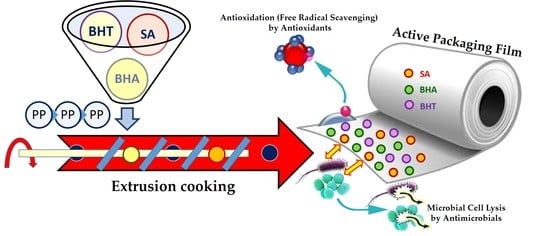
Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Active Films
2.2. Measurement of Film Properties
2.2.1. Thickness Measurement
2.2.2. Mechanical Properties
2.2.3. Fourier-Transform Infrared (FTIR) Spectrum Analysis
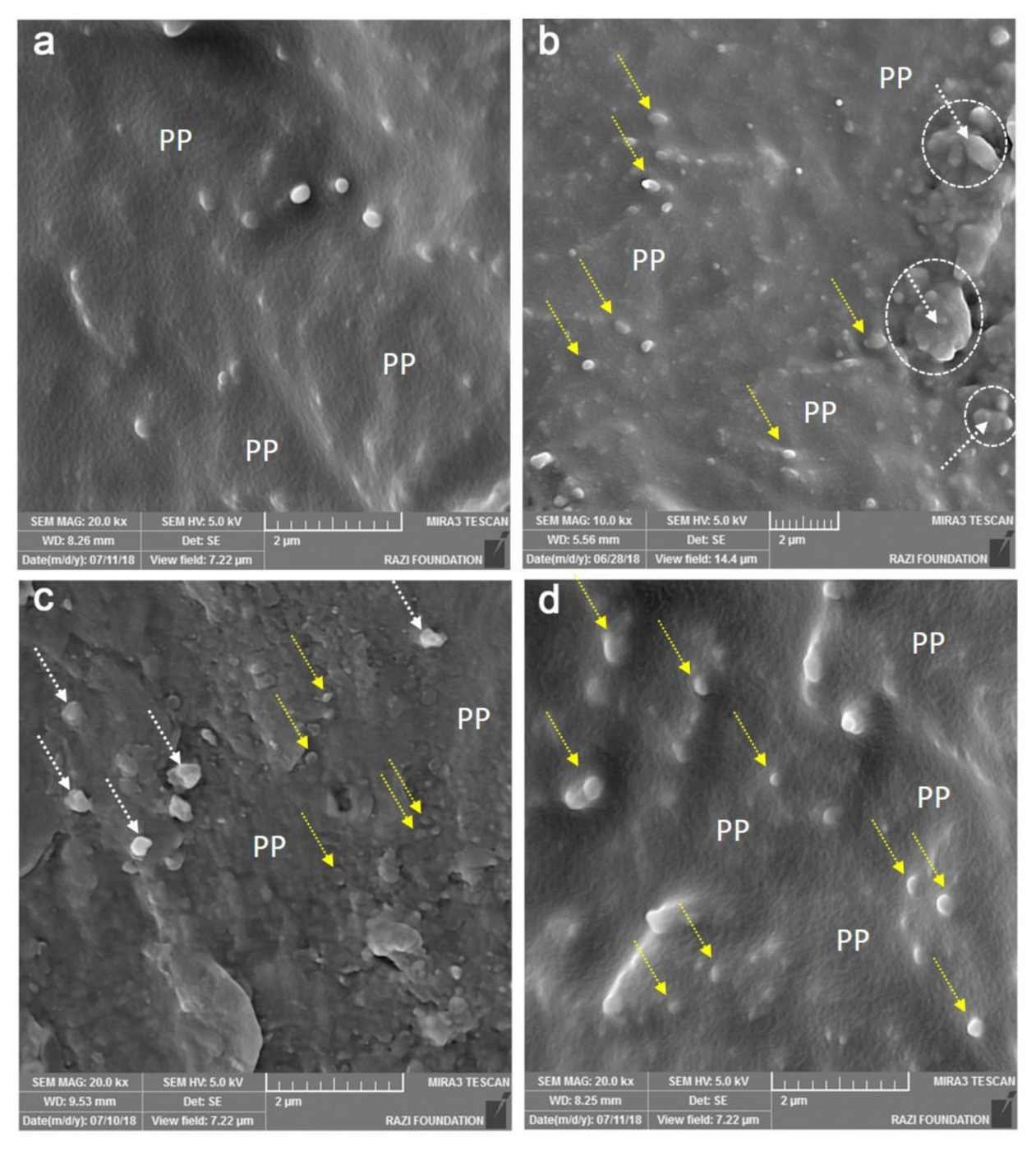
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Dynamic Mechanical Thermal Analysis (DMTA)
2.2.6. Film Transparency
2.2.7. Contact Angle
2.2.8. Colour Measurement
2.2.9. Antioxidant Capacity
2.2.10. In Vitro Antimicrobial Properties
2.2.11. Migration Behaviour
2.3. Statistical Analyses
3. Results and Discussion
3.1. Film Thickness
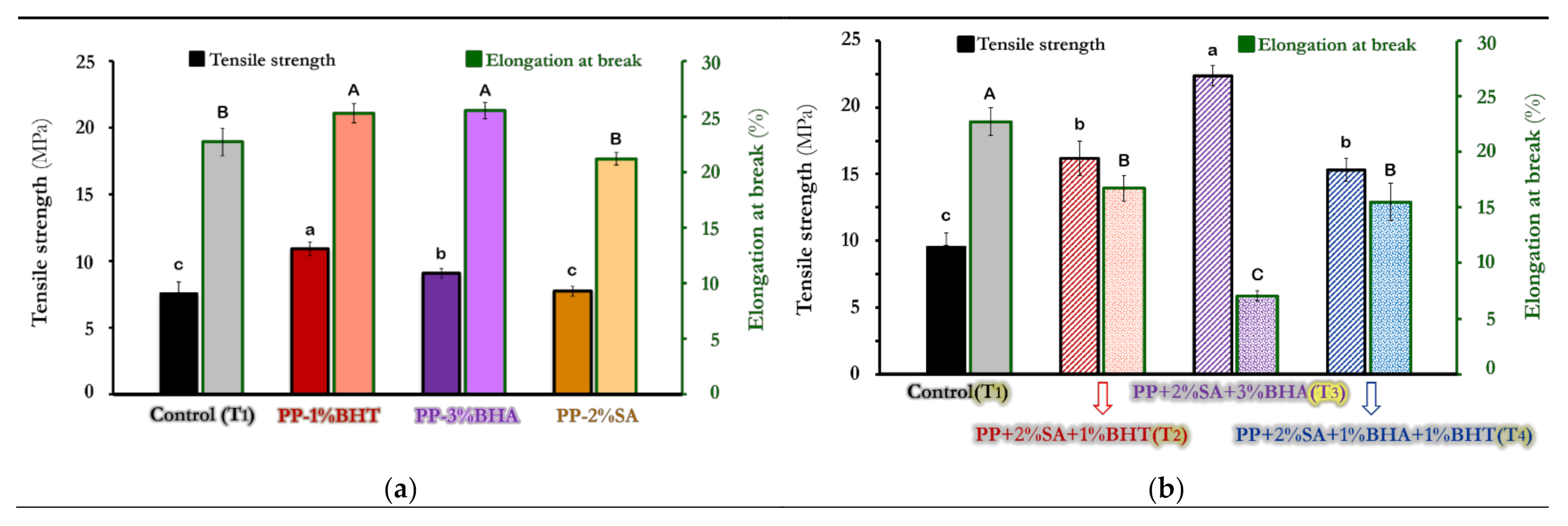
3.2. Mechanical Properties
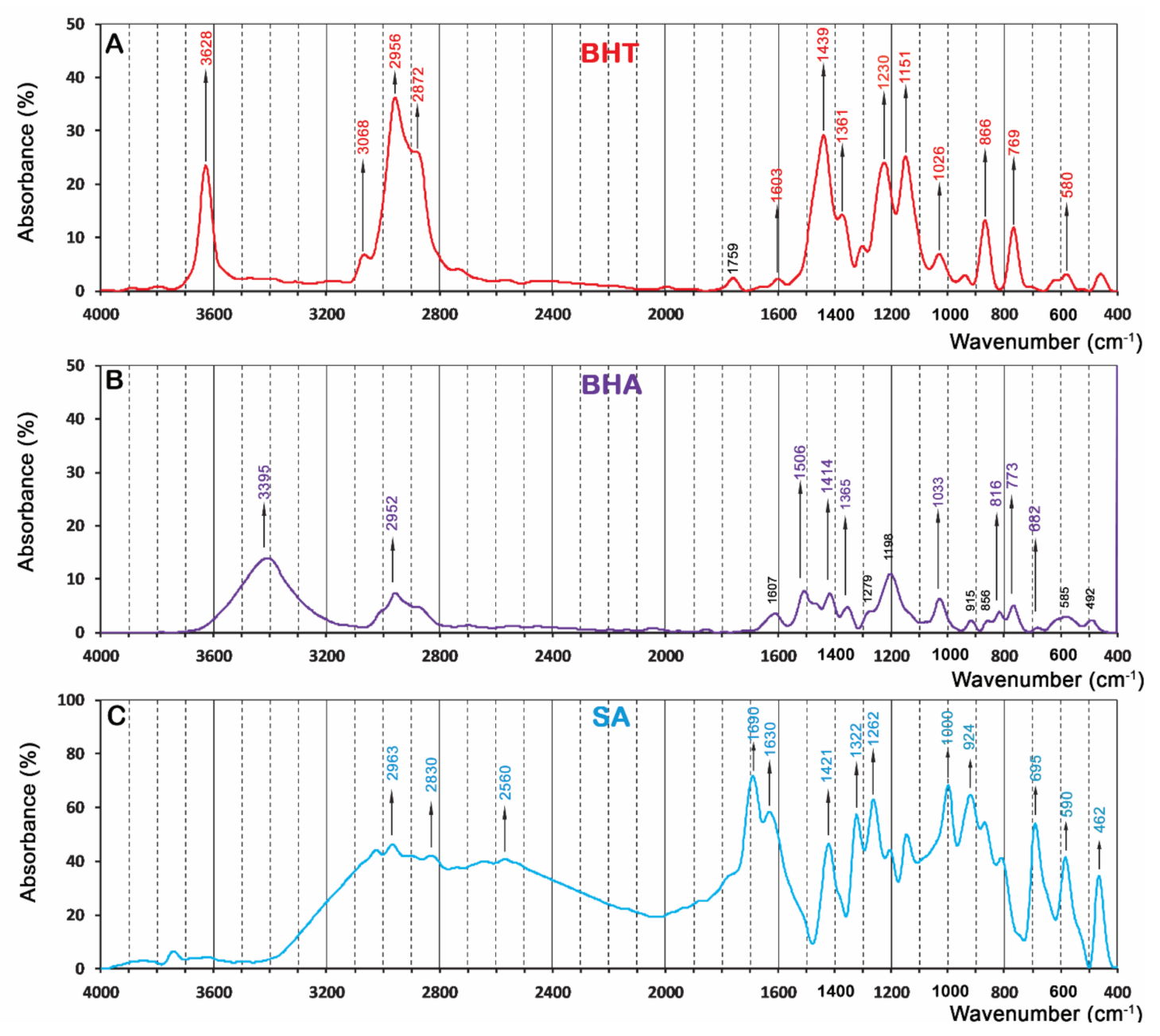
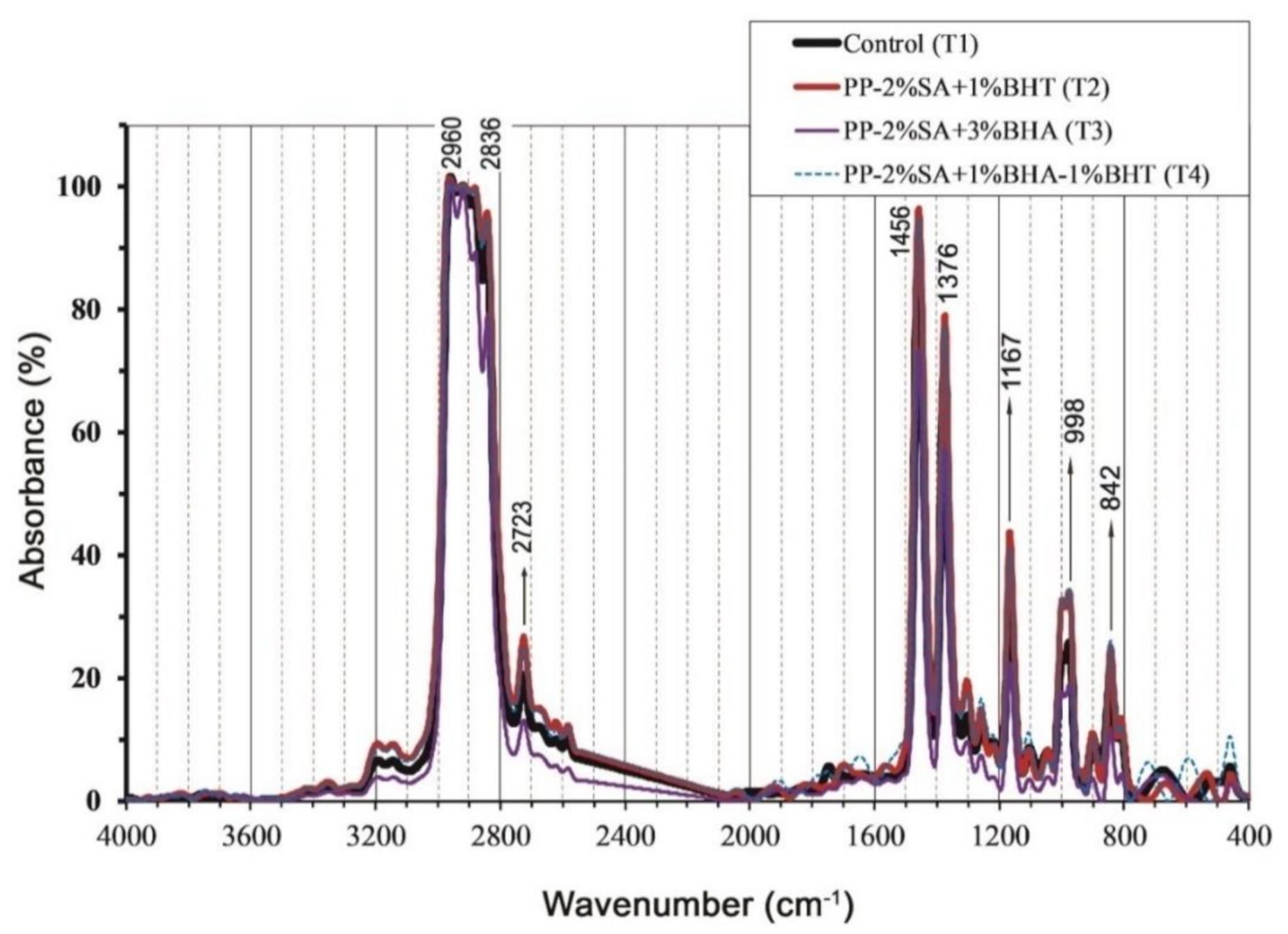
3.3. Fourier-Transform Infrared (FTIR) Spectrum
3.4. Scanning Electron Microscopy (SEM)
3.5. Dynamic Mechanical Thermal Analysis (DMTA)
3.6. Film Transparency
3.7. Contact Angle
3.8. Colour Attributes
3.9. Antioxidant Properties
3.10. Antimicrobial Properties
3.11. Migration Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.R.S.; Samaei, S.P. Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J. Food Sci. Technol. 2018, 55, 3098–3109. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.S.; Samaei, S.P. Effect of carrier types and compositions on the production yield, microstructure and physical characteristics of spray dried sour cherry juice concentrate. J. Food Meas. Charact. 2017, 11, 1602–1612. [Google Scholar] [CrossRef]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Development of a biodegradable coating formulation based on the biological characteristics of the Iranian Ultra-filtrated cheese. Biologia 2018, 73, 403–413. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol. Biotechnol. 2020, 58, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, Ş. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat. Res. Toxicol. Environ. Mutagenesis 2007, 626, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of novel active polypropylene based packaging films containing different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Leslie, S.W.; Gad, S.C.; Acosta, D. Cytotoxicity of butylated hydroxytoluene and butylated hydroxyanisole in cultured heart cells. Toxicology 1978, 10, 281–289. [Google Scholar] [CrossRef]
