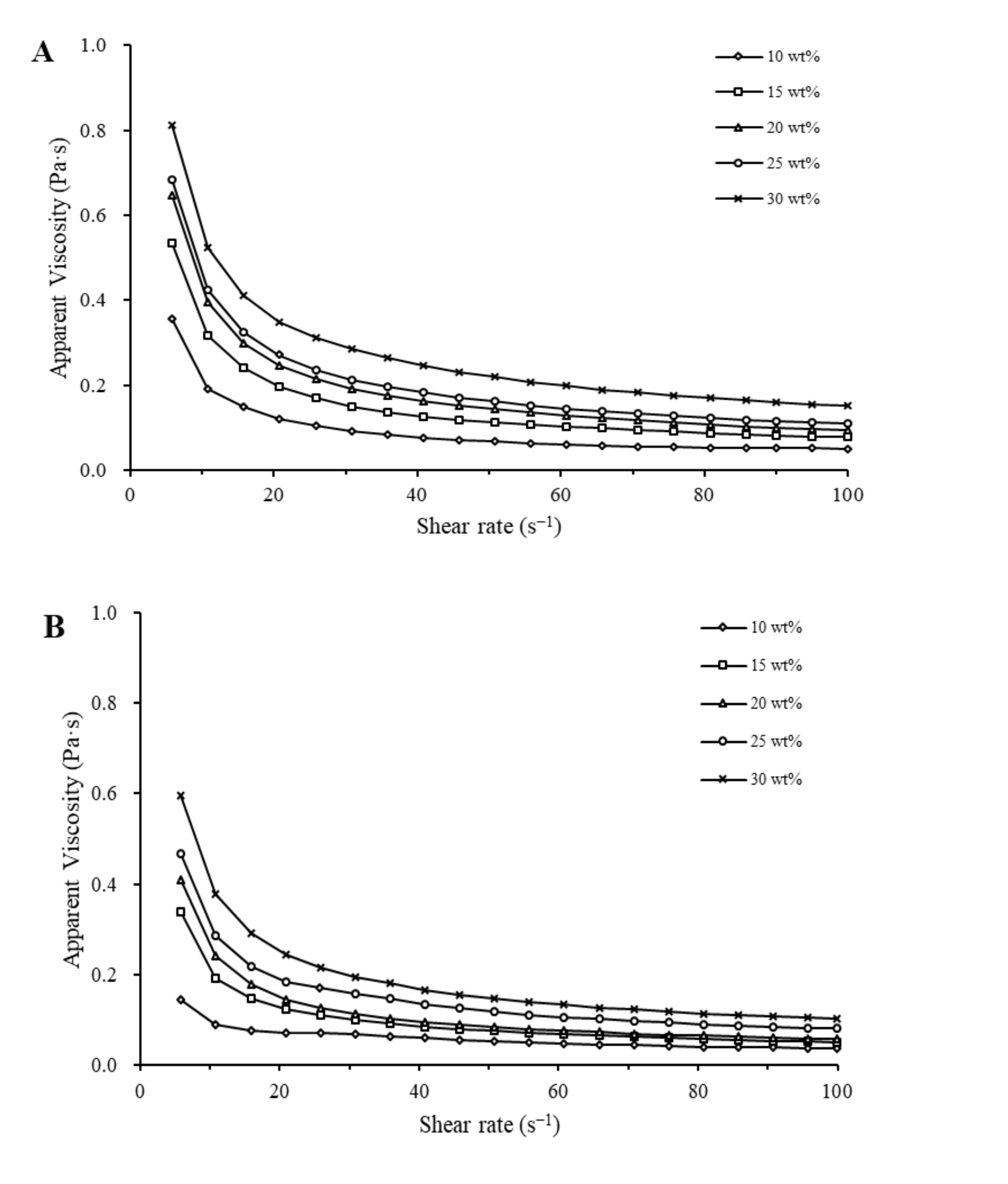
3.1.3. Apparent Viscosity of Emulsion
3.2. Whipped Cream Properties
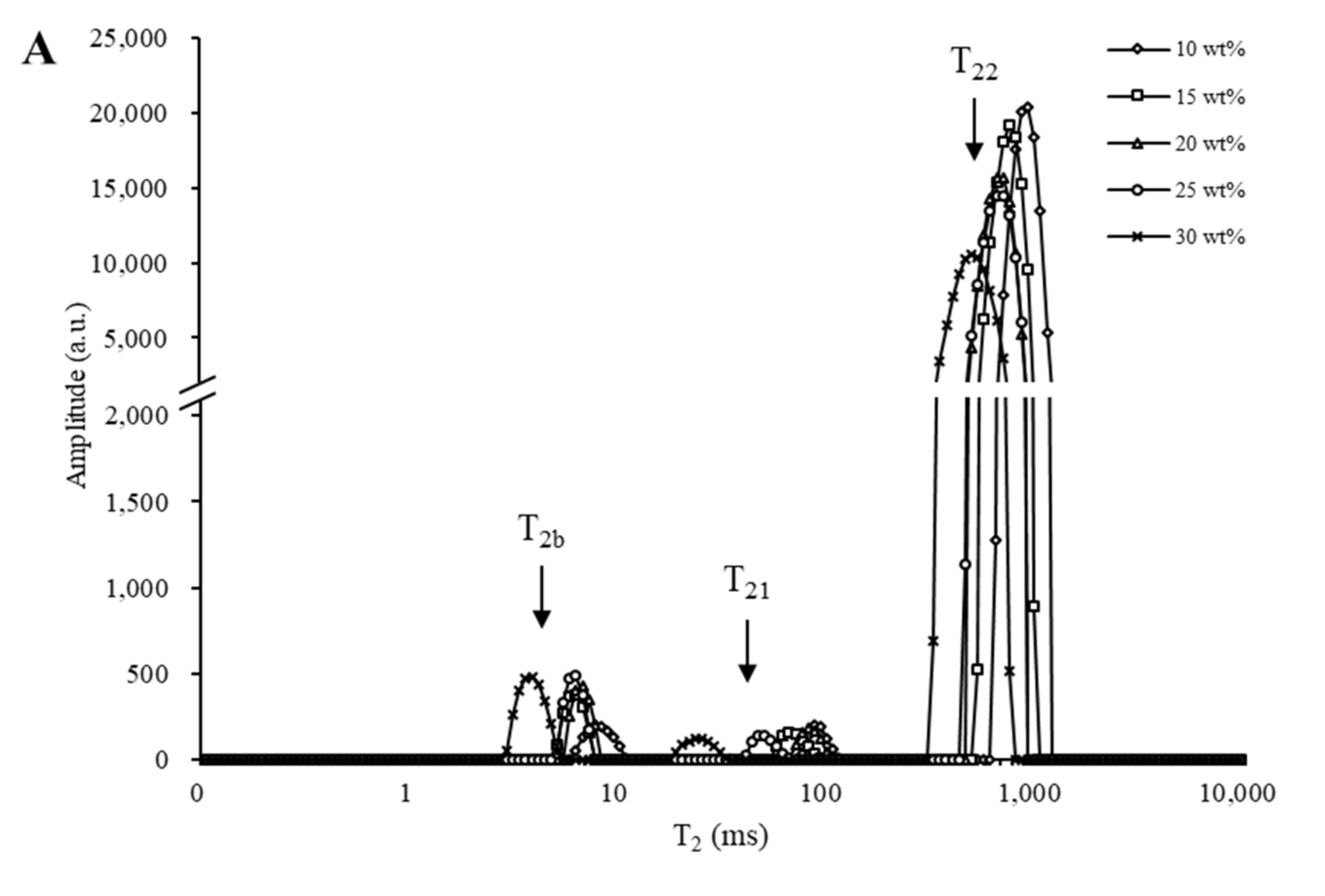
3.2.1. Relaxation Time Analysis
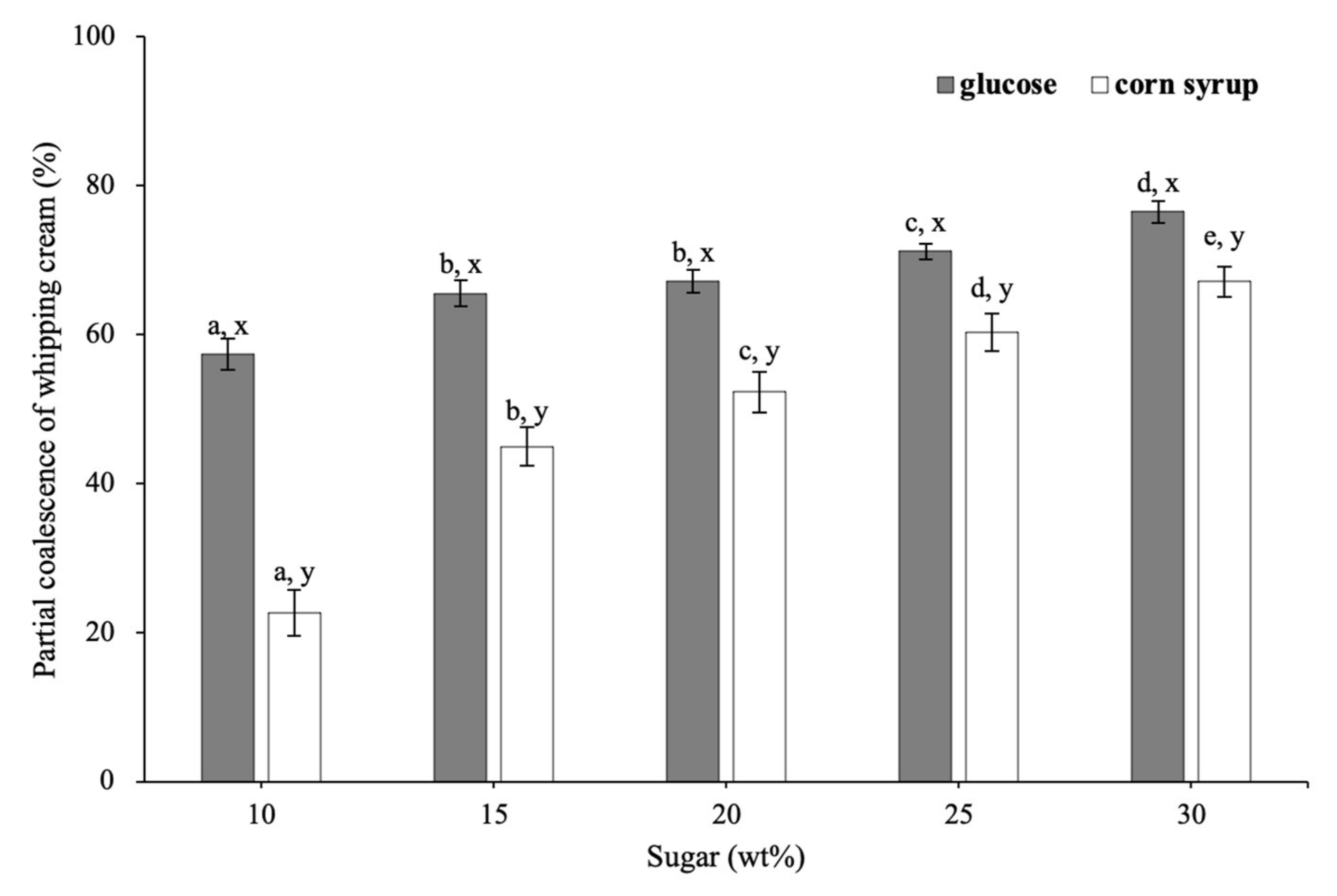
3.2.2. Partial Coalescence of Whipped Cream
3.2.3. Interfacial Protein Concentration of Whipped Cream
3.2.4. The Optimum Whipping Time of Whipped Cream
3.2.5. Overrun of Whipped Cream
3.2.6. Firmness of Whipped Cream
3.2.7. Microstructure of Whipped Cream
3.2.8. Serum Loss and Performance of Whipped Cream after Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Yuan, D.; Li, Y.; Li, M.; Wang, Y.; Li, Y.; Zhang, L. Thermodynamic and whipping properties of milk fat in whipped cream: A Study based on DSC and TD-NMR. Int. Dairy J. 2019, 97, 149–157. [Google Scholar] [CrossRef]
- Zeng, D.; Cai, Y.; Liu, T.; Huang, L.; Liu, P.; Zhao, M.; Zhao, Q. Effect of sucrose ester S370 on interfacial layers and fat crystals network of whipped cream. Food Hydrocoll. 2021, 113, 106541. [Google Scholar] [CrossRef]
- Li, S.; Ye, A.; Singh, H. Seasonal variations in the characteristics of milk fat and the whipping properties of cream. Int. Dairy J. 2022, 127, 105288. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yuan, D.; Wang, Y.; Li, M.; Zhang, L. The effect of caseins on the stability and whipping properties of recombined dairy creams. Int. Dairy J. 2020, 105, 104658. [Google Scholar] [CrossRef]
- Fuller, G.T.; Considine, T.; MacGibbon, A.; Golding, M.; Matia-Merino, L. Effect of Tween emulsifiers on the shear stability of partially crystalline oil-in-water emulsions stabilized by sodium caseinate. Food Biophys. 2018, 13, 80–90. [Google Scholar] [CrossRef]
