Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Micro-Organisms
2.2. Raw Materials Extraction
2.3. Chemical Composition of Raw Materials
2.4. Determination of β-Carotene, Vitamin C, and Tocopherol Contents
2.5. Determination of Antioxidant Activity
2.6. Determination of Fatty Acid Contents
2.7. Determination of Bioactive Content Using the FTIR
2.8. Determination of the Antimicrobial Effect
2.8.1. Antibacterial Effect
2.8.2. Antifungal Effect
2.9. Cytotoxicity and Anti-Cytotoxic Effect of Utilized Extracts
2.10. Determination of Mycotoxin
2.11. Snack-Bar Manufacturing as a Model Application
2.12. Evaluation of the Snack-Bar Characteristics
2.13. Calorimetric Estimation of β-Glucan Content
2.14. Simulated Experiment
2.15. Statistical Analysis
3. Results
3.1. Chemical Composition of Raw Materials
3.2. Fatty Acids Composition of Raw Materials
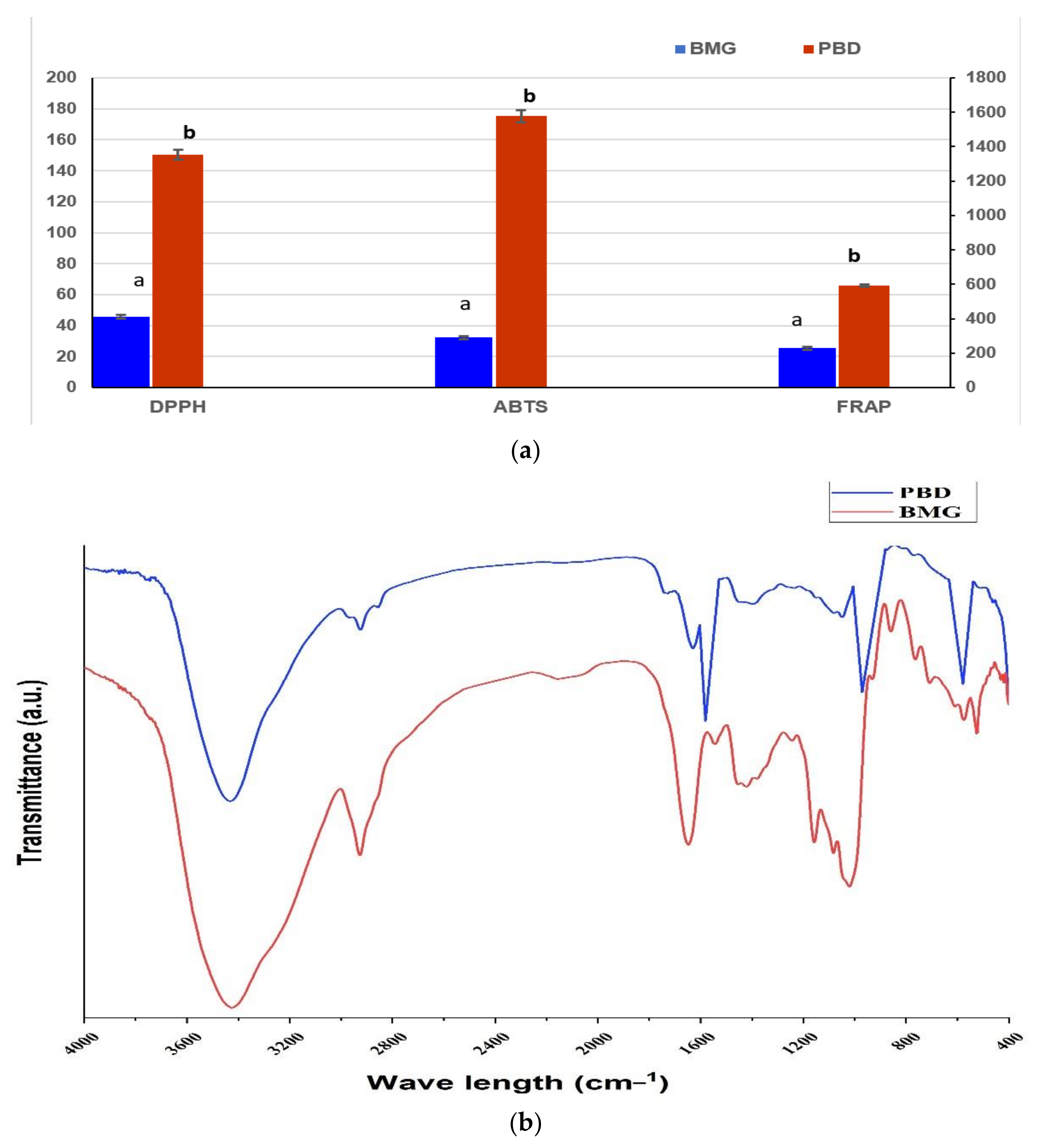
3.3. Antioxidant Activity and Active Groups of Raw Materials
3.4. Determination of Phenolic Fractions
3.5. Determination of Antimicrobial Activity
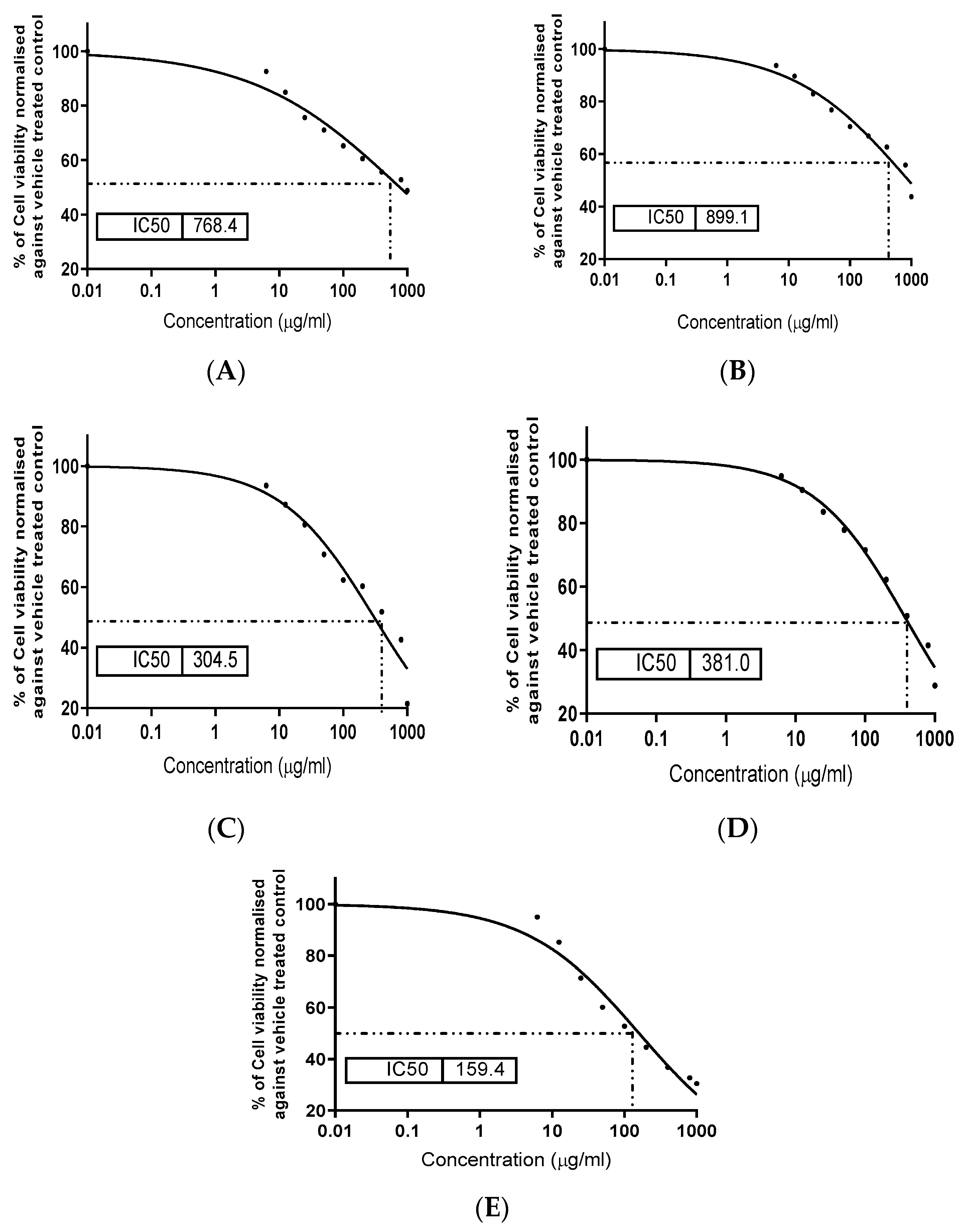
3.6. Determination of Extracts Cytotoxicity
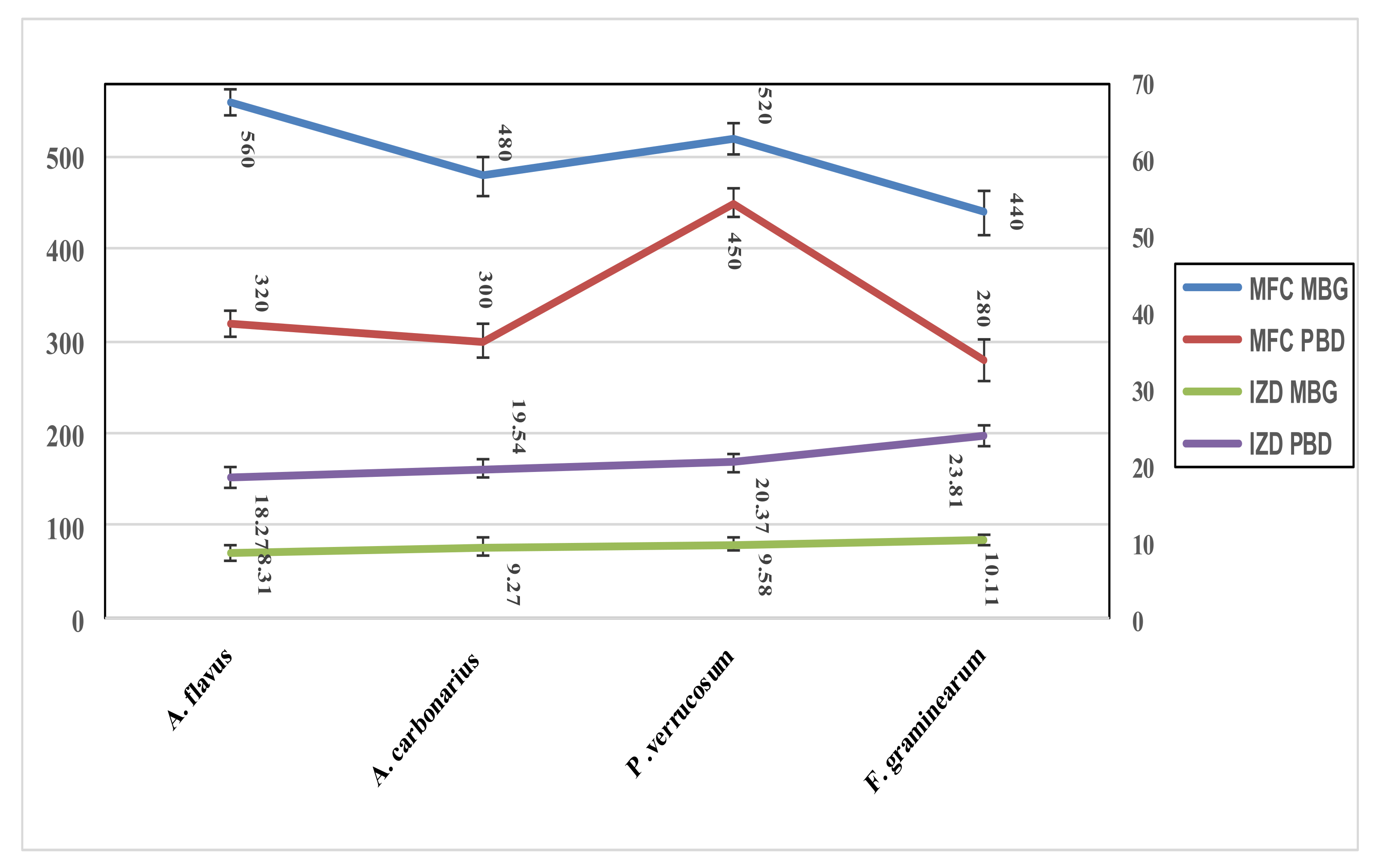
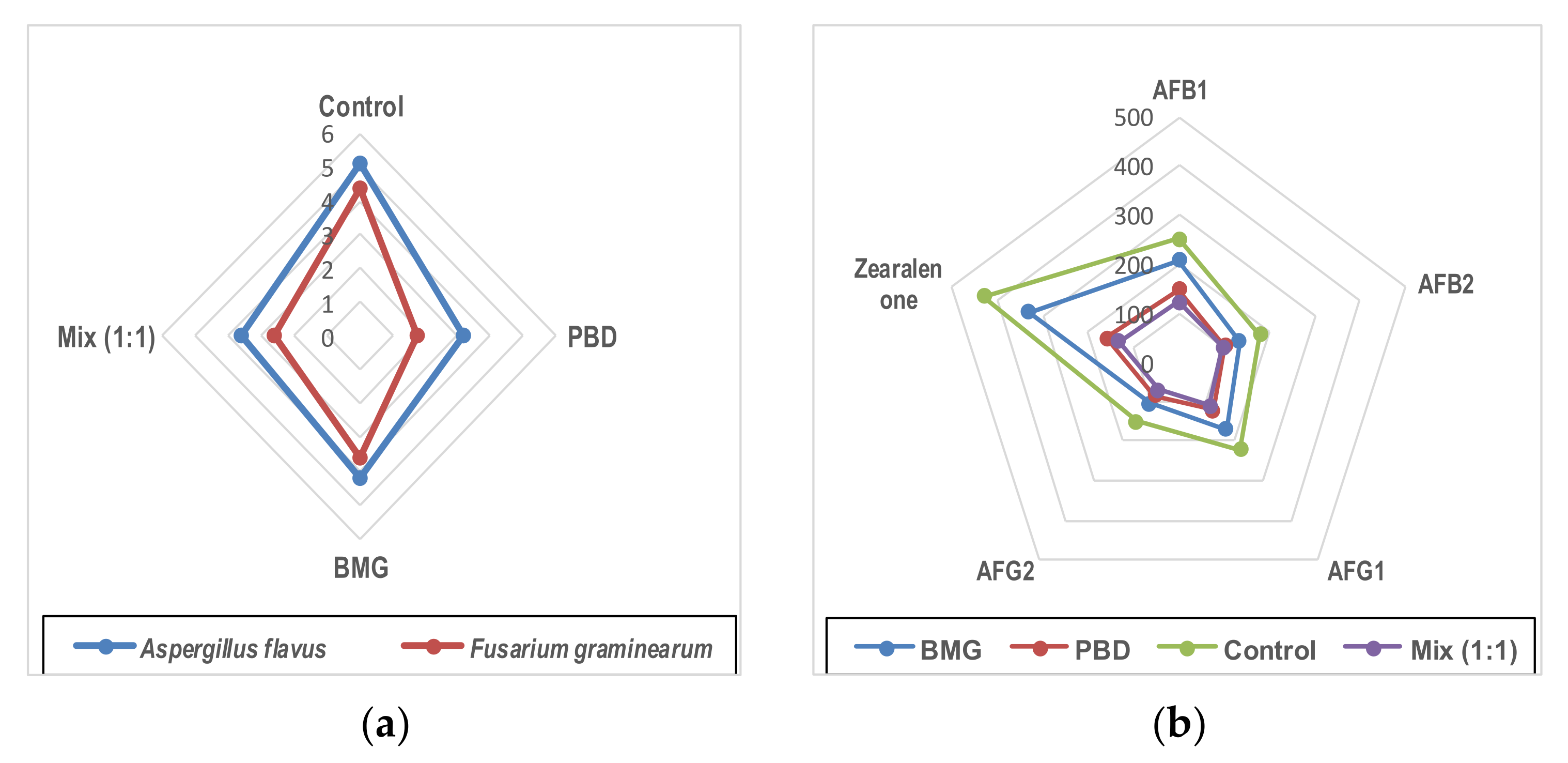
3.7. Determination of Extracts Role as an Anti-Mycotoxigenic Preservative
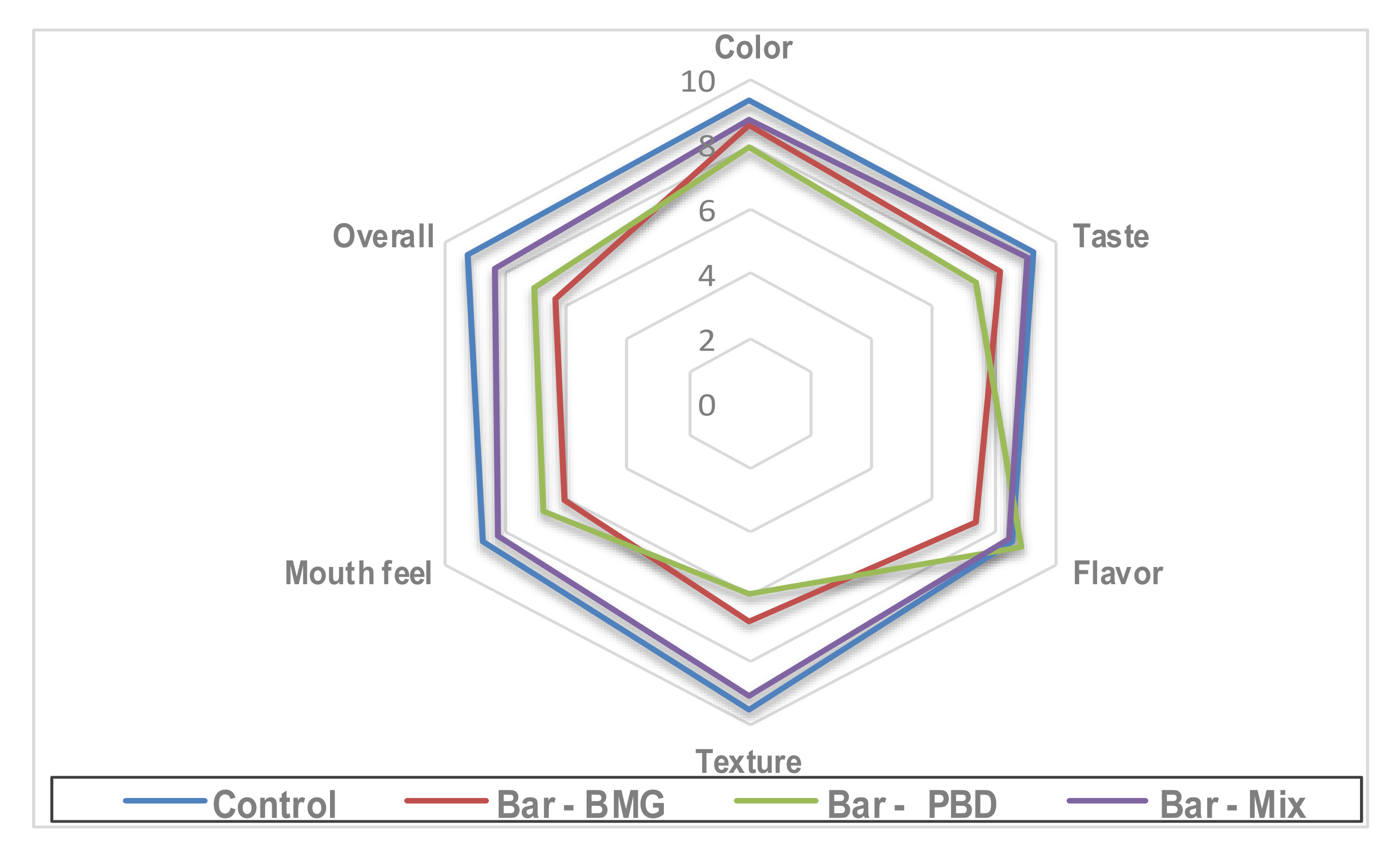
3.8. Characterization of Snack Bar Manufactured Targeted Materials
3.9. Simulated Experiment for Bar Resistance of Fungal Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unnevehr, L.J. Food safety issues and fresh food product exports from LDCs. Agric. Econ. 2000, 23, 231–240. [Google Scholar] [CrossRef]
- Da Rocha, M.E.B.; Freire, F.D.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Jayashree, T.; Subramanyam, C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free. Radic. Biol. Med. 2000, 29, 981–985. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; pp. 1–85. [Google Scholar] [CrossRef]