- Kamemura, N. Butylated hydroxytoluene, a food additive, modulates membrane potential and increases the susceptibility of rat thymocytes to oxidative stress. Comput. Toxicol. 2018, 6, 32–38. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-Y.; Lim, W.; You, S.; Song, G. Butylated hydroxyanisole exerts neurotoxic effects by promoting cytosolic calcium accumulation and endoplasmic reticulum stress in Astrocytes. J. Agric. Food Chem. 2019, 67, 9618–9629. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Parija, S.C. Food additives: Potential risk for cancer. World J. Pharm. Res. 2018, 7, 405–410. [Google Scholar] [CrossRef]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef]
- Khodaeimehr, R.; Peighambardoust, S.J.; Peighambardoust, S.H. Preparation and characterization of corn starch/clay nanocomposite films: Effect of clay content and surface modification. Starch/Stärke 2018, 70, 1700251. [Google Scholar] [CrossRef]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Effectiveness of edible coating based on chitosan and natamycin on biological, physico-chemical and organoleptic attributes of Iranian ultra-filtrated cheese. Biologia 2020, 75, 605–611. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Zahed-Karkaj, S.; Peighambardoust, S.H.; Ebrahimi, Y.; Peressini, D. Characterization of carboxymethyl cellulose-based active films incorporating non-modified and Ag or Cu-modified Cloisite 30B and montmorillonite nanoclays. Iran. Polym. J. 2020, 29, 1087–1097. [Google Scholar] [CrossRef]
- Golshan Tafti, A.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Shakuoie Bonab, E. Physico-chemical and functional properties of spray-dried sourdough in breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J. Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 2017, 42, e13488. [Google Scholar] [CrossRef]
- Golshan Tafti, A.; Peighardoust, S.H.; Behnam, F.; Bahrami, A.; Aghagholizadeh, R.; Ghamari, M.; Rafat, S.A. Effects of spray-dried sourdough on flour characteristics and rheological properties of dough. Czech J. Food Sci. 2013, 31, 361–367. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Niakosari, M. Effect of infrared-assisted spouted bed drying of flaxseed on the quality characteristics of its oil extracted by different methods. J. Sci. Food Agric. 