Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Tea Samples
2.2. Preparation of Green Tea Infusions
2.3. Extraction of Green Tea Extracts
2.3.1. Conventional Solid–Liquid Extraction
2.3.2. Solid–Liquid Extraction with Soxhlet Apparatus
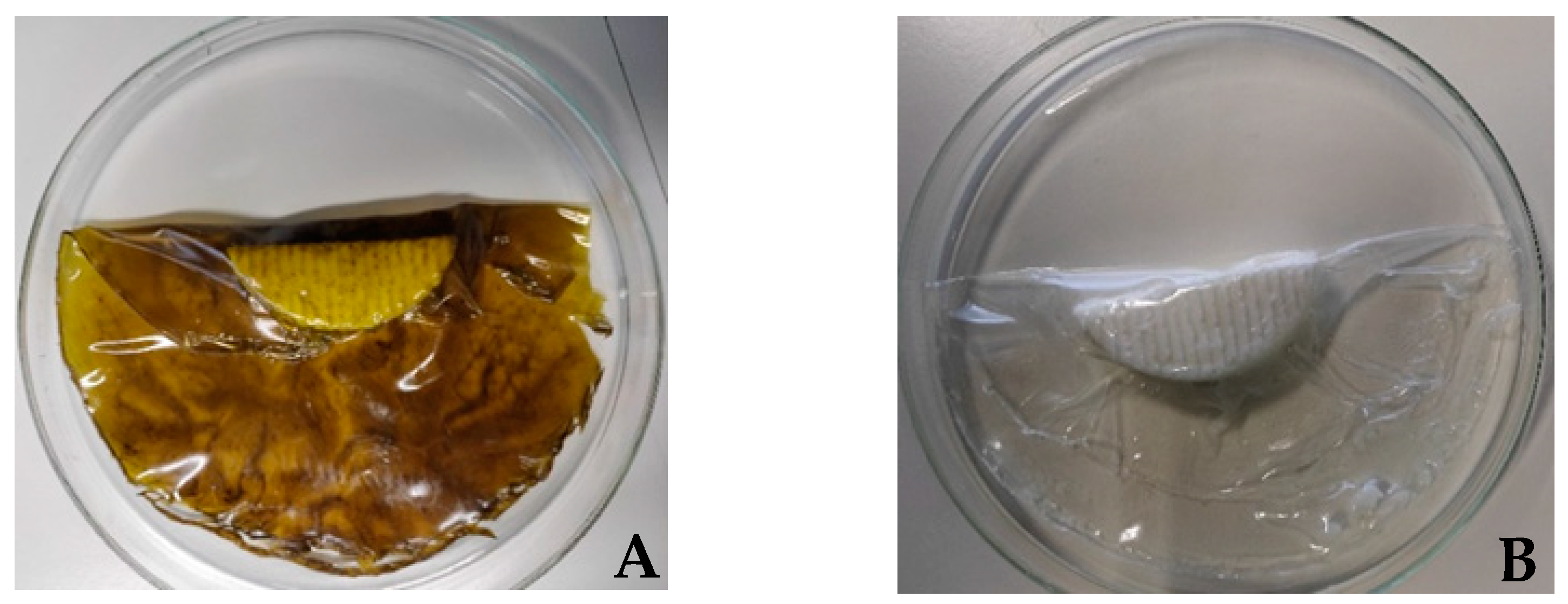
2.4. Preparation of Whey-Based Protein Films
2.5. Evaluation of the Antioxidant Properties
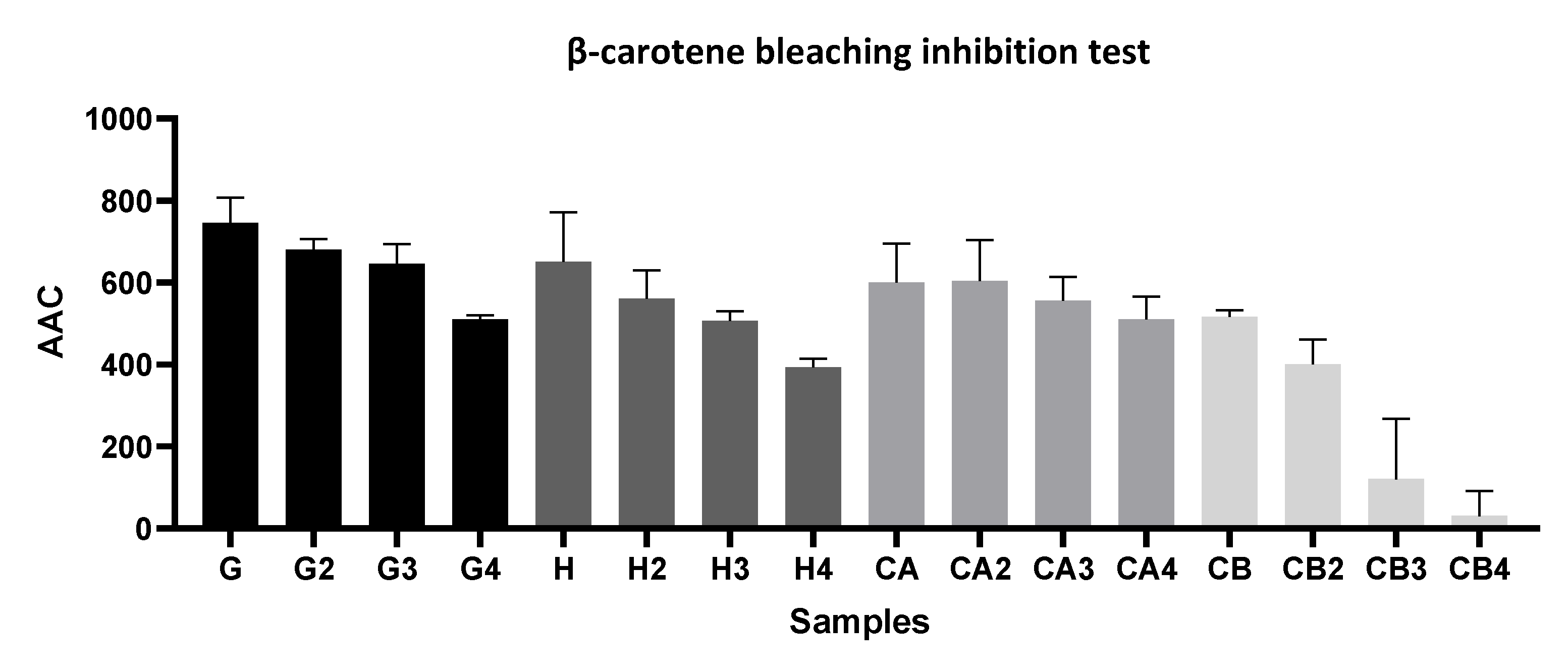
2.5.1. β-Carotene Bleaching Assay
2.5.2. DPPH Radical-Scavenging Method
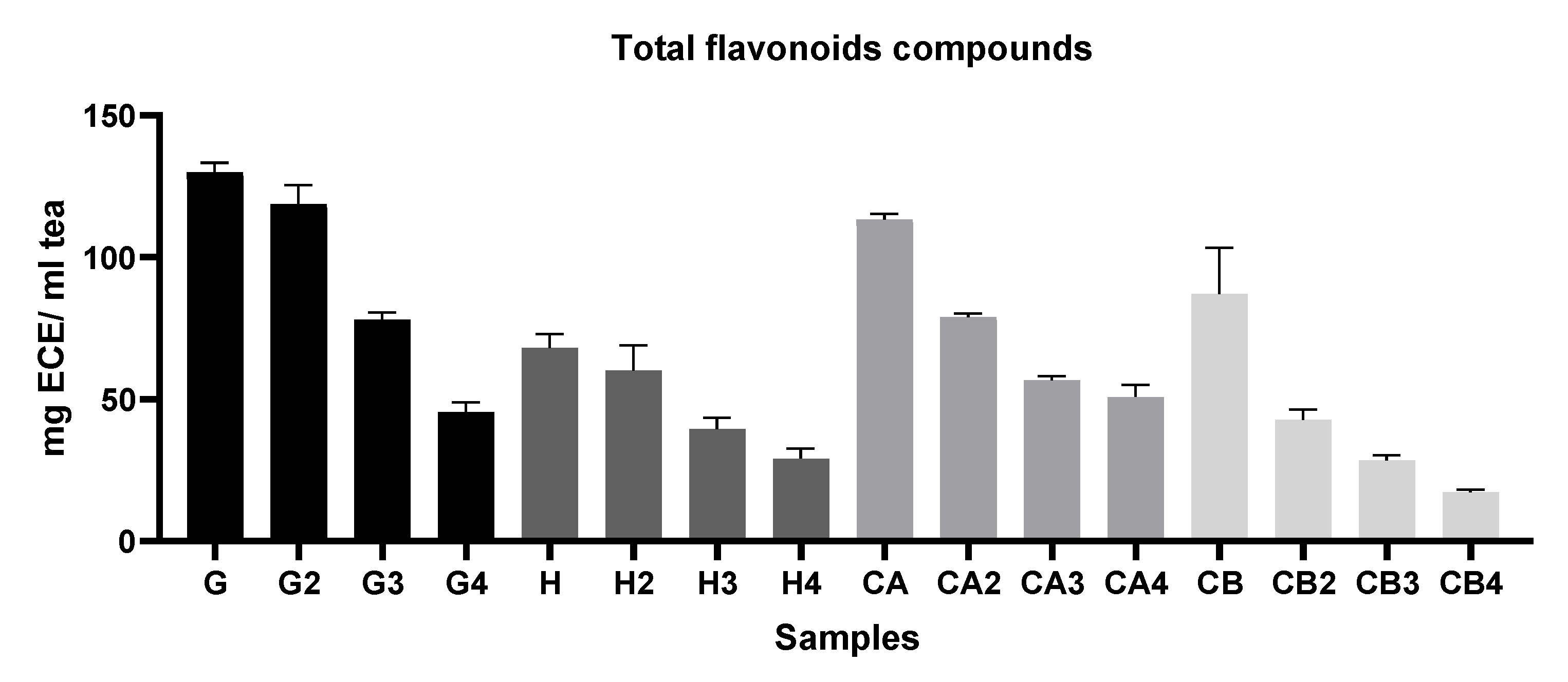
2.5.3. Determination of Total Flavonoid Content (TFC)
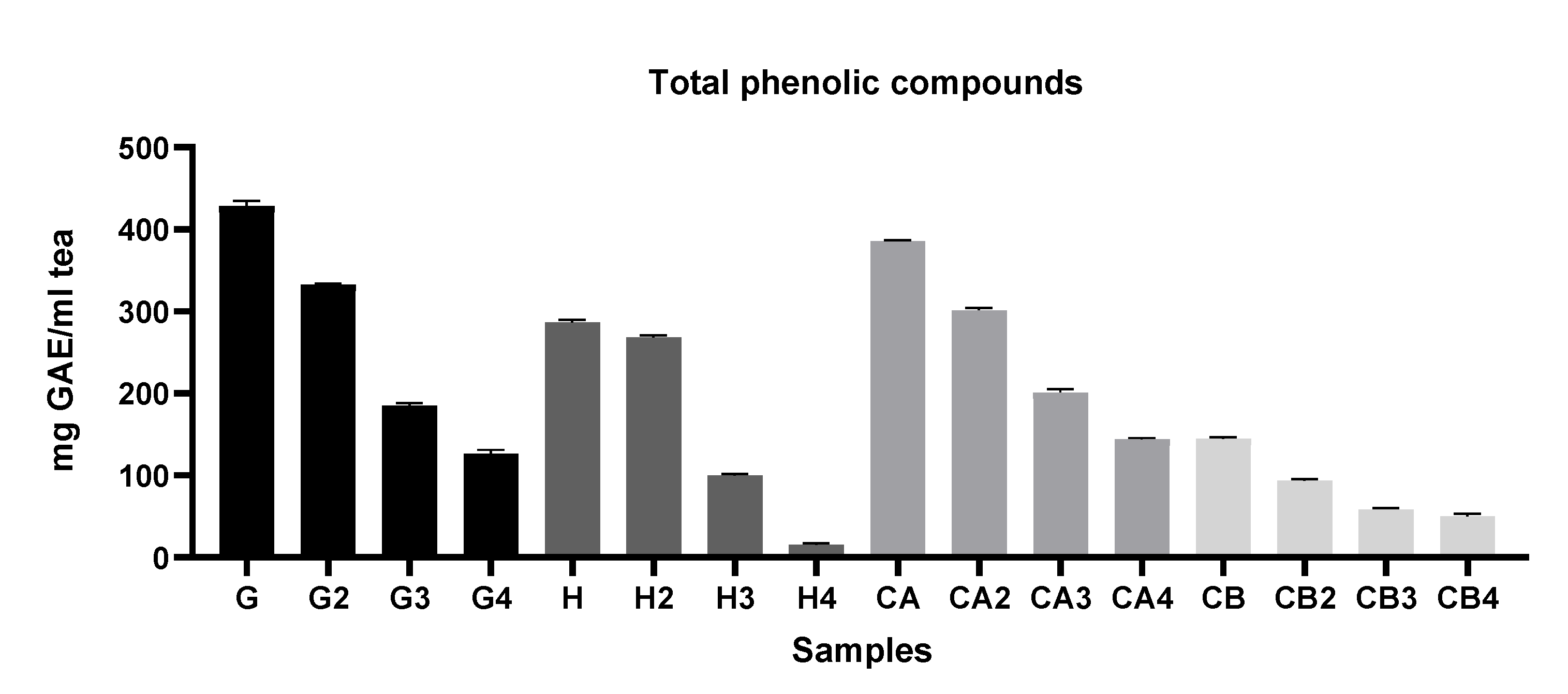
2.5.4. Determination of Total Phenolic Compounds (TPC)
2.6. Fresh Cheese Samples
2.7. Evaluation of the Lipid Oxidation Status (TBARS Assay)
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the In Vitro Antioxidant Capacity, TPC, and TFC
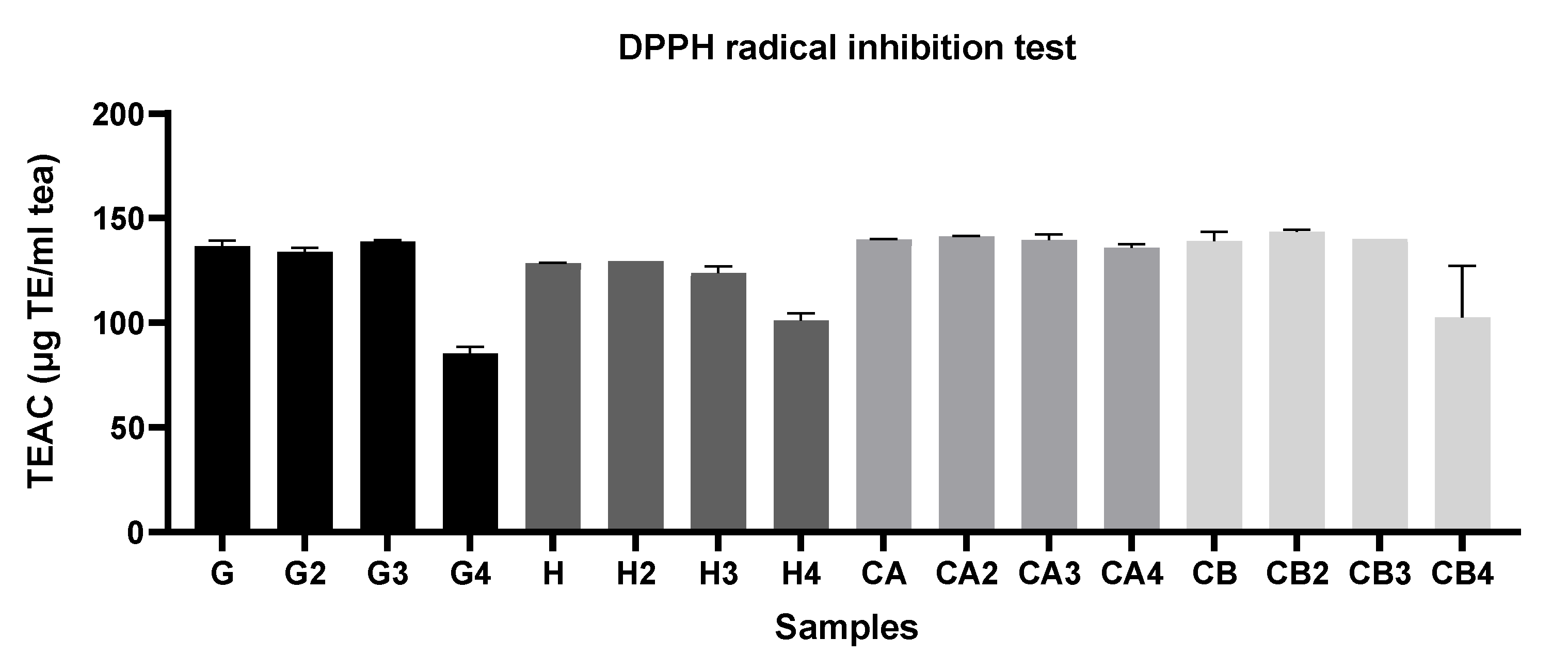
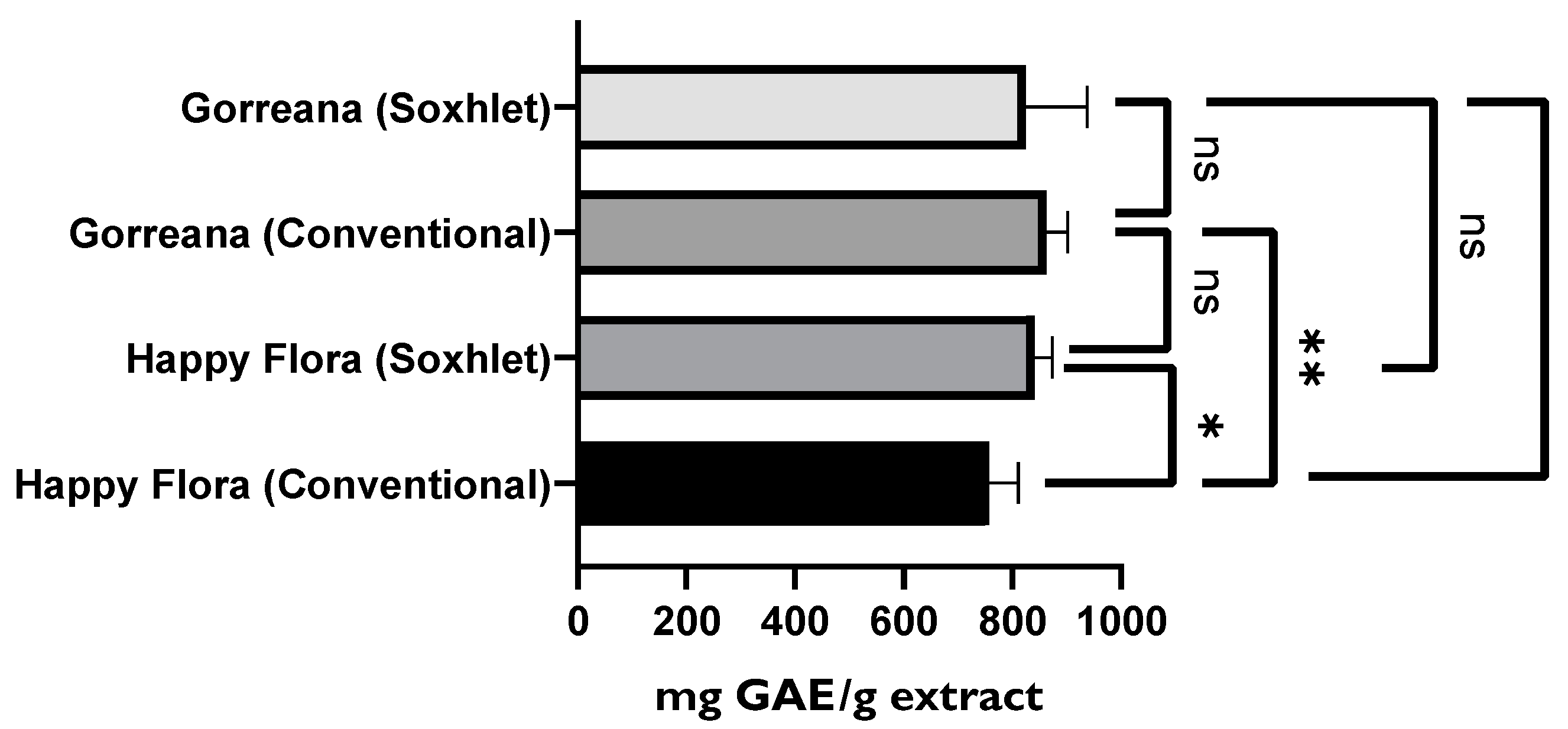
3.1.1. Evaluation of the Antioxidant Capacity, TPC, and TFC among Different Green Tea Infusions
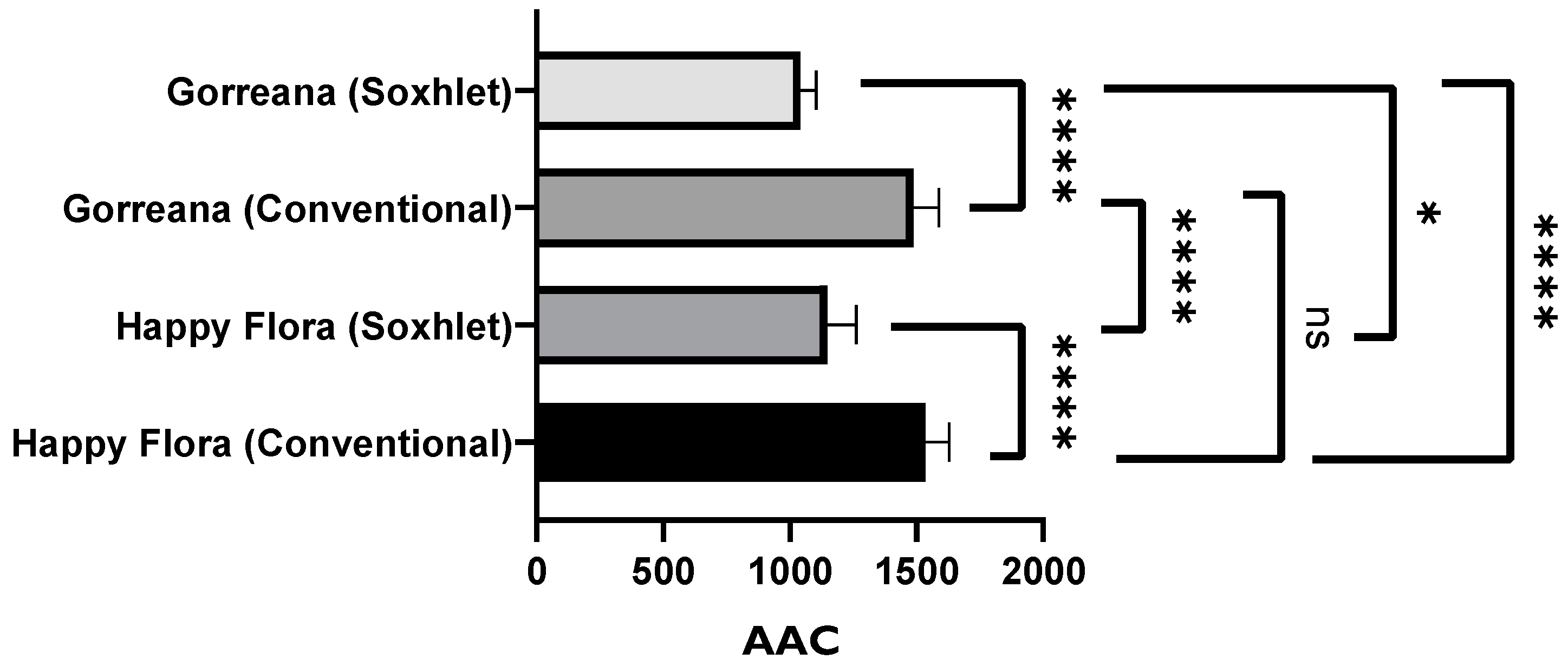
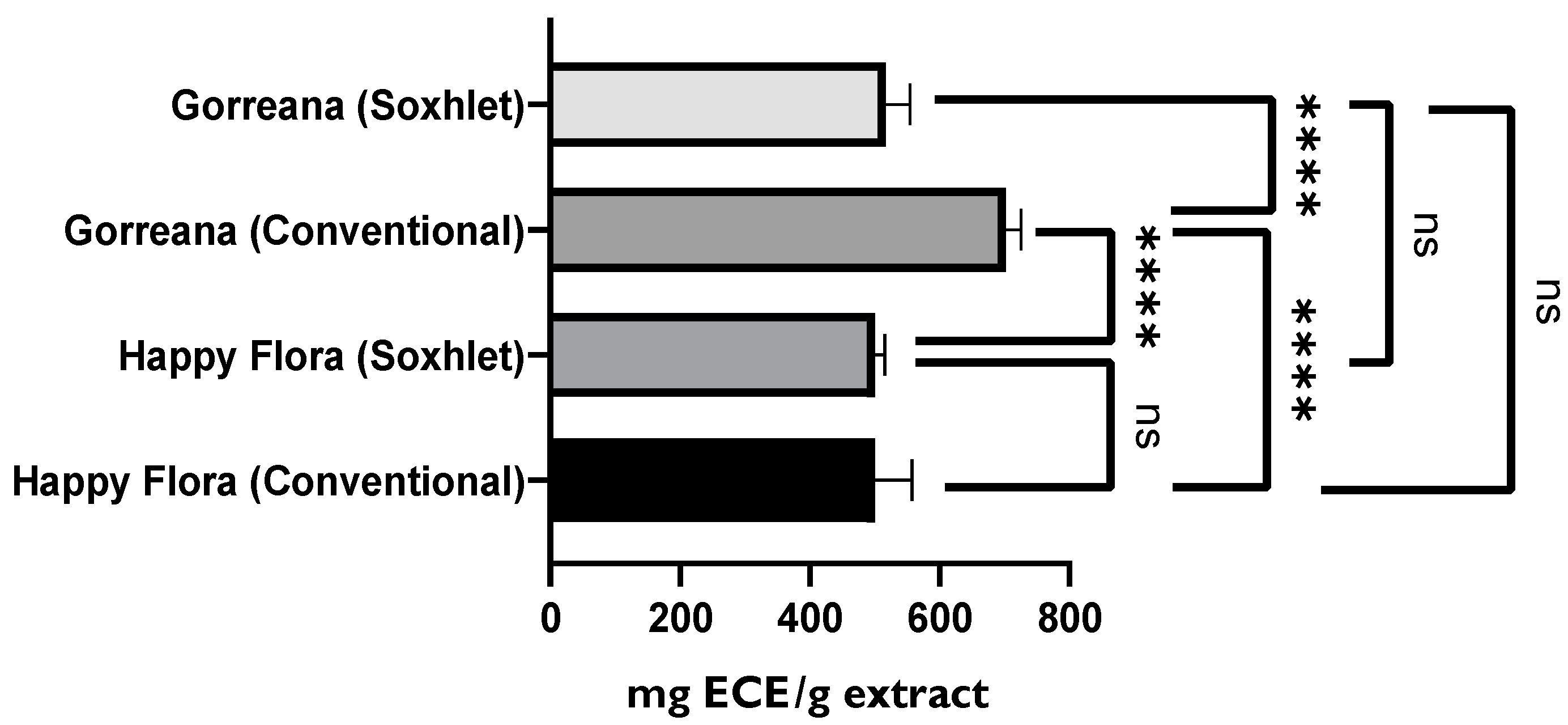
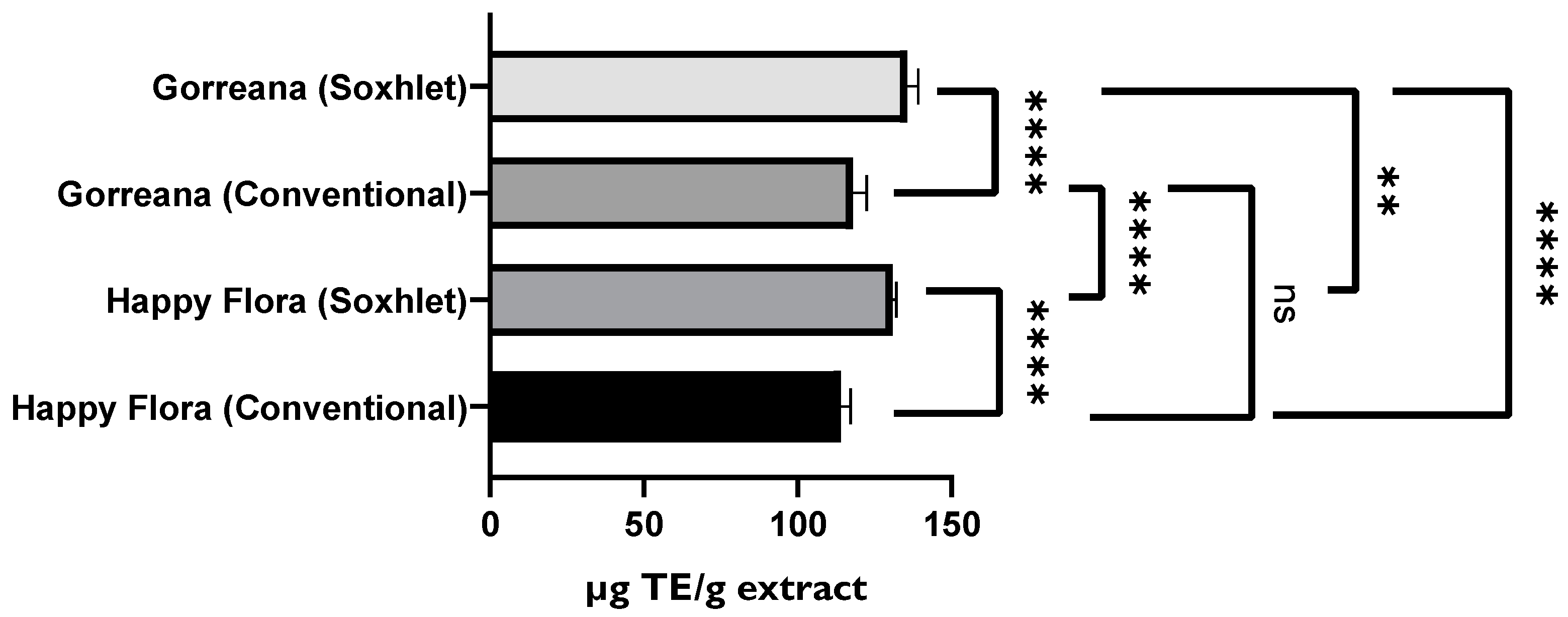
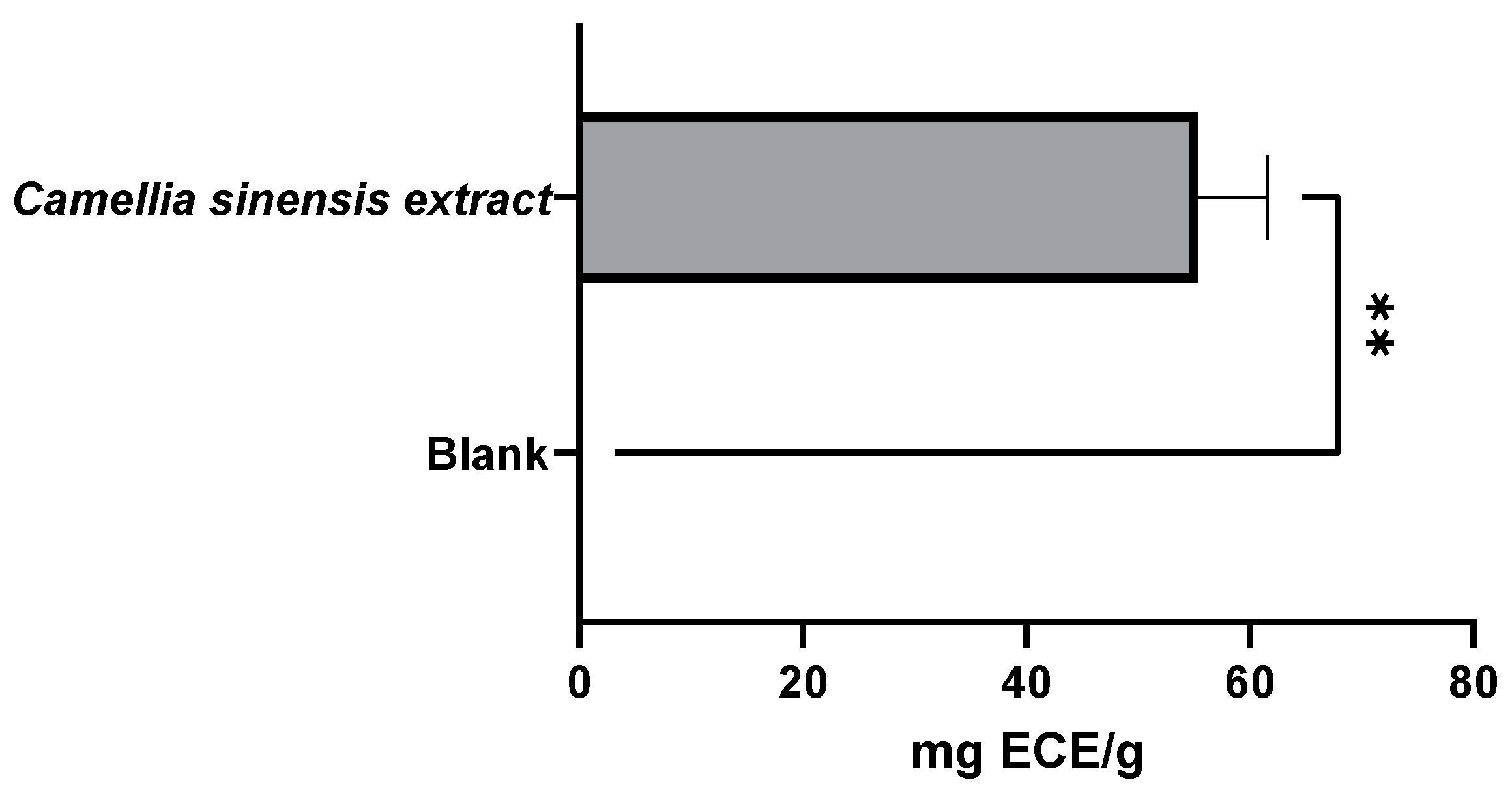
3.1.2. Evaluation of the Antioxidant Capacity of Green Tea Extracts
3.2. Antioxidant and Antimicrobial Properties of the Active Film