- Collins, A.R. Oxidative DNA damage, antioxidants, and cancer. BioEssays 1999, 21, 238–246. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Qingming, Y.; Xianhui, P.; Weibao, K.; Hong, Y.; Yidan, S.; Li, Z.; Yanan, Z.; Yuling, Y.; Lan, D.; Guoan, L. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010, 118, 84–89. [Google Scholar] [CrossRef]
- Duke, S.H.; Henson, C.A. A Comparison of Barley Malt Quality Measurements and Malt Sugar Concentrations. J. Am. Soc. Brew. Chem. 2008, 66, 151–161. [Google Scholar] [CrossRef]
- Aniche, G.N.; Palmer, G.H. Development of amylolytic activities in sorghum and barley malt. J. Inst. Brew. 1990, 96, 377–379. [Google Scholar] [CrossRef]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind. Crops Prod. 2011, 34, 1523–1527. [Google Scholar] [CrossRef]
- Heber, D.; Schulman, R.N.; Seeram, N.P. Pomegranates: Ancient Roots to Modern Medicine; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Casanova, E.; García-Mina, J.M.; Calvo, M.I. Antioxidant and Antifungal Activity of Verbena officinalis L. Leaves. Plant Foods Hum. Nutr. 2008, 63, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Evans, D.E.; Wallace, W.; Lance, R.C.M.; MacLeod, L.C. Measurement of Beta-amylase in Malting Barley (Hordeum vulgareL.). II. The Effect of Germination and Kilning. J. Cereal Sci. 1997, 26, 241–250. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, C. Determination of Fat Content. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–37. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Shehata, M.G.; Badr, A.N.; Gromadzka, K.; Stępień, L. The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Brunt, K.; Sanders, P. Improvement of the AOAC 2009.01 total dietary fibre method for bread and other high starch containing matrices. Food Chem. 2013, 140, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- Nielsen, S.S. Determination of Moisture Content. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–27. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Rogoziński, T.; Perkowski, J. Contamination of pine and birch wood dust with microscopic fungi and determination of its sterol contents. Arch. Ind. Hyg. Toxicol. 2017, 68, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of methodology to analyze ascorbic and dehydroascorbic acid in vegetables. Química Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Noah Badr, A.; Shehata, M.G. Characterization of olive oil by-products: Antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Šimić, G.; Horvat, D.; Dvojković, K.; Abičić, I.; Vuletić, M.V.; Tucak, M.; Lalić, A. Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J. Food Sci. 2017, 35, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Fogarasi, A.-L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, É.; Hegyesné-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef] [Green Version]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Vajrabhaya, L.-O.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qin, S.; Liu, Z.; Zheng, Y.; Han, L.; Zhang, M.; Luo, N.; Gu, Y.; Zhang, Y.; Gu, N. Synergistic Anti-Proliferation and Anti-Angiogenesis Effects of Sevacizumab on Hepatocellular Carcinoma Cells in Combination with Chemotherapy. Res. Sq. 2021, 3, 1–24. [Google Scholar] [CrossRef]
- Corrigan, V.; Hedderley, D.; Harvey, W. Modeling the Shelf Life of Fruit-Filled Snack Bars Using Survival Analysis and Sensory Profiling Techniques. J. Sens. Stud. 2012, 27, 403–416. [Google Scholar] [CrossRef]