2020, 100, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Manshadi, A.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Niakosari, M. Oxidative and physical stability, rheological properties and sensory characteristics of ‘salad dressing’ samples formulated with flaxseed oil and n-OSA starch. J. Food Meas. Charact. 2019, 13, 26–33. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Fasihnia, S.H.; Karimian Khosrowshahi, N.; Gullón, B.; Lorenzo, J.M. Optimization of the amount of ZnO, CuO, and Ag nanoparticles on antibacterial properties of low-density polyethylene (LDPE) films using the response surface method. Food Anal. Methods 2020. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Zahed-Karkaj, S. Development of antibacterial carboxymethyl cellulose-based nanobiocomposite films containing various metallic nanoparticles for food packaging applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Beigmohammadi, F.; Peighambardoust, S.J. Application of organoclay nanoparticle in low-density polyethylene films for packaging of UF cheese. Packag. Technol. Sci. 2016, 29, 355–363. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Hosseini, S.V.; Regenstein, J.M. Improved mechanical and antibacterial properties of active LDPE films prepared with combination of Ag, ZnO and CuO nanoparticles. Food Packag. Shelf Life 2019, 22, 100391. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Iheaturu, N.C.; Nwakaudu, A.A.; Nwakaudu, M.S.; Owuamanam, C.I. The use of natural antioxidant active polymer packaging for food preservation: A review. Futo J. Ser. 2018, 4, 94–112. [Google Scholar]
- Ortiz-Vazquez, H.; Shin, J.; Soto-Valdez, H.; Auras, R. Release of butylated hydroxytoluene (BHT) from Poly(lactic acid) films. Polym. Test. 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Hauser, C.; Wunderlich, J. Antimicrobial packaging films with a sorbic acid based coating. Procedia Food Sci. 2011, 1, 197–202. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Wessling, C.; Nielsen, T.; Giacin, J.R. Antioxidant ability of BHT-and α-tocopherol-impregnated LDPE film in packaging of oatmeal. J. Sci. Food Agric. 2001, 81, 194–201. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; De Quirós, A.R.B.; Ares, A.N.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Castro-López, M.D.M.; López-Vilariño, J.M.; González-Rodríguez, M.V. Immobilization of green tea extract on polypropylene films to control the antioxidant activity in food packaging. Food Res. Int. 2013, 53, 522–528. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, C.; Guo, Y.; Ahmed, I.; Pavase, T.R.; Lv, L.; Li, Z.; Lin, H. Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets. Food Packag. Shelf Life 2019, 20, 100320. [Google Scholar] [CrossRef]
- Torres-Arreola, W.; Soto-Valdez, H.; Peralta, E.; Cárdenas-López, J.L.; Ezquerra-Brauer, J.M. Effect of a low-density polyethylene film containing butylated hydroxytoluene on lipid oxidation and protein quality of Sierra fish (Scomberomorus sierra) muscle during frozen storage. J. Agric. Food Chem. 2007, 55, 6140–6146. [Google Scholar] [CrossRef]
- Soto-Cantú, C.; Graciano-Verdugo, A.; Peralta, E.; Islas-Rubio, A.; González-Córdova, A.; Gonzalez-Leon, A.; Soto-Valdez, H. Release of butylated hydroxytoluene from an active film packaging to Asadero cheese and its effect on oxidation and odor stability. J. Dairy Sci. 2008, 91, 11–19. [Google Scholar] [CrossRef]
- Kuplennik, N.; Tchoudakov, R.; Zelas, Z.B.-B.; Sadovski, A.; Fishman, A.; Narkis, M. Antimicrobial packaging based on linear low-density polyethylene compounded with potassium sorbate. LWT 2015, 62, 278–286. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Pateiro, M.; Lorenzo, J. Properties and application of multifunctional composite polypropylene-based films incorporating a combination of BHT, BHA and sorbic acid in extending donut shelf-life. Molecules 2020, 25, 5197. [Google Scholar] [CrossRef]
- ASTM. ASTM D882-18, standard test method for tensile properties of thin plastic sheeting. In Annual Book of American Standard Testing Methods; American Society for Testing and Materials: West Conshohocken, PA, USA, 2018; Volume 8, pp. 182–190. [Google Scholar]