- Zhao, Q.; Kuang, W.; Long, Z.; Fang, M.; Liu, D.; Yang, B.; Zhao, M. Effect of sorbitan monostearate on the physical characteristics and whipping properties of whipped cream. Food Chem. 2013, 141, 1834–1840. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the Influence on the properties of dispersed Systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Ellis, A.L.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. The effect of sugars on agar fluid gels and the stabilisation of their foams. Food Hydrocoll. 2019, 87, 371–381. [Google Scholar] [CrossRef]
- Clemens, R.A.; Jones, J.M.; Kern, M.; Lee, S.-Y.; Mayhew, E.J.; Slavin, J.L.; Zivanovic, S. Functionality of sugars in foods and health: Functionality of sugars in foods and health. Compr. Rev. Food Sci. Food Safety 2016, 15, 433–470. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.K.; Dickinson, E. Instability and structural change in an aerated system containing egg albumen and invert sugar. Food Hydrocoll. 2005, 19, 111–121. [Google Scholar] [CrossRef]
- Thanasukarn, P.; Pongsawatmanit, R.; McClements, D.J. Impact of fat and water crystallization on the stability of hydrogenated palm oil-in-water emulsions stabilized by whey protein isolate. Colloids Surf. A Physicochem. Eng. Asp. 2004, 246, 49–59. [Google Scholar] [CrossRef]
- Du, Q.; Tang, J.; Xu, M.; Lyu, F.; Zhang, J.; Qiu, Y.; Liu, J.; Ding, Y. Whey protein and maltodextrin-stabilized oil-in-water emulsions: Effects of dextrose equivalent. Food Chem. 2021, 339, 128094. [Google Scholar] [CrossRef]
- Thiel, A.E.; Hartel, R.W.; Spicer, P.T.; Hendrickson, K.J. Coalescence behavior of pure and natural fat droplets characterized via micromanipulation. J. Am. Oil Chem. Soc. 2016, 93, 1467–1477. [Google Scholar] [CrossRef]