- Grillo, O.; Rizzo, V.; Saccone, R.; Fallico, B.; Mazzaglia, A.; Venora, G.; Muratore, G. Use of image analysis to evaluate the shelf life of bakery products. Food Res. Int. 2014, 62, 514–522. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1→3),(1→4)-β-D-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Badr, A.N.; Ali, H.S.; Abd-Elsalam, I.S.; Hussein, A.M.S.; Al-Khalifa, A.S. Anti-mycotoxigenic properties of “Fino” using the modified zinc-yeast. CyTA J. Food 2019, 17, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Edge, M.S.; Jones, J.M.; Marquart, L. A New Life for Whole Grains. J. Am. Diet. Assoc. 2005, 105, 1856–1860. [Google Scholar] [CrossRef]
- Huang, G.L. Extraction of Two Active Polysaccharides from the Yeast Cell Wall. Z. Für Nat. C 2008, 63, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Razzaq, H.A.A.; Gomez d’Ayala, G.; Santagata, G.; Bosco, F.; Mollea, C.; Larsen, N.; Duraccio, D. Bioactive films based on barley β-glucans and ZnO for wound healing applications. Carbohydr. Polym. 2021, 272, 118442. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Mousavi Khaneghah, A.; Borges, L.L.; Bonato, M.A.; Fakhri, Y.; Barbalho, C.B.; Barbalho, R.L.C.; Corassin, C.H.; Oliveira, C.A.F. In vitro and in vivo capacity of yeast-based products to bind to aflatoxins B1 and M1 in media and foodstuffs: A systematic review and meta-analysis. Food Res. Int. 2020, 137, 109505. [Google Scholar] [CrossRef]
- Hathout, A.S.; Aly, S.E. Biological detoxification of mycotoxins: A review. Ann. Microbiol. 2014, 64, 905–919. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Taş, A.A. Antioxidant activity of some foods containing phenolic compounds. Int. J. Food Sci. Nutr. 2001, 52, 501–508. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Microbiology 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, S.M.; Badr, A.N.; Seif, F.A.H.A.; Ali, S.M.; Hassan, R.A. Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J. Biol. Sci. 2018, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process Preserv. 2021, 45, e15112. [Google Scholar] [CrossRef]