- Durmuş, A.; Woo, M.; Kaşgöz, A.; Macosko, C.W.; Tsapatsis, M. Intercalated linear low density polyethylene (LLDPE)/clay nanocomposites prepared with oxidized polyethylene as a new type compatibilizer: Structural, mechanical and barrier properties. Eur. Polym. J. 2007, 43, 3737–3749. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van Brenk, S.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Dough processing in a Couette-type device with varying eccentricity: Effect on glutenin macro-polymer properties and dough micro-structure. J. Cereal Sci. 2007, 45, 34–48. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; van der Goot, A.; Boom, R.; Hamer, R. Mixing behaviour of a zero-developed dough compared to a flour–water mixture. J. Cereal Sci. 2006, 44, 12–20. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- ASTM. ASTM D5026-15, Standard test methods for plastics: Dynamic mechanical properties: In tension. In Annual Book of American Standard Testing Methods; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Cook, W.D.; Scott, T.F.; Quay-Thevenon, S.; Forsythe, J.S. Dynamic mechanical thermal analysis of thermally stable and thermally reactive network polymers. J. Appl. Polym. Sci. 2004, 93, 1348–1359. [Google Scholar] [CrossRef]
- Sun, C.-W.; Chuang, C.S.A.C. Hemodynamics study based on near-infrared optical assessment. Hemodyn. New Diagn. Ther. Approaches 2012, 3. [Google Scholar] [CrossRef]
- Castejon, P.; Habibi, K.; Saffar, A.; Ajji, A.; Martínez, A.B.; Arencón, D. Polypropylene-based porous membranes: Influence of polymer composition, extrusion draw ratio and uniaxial strain. Polymer 2017, 10, 33. [Google Scholar] [CrossRef]
- Williams, D.L.; Kuhn, A.T.; Amann, M.A.; Hausinger, M.B.; Konarik, M.M.; Nesselrode, E.I. Computerised measurement of contact angles. Galvanotechnik 2010, 101, 2502–2512. [Google Scholar]
- Rhim, J.W.; Wu, Y.; Weller, C.L.; Schnepf, M. Physical characteristics of a composite film of soy protein isolate and propyleneglycol alginate. J. Food Sci. 1999, 64, 149–152. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Identification and synthesis of multifunctional peptides from wheat germ hydrolysate fractions obtained by proteinase K digestion. J. Food Biochem. 2019, 43, e12800. [Google Scholar] [CrossRef]
- Pezo, D.; Salafranca, J.; Nerín, C. Determination of the antioxidant capacity of active food packagings by in situ gas-phase hydroxyl radical generation and high-performance liquid chromatography–fluorescence detection. J. Chromatogr. A 2008, 1178, 126–133. [Google Scholar] [CrossRef] [PubMed]