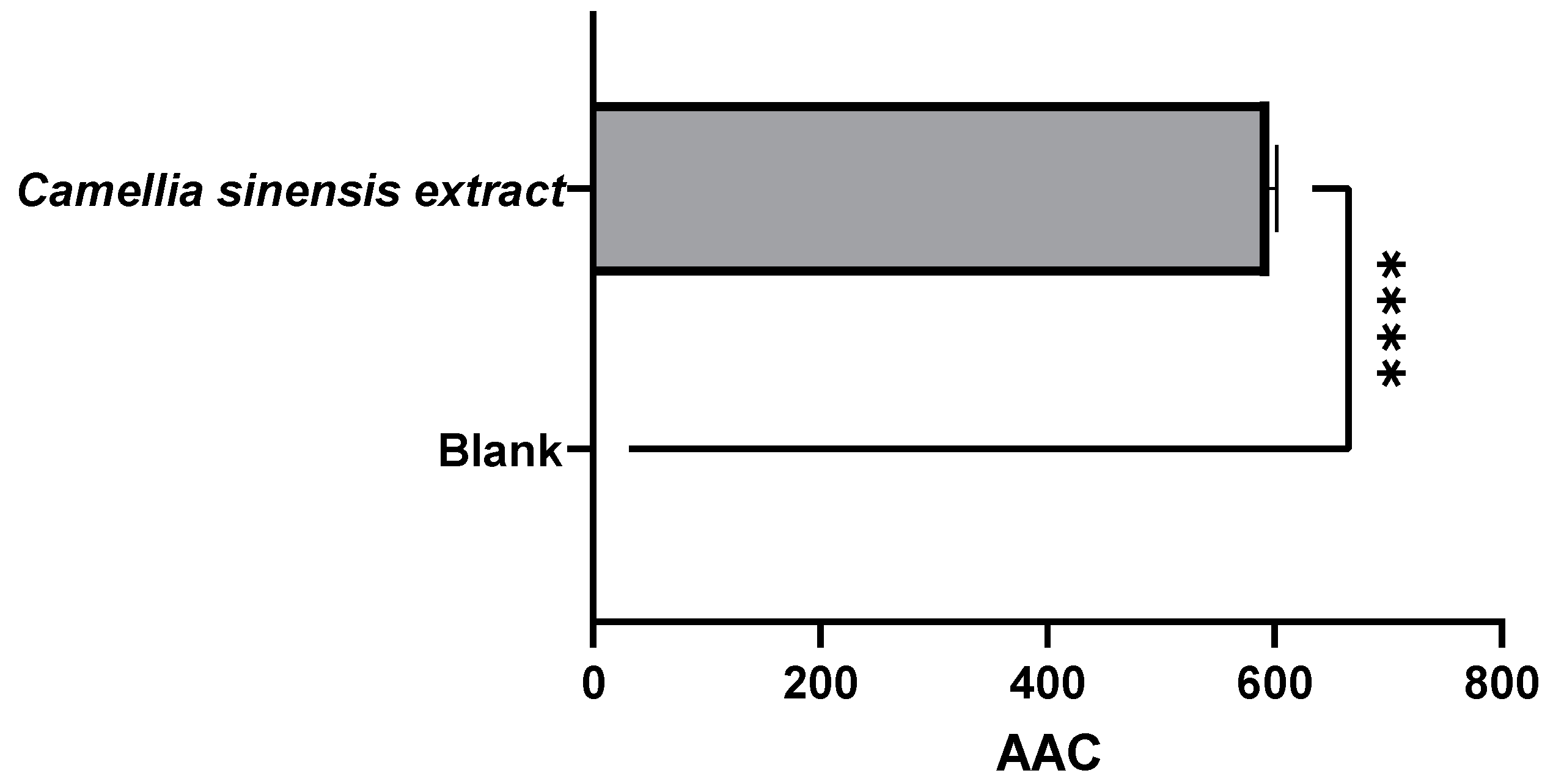
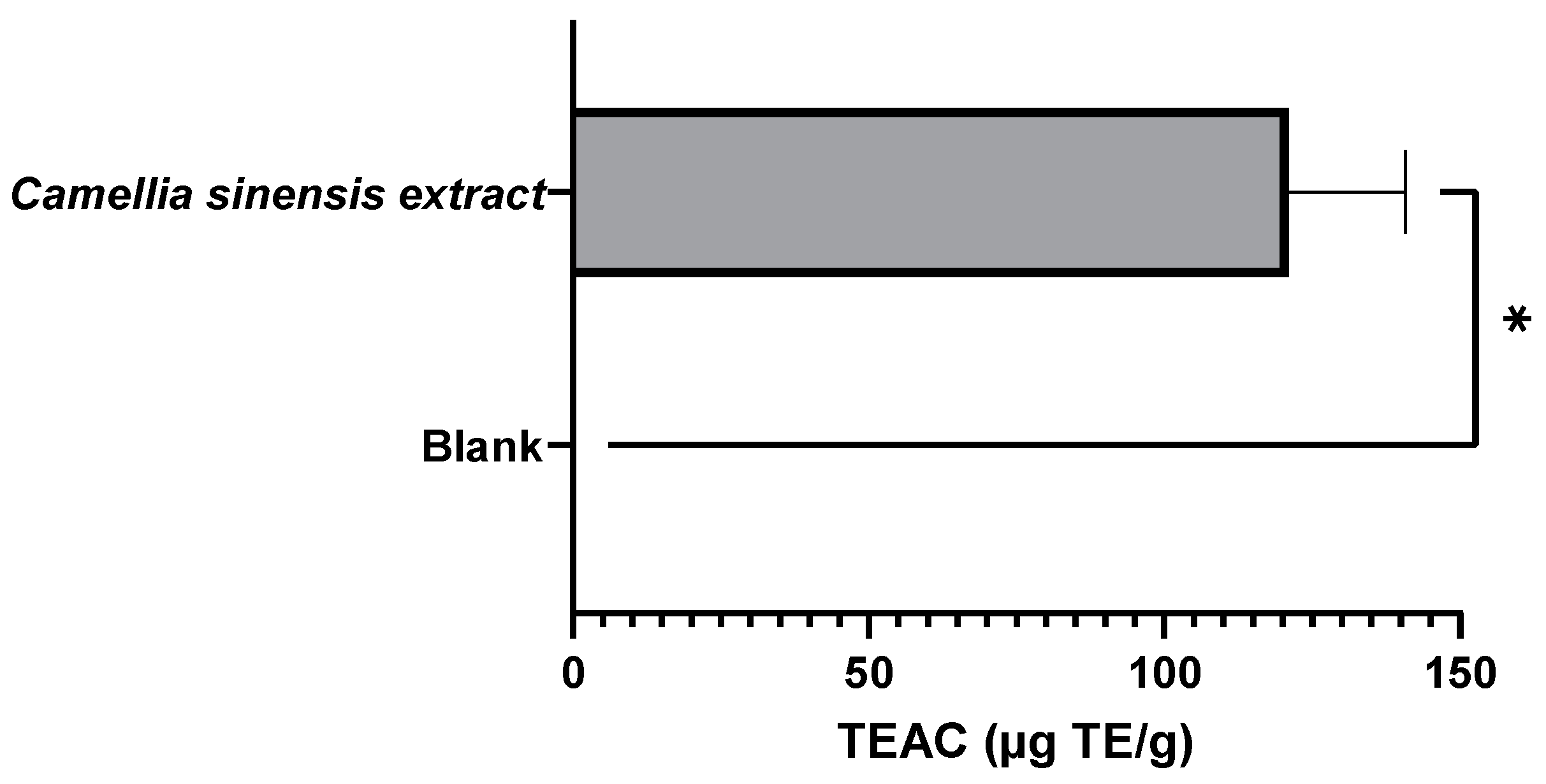
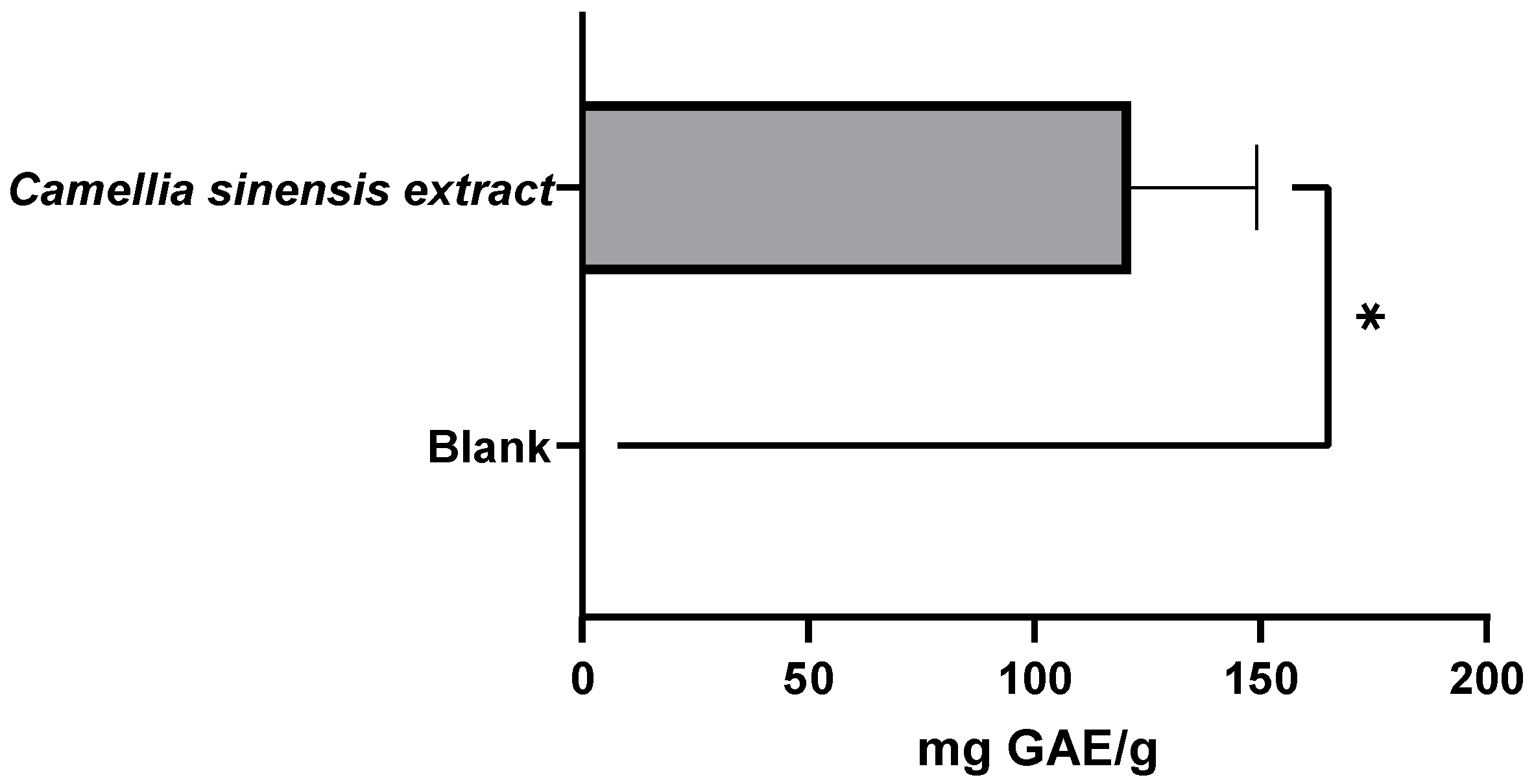
3.2.1. Evaluation of the Antioxidant Capacity of the Whey Protein-Based Films
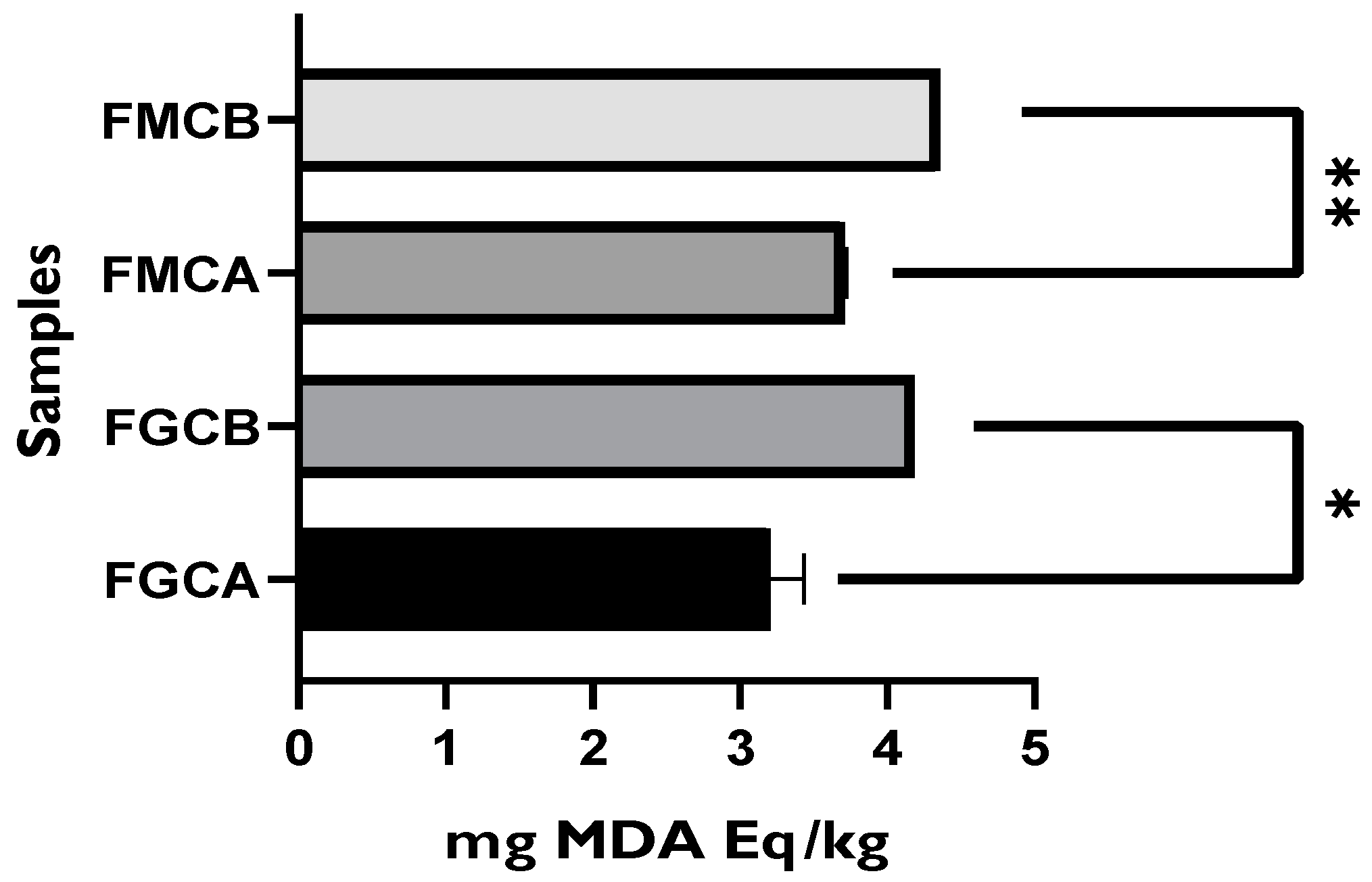
3.2.2. Evaluation of the Lipid Oxidation Status of Packaged Goat and Mixture Fresh Cheeses
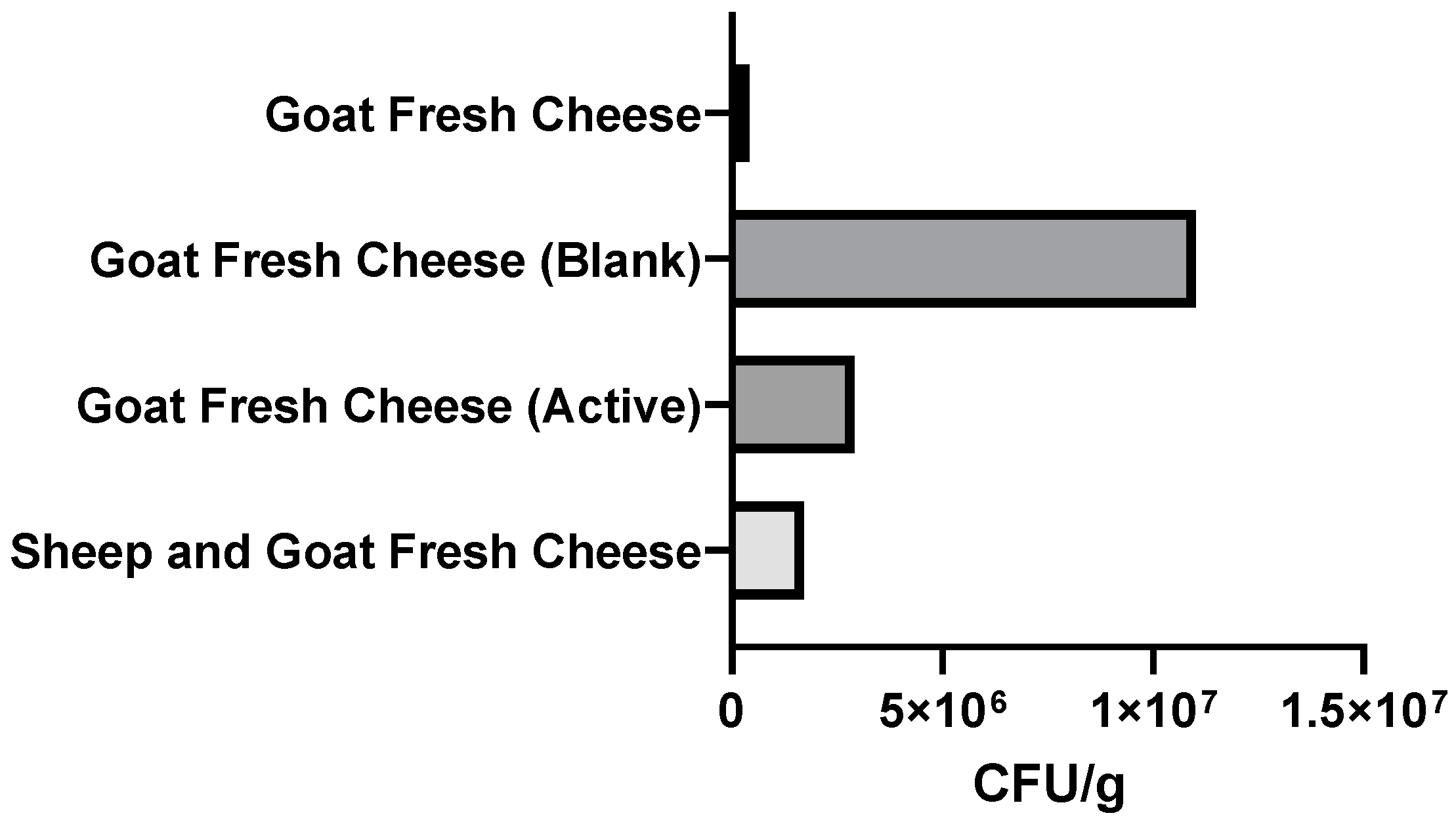
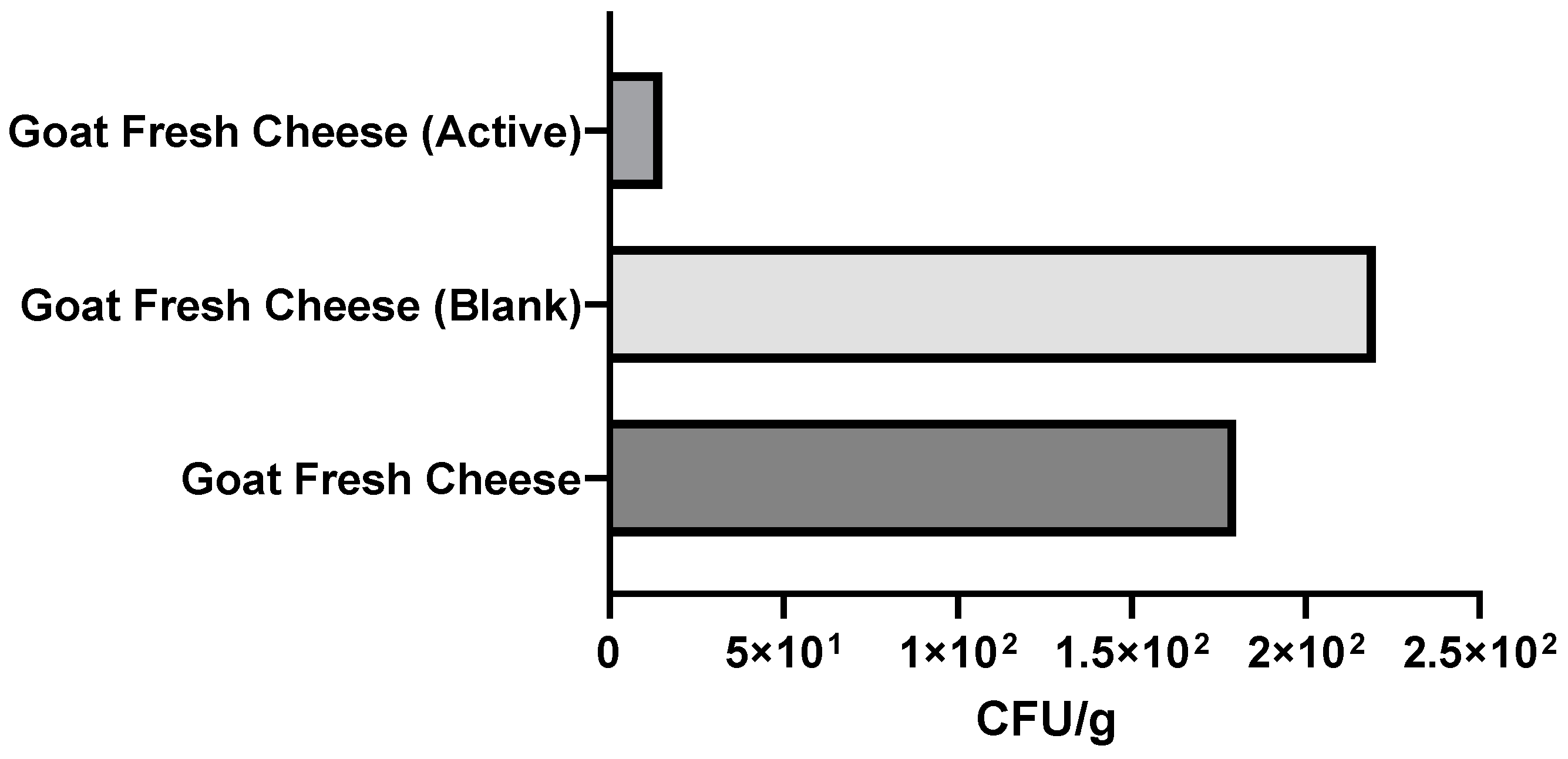
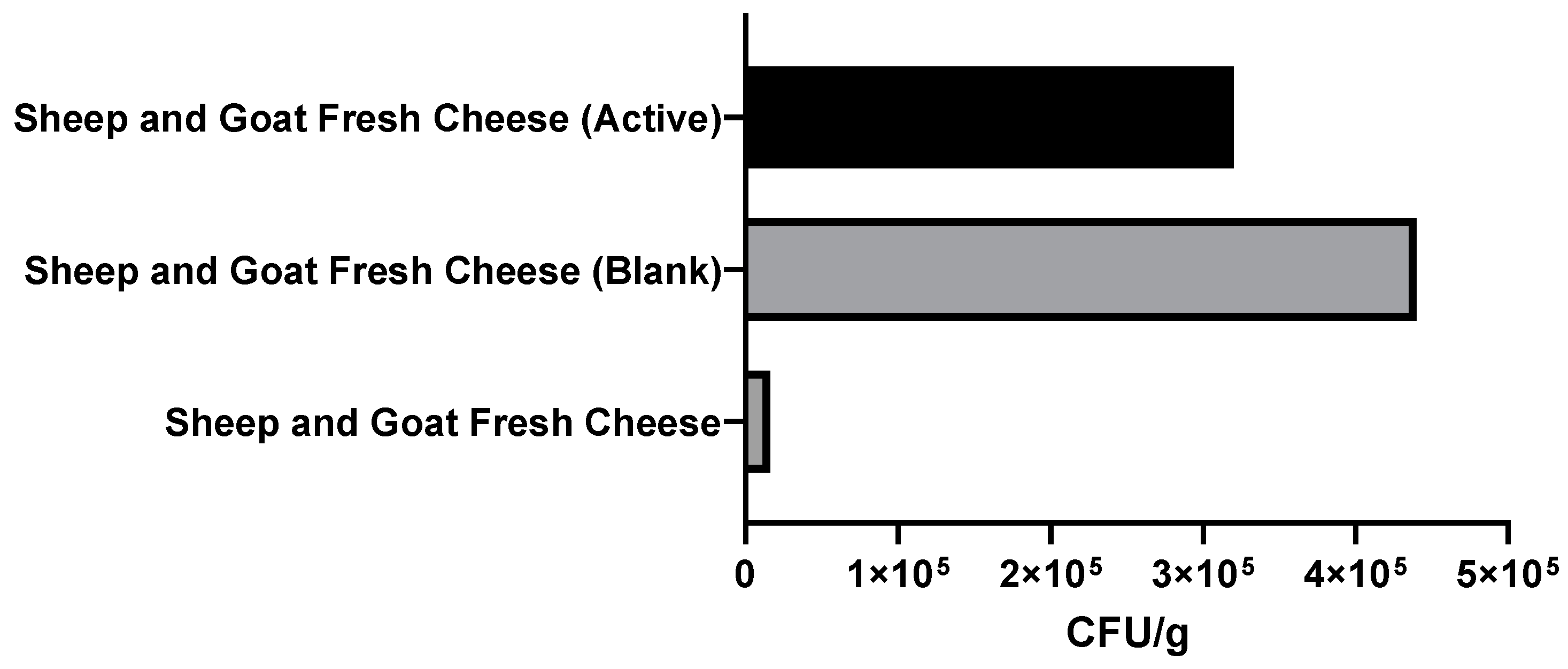
3.2.3. Microbiological Analysis of Packaged Fresh Cheeses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of Edible Films and Coatings in Cheese Preservation: Opportunities and Challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinrichs, J. Incorporation of Whey Proteins in Cheese. Int. Dairy J. 2001, 11, 495–503. [Google Scholar] [CrossRef]
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.L.N.E.; Rosa, H.J.D. Control of Listeria Monocytogenes in Fresh Cheese Using Protective Lactic Acid Bacteria. Int. J. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a Source of Peptides with Remarkable Biological Activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Asensio-Grau, A.; Peinado, I.; Heredia, A.; Andrés, A. In Vitro Study of Cheese Digestion: Effect of Type of Cheese and Intestinal Conditions on Macronutrients Digestibility. LWT 2019, 113, 108278. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and Antimicrobial Activities of (-)-Epigallocatechin-3-Gallate (EGCG) and Its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.C.G.; Domingos-Lopes, M.F.P.; Magalhães, V.A.F.; Freitas, D.A.S.R.; Coelho, M.C.; Rosa, H.J.D.; Dapkevicius, M.L.N.E. Latin-Style Fresh Cheese Enhances Lactic Acid Bacteria Survival but Not Listeria Monocytogenes Resistance under in Vitro Simulated Gastrointestinal Conditions. J. Dairy Sci. 2015, 98, 4377–4383. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.A.; Ribeiro-Santos, R.; Guerra, M.; Sanches-Silva, A. Evaluation of the Oxidative Status of Salami Packaged with an Active Whey Protein Film. Foods 2019, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial Edible Films and Coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Siso, M.I.G. The Biotechnological Utilization of Cheese Whey: A Review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of Liquid Whey Protein Concentrate–Based Edible Coating Enriched with Cinnamon Carbon Dioxide Extract on the Quality and Shelf Life of Eastern European Curd Cheese. J. Dairy Sci. 2021, 104, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active Edible Furcellaran/Whey Protein Films with Yerba Mate and White Tea Extracts: Preparation, Characterization and Its Application to Fresh Soft Rennet-Curd Cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/Whey Protein Film as Active Coating to Extend Ricotta Cheese Shelf-Life. LWT-Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Juszczak, L.; Krzyściak, P.; Zimowska, M. Characterization of Furcellaran-Whey Protein Isolate Films with Green Tea or Pu-Erh Extracts and Their Application as Packaging of an Acid-Curd Cheese. Food Bioprocess Technol. 2021, 14, 78–92. [Google Scholar] [CrossRef]
- Castro, F.V.R.; Andrade, M.A.; Silva, A.S.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.P.L.F.; Bertan, L.C.; de Rensis, C.M.V.B.; Bilck, A.P.; Vianna, P.C.B. Whey Protein-Based Films Incorporated with Oregano Essential Oil. Polimeros 2017, 27, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Mosavinezhad, K.; Shakerian, A.; Chaleshtori, R.S.; Rahimi, E. Antimicrobial and Antioxidant Effects of Thymus Daenensis and Camellia Sinensis Ethanolic Extracts of Chicken Meat During Frozen Storage. J. Med. Plants By-Prod. 2020, 9, 17–27. [Google Scholar]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Fakae, L.B.; Stevenson, C.W.; Zhu, X.Q.; Elsheikha, H.M. In Vitro Activity of Camellia sinensis (Green Tea) against Trophozoites and Cysts of Acanthamoeba Castellanii. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 59–72. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pinto, M. Tea: A New Perspective on Health Benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Colombo, F.; di Lorenzo, C.; Biella, S.; Vecchio, S.; Frigerio, G.; Restani, P. Adverse Effects to Food Supplements Containing Botanical Ingredients. J. Funct. Foods 2020, 72, 103990. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Rubab, S.; Rizwani, G.H.; Bahadur, S.; Shah, M.; Alsamadany, H.; Alzahrani, Y.; Shuaib, M.; Hershan, A.; Hobani, Y.H.; Shah, A.A. Determination of the GC–MS Analysis of Seed Oil and Assessment of Pharmacokinetics of Leaf Extract of Camellia sinensis L. J. King Saud Univ.-Sci. 2020, 32, 3138–3144. [Google Scholar] [CrossRef]
- Santacruz, S.; Castro, M. Viability of Free and Encapsulated Lactobacillus Acidophilus Incorporated to Cassava Starch Edible Films and Its Application to Manaba Fresh White Cheese. LWT 2018, 93, 570–572. [Google Scholar] [CrossRef]
- Jutinico-Shubach, A.; Gutiérrez-Cortés, C.; Suarez, H. Antilisterial Activity of Chitosan-Based Edible Coating Incorporating Cell-Free Supernatant from Pediococcus Pentosaceus 147 on the Preservation of Fresh Cheese. J. Food Process. Preserv. 2020, 44, e14715. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Azevedo, G.; Machado, A.V.; Carvalho-Costa, D.; Sanches-Silva, A. Whey Protein Active Films Incorporated with a Blend of Essential Oils: Characterization and Effectiveness. Packag. Technol. Sci. 2018, 31, 27–40. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the Antioxidant Capacity and Phenolic Content of Three Thymus Species. JAMS J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application to Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, H.; Yamakura, S.; Arastoo, R.S.; Oshima, T.; Kokubo, K. Systematic Evaluation and Mechanistic Investigation of Antioxidant Activity of Fullerenols Using β -Carotene Bleaching Assay. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of Rosemary and Thyme Extracts for Incorporation into a Whey Protein Based Film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH • Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Iqbal, M. Antioxidant Activity of Ricinus Communis. Org. Med. Chem. Int. J. 2018, 5, 107–112. [Google Scholar] [CrossRef]
- Pontis, J.A.; Costa, L.A.M.A.D.; Silva, S.J.R.D.; Flach, A. Color, Phenolic and Flavonoid Content, and Antioxidant Activity of Honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.; Pezzini, B.; Soares, L. Spectrophotometric Determination of the Total Flavonoid Content in Ocimum basilicum L. (Lamiaceae) Leaves. Pharmacogn. Mag. 2015, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.K.; Lee, C.Y. Relative Antioxidant and Cytoprotective Activities of Common Herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Kodama, D.H.; Gonçalves, A.E.D.S.S.; Lajolo, F.M.; Genovese, M.I. Flavonoids, Total Phenolics and Antioxidant Capacity: Comparison between Commercial Green Tea Preparations. Food Sci. Technol. 2010, 30, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.S.D.; Araújo, M.E.M.; Rodríguez, L.G.; Júlio, A.; Mendes, B.G.; Santos, R.M.B.D.; Simões, J.A.M. Influence of Preparation Procedures on the Phenolic Content, Antioxidant and Antidiabetic Activities of Green and Black Teas. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Lachman, J.; Hosnedl, V.; Pivec, V.; Orsák, M. Polyphenols in Cereals and Their Positive and Negative Role in Human and Animal Nutrition. In Proceedings of the Cereals for Human Health and Preventive Nutrition, Brno, Czech Republic, 7–11 July 1998; pp. 118–125. [Google Scholar]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active Polylactic Acid Film Incorporated with Green Tea Extract: Development, Characterization and Effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Chalob, K.K.; Abdul-Rahman, S.M. Antimicrobial Activity of Edible Film from Whey Protien Isolate Incorported with Green Tea Extract and Its Use in Cheese Coatin. Iraq J. Mark. Res. Consum. Prot. 2018, 10, 50–60. [Google Scholar]
- García-Díez, J.; Saraiva, C. Use of Starter Cultures in Foods from Animal Origin to Improve Their Safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