- Richardson, A.M.; Tyuftin, A.A.; Kilcawley, K.N.; Gallagher, E.; O’ Sullivan, M.G.; Kerry, J.P. The impact of sugar particle size manipulation on the physical and sensory properties of chocolate brownies. LWT-Food Sci. Technol. 2018, 95, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Sugar reduction in bakery products: Current strategies and sourdough technology as a potential novel approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef]
- Melanson, E.L.; Astrup, A.; Donahoo, W.T. The relationship between dietary fat and fatty acid intake and body weight, diabetes, and the metabolic syndrome. Ann. Nutr. Metab. 2009, 55, 229–243. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Zhao, Q.; Yang, B.; Liu, L. Effect of homogenisation and storage time on surface and rheology properties of whipping cream. Food Chem. 2012, 131, 748–753. [Google Scholar] [CrossRef]
- Fredrick, E.; Heyman, B.; Moens, K.; Fischer, S.; Verwijlen, T.; Moldenaers, P.; Van der Meeren, P.; Dewettinck, K. Monoacylglycerols in dairy recombined cream: II. The effect on partial coalescence and whipping properties. Food Res. Int. 2013, 51, 936–945. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Chen, Y.; Zhao, M.; Sun, W. Gel properties of soy protein isolate modified by lipoxygenase-catalyzed linoleic acid oxidation and their influence on pepsin diffusion and in vitro gastric digestion. J. Agric. Food Chem. 2020, 68, 5691–5698. [Google Scholar] [CrossRef]
- Bolliger, S.; Goff, H.D.; Tharp, B.W. Correlation between colloidal properties of ice cream mix and ice cream. Int. Dairy J. 2000, 10, 303–309. [Google Scholar] [CrossRef]
- Petrut, R.F.; Danthine, S.; Blecker, C. Assessment of partial coalescence in whippable oil-in-water food emulsions. Adv. Colloid Interface Sci. 2016, 229, 25–33. [Google Scholar] [CrossRef]
- Thakur, R.K.; Vial, C.; Djelveh, G. Effect of composition and process parameters on elasticity and solidity of foamed food. Chem. Eng. Processing Process Intensif. 2008, 47, 474–483. [Google Scholar] [CrossRef]