- BS-British-Standard. Part 1: Guide to the selection of conditions and test methods for overall migration. In Materials and Articles in Contact with Foodstuff-Plastics; BSI—British Standards Institution: London, UK, 2002; pp. 1–50. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Razali, I.; Nor Asimiah, A.S.; Norhaya, H. Determination of antioxidants in palm oil products by high performance liquid chromatography. Elaeis 1997, 9, 25–33. [Google Scholar]
- Ammawath, W.; Man, Y.B.C.; Rahman, R.B.A.; Baharin, B.S. A fourier transform infrared spectroscopic method for determining butylated hydroxytoluene in palm olein and palm oil. J. Am. Oil Chem. Soc. 2006, 83, 187–191. [Google Scholar] [CrossRef]
- Kang, K.; Chang, Y.; Choi, J.C.; Park, S.-J.; Han, J. Migration study of butylated hydroxytoluene and Irganox 1010 from polypropylene treated with severe processing conditions. J. Food Sci. 2018, 83, 1005–1010. [Google Scholar] [CrossRef]
- Chinna Babu, P.; Sundaraganesan, N.; Dereli, Ö.; Türkkan, E. FT-IR, FT-Raman spectra, density functional computations of the vibrational spectra and molecular geometry of butylated hydroxy toluene. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 79, 562–569. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Singh, R.; Jana, S. Physicochemical and spectroscopic characterization of biofield treated butylated hydroxytoluene. J. Food Ind. Microbiol. 2015, 1, 1. [Google Scholar]
- Li, L.; Wang, H.; Chen, M.; Jiang, S.; Jiang, S.; Li, X.; Wang, Q. Butylated hydroxyanisole encapsulated in gelatin fiber mats: Volatile release kinetics, functional effectiveness and application to strawberry preservation. Food Chem. 2018, 269, 142–149. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Infrared spectroscopy. In Introduction to Spectroscopy; Pavia, D.L., Lampman, G.M., Kriz, G.S., Vyvyan, J.A., Eds.; Cengage Learning: Belmont, CA, USA, 2008; pp. 13–101. ISBN 1111800626. [Google Scholar]
- Roeges, N.P.G. Normal vibrations and absorption regions of—CH2X. In A guide to the Complete Interpretation of Infrared Spectra of Organic Structures; Roeges, N.P.G., Baas, J.M.A., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 50–112. ISBN 0471939986. [Google Scholar]