- Abu-Sree, Y.H.; Abdel-Fattah, S.M.; Abdel-Razek, A.G.; Badr, A.N. Neoteric approach for peanuts biofilm using the merits of Moringa extracts to control aflatoxin contamination. Toxicol. Rep. 2021, 8, 1685–1692. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G. Prospective antimycotoxigenic action of wild Opuntia ficus-indica by-products. Czech J. Food Sci. 2020, 38, 308–314. [Google Scholar] [CrossRef]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Naeem, M.A. Protective efficacy using Cape- golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Youssef, M.; Abdel-Razek, A.G.; Shehata, M.G.; Hassanien, M.M.; Amra, H. Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egypt. J. Chem. 2021, 64, 285–298. [Google Scholar] [CrossRef]
- Amenu, D. Antimicrobial activity of medicinal plant extracts and their synergistic effect on some selected pathogens. Am. J. Ethnomed. 2014, 1, 18–29. [Google Scholar]
| Proximate Analysis | ||||||
| Moisture (%) | Protein (%) | Fat (%) | Ash (%) | Carbohydrates (%) | Crude fiber (%) | |
| BMG | 18.41 ± 1.34 a | 10.57 ± 0.84 a | 1.57 ± 0.37 a | 3.68 ± 0.87 a | 63.75 ± 1.27 a | 13.25 ± 0.41 a |
| PBD | 7.66 ± 1.23 b | 6.54 ± 1.05 b | 0.81 ± 0.05 b | 3.97 ± 0.67 a | 56.1 ± 2.14 b | 16.32 ± 2.73 b |
| Fiber fractions | ||||||
| Cellulose | Hemi cellulose | Lignin | NDF | ADF | ADL | |
| BMG | 9.11 ± 0.63 e | 27.67 ± 0.36 e | 1.31 ± 0.11 e | 39.02 ± 0.54 e | 11.35 ± 0.22 e | 2.25 ± 0.77 e |
| PBD | 12.32 ± 1.63 f | 10.46 ± 2.13 f | 9.67 ± 0.41 f | 26.41 ± 2.18 f | 15.95 ± 2.54 f | 3.63 ± 0.84 e |
| Mineral content (mg/kg) | ||||||
| Ca | Fe | P | Mg | Zn | Se | |
| BMG | 427.89 ± 1.21 j | 27.11 ± 0.13 j | 193.17 ± 1.31 j | 111.15 ± 1.42 j | 25.16 ± 0.31 j | 0.69 ± 0.12 j |