- Liu, P.; Huang, L.; Liu, T.; Cai, Y.; Zeng, D.; Zhou, F.; Zhao, M.; Deng, X.; Zhao, Q. Whipping properties and stability of whipping cream: The impact of fatty acid composition and crystallization properties. Food Chem. 2021, 347, 128997. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Cao, C.; Liu, Y. The partial coalescence behavior of oil-in-water emulsions: Comparison between refrigerated and room temperature storage. Food Chem. 2019, 300, 125219. [Google Scholar] [CrossRef]
- Cornacchia, L.; Roos, Y.H. Lipid and water crystallization in protein-stabilised oil-in-water emulsions. Food Hydrocoll. 2011, 25, 1726–1736. [Google Scholar] [CrossRef]
- Saito, R.; Akiyama, Y.; Tanaka, M.; Ishizu, K. Synthesis of the flower type microgels. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 305–310. [Google Scholar] [CrossRef]
- Degner, B.M.; Chung, C.; Schlegel, V.; Hutkins, R.; McClements, D.J. Factors influencing the freeze-thaw stability of emulsion-based foods. Compr. Rev. Food Sci. Food Safety 2014, 13, 98–113. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Arzeni, C.; Pilosof, A.M.R.; Rubiolo, A.C.; Santiago, L.G. Influence of freezing temperature and maltodextrin concentration on stability of linseed oil-in-water multilayer emulsions. J. Food Eng. 2015, 156, 31–38. [Google Scholar] [CrossRef]
- Ghosh, S.; Cramp, G.L.; Coupland, J.N. Effect of aqueous composition on the freeze-thaw stability of emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 82–88. [Google Scholar] [CrossRef]
- Chronakis, I.S. On the molecular characteristics, compositional properties, and structural-functional mechanisms of maltodextrins: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 599–637. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, D.; Long, Z.; Yang, B.; Fang, M.; Kuang, W.; Zhao, M. Effect of sucrose ester concentration on the interfacial characteristics and physical properties of sodium caseinate-stabilized oil-in-water emulsions. Food Chem. 2014, 151, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes-A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Matia Merino, L. Effect of sugars on the rheological properties of acid caseinate-stabilized emulsion gels. Food Hydrocoll. 2002, 16, 321–331. [Google Scholar] [CrossRef]
- Kanyuck, K.M.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. Temperature influences on network formation of low DE maltodextrin gels. Carbohydr. Polym. 2019, 218, 170–178. [Google Scholar] [CrossRef]
- Hotrum, N.E.; Stuart, M.A.C.; van Vliet, T.; Avino, S.F.; van Aken, G.A. Elucidating the relationship between the spreading coefficient, surface-mediated partial coalescence and the whipping time of artificial cream. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 71–78. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goff, H.D.; Smith, A.K. Texture-structure relationships in foamed dairy emulsions. Food Res. Int. 1996, 29, 1–13. [Google Scholar] [CrossRef]
- van Aken, G.A. Aeration of emulsions by whipping. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 333–354. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Yang, B.; Cui, C. Effect of xanthan gum on the physical properties and textural characteristics of whipped cream. Food Chem. 2009, 116, 624–628. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Z.; Zhang, H.; Wang, J.; Ren, S. Protein particles ameliorate the mechanical properties of highly polyunsaturated oil-based whipped cream: A possible mode of action. Food Hydrocoll. 2020, 99, 105350. [Google Scholar] [CrossRef]
- Dhungana, P.; Truong, T.; Bansal, N.; Bhandari, B. Effect of fat globule size and addition of surfactants on whippability of native and homogenised dairy creams. Int. Dairy J. 2020, 105, 104671. [Google Scholar] [CrossRef]