- Mirghani, M.E.S.; Man, Y.B.C.; Jinap, S.; Baharin, B.S.; Bakar, J. A new method for determining aflatoxins in groundnut and groundnut cake using fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 2001, 78, 985–992. [Google Scholar] [CrossRef]
- Singh, S.; Lee, M.H.; Park, I.; Shin, Y.J. Antimicrobial properties of polypropylene films containing AgSiO2, AgZn and AgZ for returnable packaging in seafood distribution. J. Food Meas. Charact. 2016, 10, 781–793. [Google Scholar] [CrossRef]
- Goncalves, C.M.B.; Tomé, L.C.; Garcia, H.; Brandão, L.; Mendes, A.M.; Marrucho, I.M. Effect of natural and synthetic antioxidants incorporation on the gas permeation properties of poly(lactic acid) films. J. Food Eng. 2013, 116, 562–571. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis in the Analysis of Polymers and Rubbers. In Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2015; pp. 1–33. [Google Scholar]
- Idicula, M.; Malhotra, S.; Joseph, K.; Thomas, S. Dynamic mechanical analysis of randomly oriented intimately mixed short banana/sisal hybrid fibre reinforced polyester composites. Compos. Sci. Technol. 2005, 65, 1077–1087. [Google Scholar] [CrossRef]
- Hwang, S.W.; Shim, J.K.; Selke, S.E.; Soto-Valdez, H.; Matuana, L.; Rubino, M.; Auras, R. Poly(L-lactic acid) with added α-tocopherol and resveratrol: Optical, physical, thermal and mechanical properties. Polym. Int. 2012, 61, 418–425. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Cleymand, F.; Leconte, S.; Falher, T.; Desobry, S. Effects of synthetic phenolic antioxidants on physical, structural, mechanical and barrier properties of poly lactic acid film. Carbohydr. Polym. 2012, 87, 1763–1773. [Google Scholar] [CrossRef]
- Ramis-Ramos, G. Antioxidants|Synthetic antioxidants. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, UK, 2003; pp. 265–275. [Google Scholar]
- Allen, N.; Fatinikun, K.; Henman, T. Influence of transition metal ions on the photo-behaviour of BHT in polypropylene: A uv-study. Polym. Degrad. Stab. 1987, 17, 81–88. [Google Scholar] [CrossRef]