| PBD | 1143.2 ± 7.24 k | 63.15 ± 2.37 k | 275.14 ± 4.61 k | 655.37 ± 8.41 k | 4.77 ± 0.82 k | 0.11 ± 0.04 k |
| Tocopherol, vitamin C, and β-carotene contents (mg/100 g) | ||||||
| Vitamin C | α-tocopherol | β-tocopherol | γ-tocopherol | δ-tocopherol | β-carotene | |
| BMG | 5.71 ± 1.17 r | 0.84 ± 0.74 r | 0.081 ± 0.05 | 0.54 ± 0.023 r | 0.93 ± 0.057 r | 0.17 ± 0.008 r |
| PBD | 136.3 ± 2.58 u | 179.92 ± 1.75 u | ND | 99.73 ± 0.87 u | 29.64 ± 0.58 u | 14.43 ± 0.36 u |
| Fatty Acids | BMG | PBD | Mix | |
|---|---|---|---|---|
| (%) | (%) | (1:1) | ||
| C12:0 | Lauric | 4.57 ± 0.001 | ND | ND |
| C14:1 | Myristoleic | ND | ND | ND |
| C16:0 | Palmetic | 15.14 ± 0.09 a | 2.33 ± 0.84 b | 3.52 ± 0.37 b |
| C16:1 | Palmitoleic | 1.74 ± 0.22 a | ND | 0.31 ± 0.02 b |
| C18:0 | Stearic | 1.63 ± 0.005 a | 1.37 ± 0.54 b | 1.60 ± 0.41 a |
| C18:1 | Oleic | 14.28 ± 0.67 a | 5.27 ± 0.76 b | 5.36 ± 0.89 b |
| C18:2 n-6 | linoleic | 50.85 ± 1.08 a | 5.05 ± 0.81 b | 9.35 ± 1.12 c |
| C18:3 n-3 | linolenic | 3.59 ± 0.34 a | 0.54 ± 0.05 b | 0.61 ± 0.05 b |
| C18:3 n-5 | punicic | ND | 85.44 ± 2.23 a | 74.69 ± 1.63 a |
| C20:0 | Arachidic | 0.72 ± 0.002 a | ND | 1.93 ± 0.14 b |
| C20:1 | Gadoleic | 1.17 ± 0.004 a | ND | 0.48 ± 0.03 b |
| C20:5 n-3 | Eicosapentaenoic | 6.31 ± 0.002 a | ND | 2.15 ± 0.87 b |
| Significant oil parameters | ||||
| SFA | 22.06 ± 3.16 a | 3.70 ± 1.05 b | 7.05 ± 1.88 c | |
| MUFA | 17.19 ± 2.54 a | 5.27 ± 1.66 b | 6.15 ± 2.02 b | |
| PUFA | 60.75 ± 5.02 a | 91.03 ± 5.73 b | 86.8 ± 4.79 b | |
| Phenolic Acids | BMG | PBD | Flavonoids | BMG | PBD |
|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||
| Gallic acid | 9.24 ± 1.05 a | 91.13 ± 2.61 b | Catechin | 1.05 ± 0.84 d | 16.94 ± 2.28 e |
| Ellagic acid | ND | 39.21 ± 5.42 | Catechol | ND | ND |
| Protocatechuic acid | 7.62 ± 1.21 a | 11.28 ± 1.37 b | Epicatechin | ND | ND |