| Nutrition Declaration per 100 g of Product (Queijaria Licínia, Rabaçal, Portugal) | Goat Fresh Cheese | Mixture Fresh Cheese |
|---|---|---|
| Energy (kJ)–Energy (kcal) | 10.39–259 | 10.82–261 |
| Fat (g) Of which saturated fatty acids (g) | 18.8 | 20.8 |
| 12.98 | 13.93 | |
| Carbohydrates Of which sugars | 4.2 | 4.6 |
| 0.5 | 0.9 | |
| Protein (g) | 16.6 | 13.8 |
| Salt (g) | 1.10 | 0.55 |
| Water content (g) | 45.82 | 45.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robalo, J.; Lopes, M.; Cardoso, O.; Sanches Silva, A.; Ramos, F. Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese. Foods 2022, 11, 1158. https://doi.org/10.3390/foods11081158
Robalo J, Lopes M, Cardoso O, Sanches Silva A, Ramos F. Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese. Foods. 2022; 11(8):1158. https://doi.org/10.3390/foods11081158
Chicago/Turabian StyleRobalo, João, Maria Lopes, Olga Cardoso, Ana Sanches Silva, and Fernando Ramos. 2022. "Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese" Foods 11, no. 8: 1158. https://doi.org/10.3390/foods11081158
APA StyleRobalo, J., Lopes, M., Cardoso, O., Sanches Silva, A., & Ramos, F. (2022). Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese. Foods, 11(8), 1158. https://doi.org/10.3390/foods11081158