| Sample | Sugar Level (wt%) | top (s) | Overrun (%) | Firmness (N) | Serum Loss (%) |
|---|---|---|---|---|---|
| Creams-glucose | 10 | 980 ± 15 d,x | 264 ± 13 a,x | 1.25 ± 0.10 a,x | 26.09 ± 1.92 d,x |
| 15 | 611 ± 9 c,x | 276 ± 10 a,x | 2.01 ± 0.09 b,x | 10.26 ± 0.51 c,x | |
| 20 | 447 ± 5 b,x | 335 ± 7 c,x | 2.61 ± 0.06 c,x | 6.31 ± 0.21 b,x | |
| 25 | 433 ± 5 b,x | 327 ± 10 b,c,x | 3.14 ± 0.03 d,x | 0.42 ± 0.15 a,x | |
| 30 | 390 ± 4 a | 306 ± 4 b,x | 3.82 ± 0.10 e,x | 0.23 ± 0.11 a,x | |
| Creams-corn syrup | 10 | 1033 ± 17 e,y | 376 ± 4 e,y | 1.23 ± 0.03 a,x | 35.50 ± 1.13 d,y |
| 15 | 838 ± 13 d,y | 330 ± 6 d,y | 2.02 ± 0.02 b,x | 20.81 ± 1.04 c,y | |
| 20 | 758 ± 10 c,y | 300 ± 6 c,y | 2.60 ± 0.09 c,x | 14.12 ± 0.87 b,y | |
| 25 | 711 ± 12 b,y | 261 ± 4 b,y | 3.06 ± 0.02 d,y | 0.54 ± 0.17 a,x | |
| 30 | 510 ± 14 a,y | 235 ± 9 a,y | 3.19 ± 0.03 d,y | 0.28 ± 0.13 a,x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Zeng, D.; Liu, T.; Cai, Y.; Li, Y.; Zhao, M.; Zhao, Q. Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams. Foods 2022, 11, 1195. https://doi.org/10.3390/foods11091195
Zeng Y, Zeng D, Liu T, Cai Y, Li Y, Zhao M, Zhao Q. Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams. Foods. 2022; 11(9):1195. https://doi.org/10.3390/foods11091195
Chicago/Turabian StyleZeng, Yongchao, Di Zeng, Tongxun Liu, Yongjian Cai, Yonghao Li, Mouming Zhao, and Qiangzhong Zhao. 2022. "Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams" Foods 11, no. 9: 1195. https://doi.org/10.3390/foods11091195
APA StyleZeng, Y., Zeng, D., Liu, T., Cai, Y., Li, Y., Zhao, M., & Zhao, Q. (2022). Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams. Foods, 11(9), 1195. https://doi.org/10.3390/foods11091195