| trans-Ferulic acid | ND | 16.17 ± 1.91 | Rutin | ND | 8.42± 1.08 |
| Cinnamic acid | ND | 43.16 ± 1.05 | Apigenin | 0.74 ± 0.16 d | 29.41 ± 2.27 e |
| Syringic acid | 3.08 ± 0.67 a | 17.39 ± 2.51 b | Quercetin | 0.38 ± 0.09 d | 28.34 ± 1.54 e |
| Caffeic acid | 4.18 ± 0.54 a | 18.44 ± 2.74 b | Luteolin | ND | 18.52 ± 1.05 |
| Ferulic acid | 15.11 ± 2.16 a | 8.41 ± 1.34 b | Hesperidin | ND | 3.55 ± 1.28 |
| p-Hydroxybenzoic acid | 5.13 ± 0.74 a | 2.88 ± 0.73 b | Naringenin | ND | 13.53 ± 1.44 |
| p-Coumaric acid | 22.61 ± 2.37 a | 20.08 ± 1.41 b | Kaempferol | ND | 4.21 ± 0.81 |
| Vanillic acid | 5.06 ± 0.33 a | 34.17 ± 1.02 b | Chrysin | ND | ND |
| MIC (mg/L) | MBC (mg/L) | IZD (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BMG | PBD | Mix (1:1) | BMG | PBD | Mix (1:1) | BMG | PBD | Mix (1:1) | |
| Gram-positive bacteria | |||||||||
| Bacillus cereusEMCC 1080 | 180 | 100 | 80 | 220 | 150 | 140 | 7.34 ± 0.54 c | 12.16 ± 1.02 b | 14.9 ± 1.34 a |
| Streptomyces avermitilisATCC 31267 | 170 | 80 | 50 | 200 | 150 | 150 | 7.61 ± 0.39 b | 13.84 ± 0.93 a | 15.1 ± 1.66 a |
| Micrococcus luteus ATCC 15176 | 180 | 90 | 80 | 220 | 160 | 150 | 8.12 ± 0.23 b | 12.55 ± 1.1 a | 14.6 ± 1.41 a |
| Staphylococcus aureusATCC 13565 | 200 | 80 | 70 | 220 | 150 | 160 | 7.44 ± 0.87 b | 12.71 ± 1.23 a | 13.8 ± 1.19 a |
| Gram-negative bacteria | |||||||||
| Escherichia coliATCC 51659 | 250 | 90 | 90 | 280 | 180 | 160 | 5.37 ± 0.97 c | 10.97 ± 0.94 b | 12.05 ± 1.05 a |
| Salmonella typhiATCC 15566 | 250 | 70 | 70 | 280 | 200 | 180 | 5.08 ± 1.02 b | 11.02 ± 0.81 a | 12.37 ± 1.15 a |
| Pseudomonas aeruginosaNRRL 272 | 230 | 90 | 90 | 260 | 180 | 180 | 4.88 ± 0.83 c | 11.54 ± 0.79 b | 14.21 ± 1.02 a |
| Klebsiella pneumonia | 230 | 90 | 90 | 280 | 180 | 160 | 5.67 ± 0.67 b | 11.81 ± 0.88 a | 12.4 ± 1.21 a |
| LMD 7726 | |||||||||
| L * | A * | B * | ΔE | aw | Hardness (g) | Stickiness (g) | |
|---|---|---|---|---|---|---|---|
| Control | 76.3 ± 0.87 a | 4.17 ± 0.54 a | 23.37 ± 0.87 a | -- | 0.29 | 2469.3 ± 0.54 a | 1205.8 ± 1.02 a |
| Bar—BMG | 49.77 ± 1.02 b | 10.39 ± 1.05 b | 13.62 ± 0.97 b | 28.94 ± 1.02 a | 0.36 | 2671.6 ± 1.41 b | 1681.4 ± 0.71 b |
| Bar—PBD | 36.61 ± 0.62 c | 13.29 ± 0.74 c | 17.51 ± 1.08 c | 41.14 ± 0.81 c | 0.34 | 2089.4 ± 1.37 c | 1802.6 ± 0.84 c |
| Bar—Mix | 46.24 ± 0.82 b | 11.94 ± 0.88 b | 12.74 ± 0.69 b | 32.82 ± 0.73 b | 0.31 | 2254.9 ± 0.89 d | 1354.6 ± 1.08 d |
| Days | 1 | 3 | 5 | 7 | 9 | 11 | 15 |
|---|---|---|---|---|---|---|---|
| Inoculated bar | 7.9 × 102 | 1.6 × 103 | 4.4 × 103 | 7.9 × 104 | 1.4 × 105 | 5.7 × 105 | 9.8 × 106 |
| Bar-BMG | 1.4 × 102 | 2.8 × 102 | 5.6 × 102 | 2.1 × 103 | 6.8 × 103 | 1.9 × 104 | 5.2 × 104 |
| Bar-PBD | 0.16 × 102 | 0.41 × 102 | 0.89 × 102 | 1.3 × 102 | 7.3 × 102 | 2.3 × 103 | 6.9 × 103 |
| Bar-Mix | 0.54 × 102 | 0.96 × 102 | 1.05 × 102 | 3.1 × 102 | 8.9 × 102 | 3.5 × 103 | 1.04 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khojah, E.Y.; Badr, A.N.; Mohamed, D.A.; Abdel-Razek, A.G. Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes. Foods 2022, 11, 119. https://doi.org/10.3390/foods11010119
Khojah EY, Badr AN, Mohamed DA, Abdel-Razek AG. Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes. Foods. 2022; 11(1):119. https://doi.org/10.3390/foods11010119
Chicago/Turabian StyleKhojah, Ebtihal Yaqoob, Ahmed Noah Badr, Dalia Amin Mohamed, and Adel Gabr Abdel-Razek. 2022. "Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes" Foods 11, no. 1: 119. https://doi.org/10.3390/foods11010119
APA StyleKhojah, E. Y., Badr, A. N., Mohamed, D. A., & Abdel-Razek, A. G. (2022). Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes. Foods, 11(1), 119. https://doi.org/10.3390/foods11010119