- Granda-Restrepo, D.; Peralta, E.; Troncoso-Rojas, R.; Soto-Valdez, H. Release of antioxidants from co-extruded active packaging developed for whole milk powder. Int. Dairy J. 2009, 19, 481–488. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Liu, Q.S.; Li, C.; Zhou, Q.; Fiedler, H.; Liao, C.; Zhang, J.; Jiang, G. Butylated hydroxyanisole isomers induce distinct adipogenesis in 3T3-L1 cells. J. Hazard. Mater. 2019, 379, 120794. [Google Scholar] [CrossRef]
- Borsato, D.; Cini, J.R.D.M.; Da Silva, H.C.; Coppo, R.L.; Angilelli, K.G.; Moreira, I.; Maia, E.C.R. Oxidation kinetics of biodiesel from soybean mixed with synthetic antioxidants BHA, BHT and TBHQ: Determination of activation energy. Fuel Process. Technol. 2014, 127, 111–116. [Google Scholar] [CrossRef]
- Senanayake, S.N.; Wanasundara, P.J.P.; Shahidi, F. Antioxidants: Science, Technology, and Applications. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–61. [Google Scholar]
- Allam, S.S.M.; Mohamed, H.M.A. Thermal stability of some commercial natural and synthetic antioxidants and their mixtures. J. Food Lipids 2002, 9, 277–293. [Google Scholar] [CrossRef]
- Fernández-Álvarez, L.; Del Valle, P.; De Arriaga, D.; García-Armesto, M.R.; Rúa, J. Binary combinations of BHA and other natural and synthetic phenolics: Antimicrobial activity against Staphylococcus aureus and antioxidant capacity. Food Control 2014, 42, 303–309. [Google Scholar] [CrossRef]



| Film Samples | PP | SA (phr) | BHT (phr) | BHA (phr) |
|---|---|---|---|---|
| Pure PP (control) (T1) | 100 | - | - | - |
| PP-2%SA-1%BHT (T2) | 97 | 2 | 1 | - |
| PP-2%SA-3%BHA (T3) | 95 | 2 | - | 3 |
| PP-2%SA-1%BHT-1%BHA (T4) | 96 | 2 | 1 | 1 |
| SA | BHA | BHT | |
|---|---|---|---|
| Chemical structure | C6H8O2 | C11H16O2 | C15H24O |
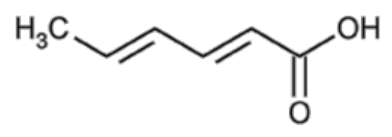 | 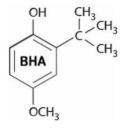 |  | |
| Density (g·cm−3) | 1.204 | 1.06 | 1.048 |
| Solubility in water (g·L−1) | 1.6 | 0 | 1.1 |
| Solubility in ethanol (g·L−1) | 12.9 | Freely soluble | Freely soluble |
| Molar mass (g·mol−1) | 112.1 | 180.2 | 220.4 |
| Molar volume (cm3·mol−1) | 109.4 | 182.4 | 256.0 |
| Polarity | High | Medium | Low |
| Film Samples | Transparency (%) | Contact Angle (θ°) |
|---|---|---|
| Pure PP (control) (T1) | 76.80 ± 0.001 * b | 100.15 ± 2.20 a |
| PP-2%SA-1%BHT (T2) | 80.57 ± 0.004 a | 108.15 ± 6.30 a |
| PP-2%SA-3%BHA (T3) | 75.23 ± 0.007 c | 107.24 ± 1.67 a |
| PP-2%SA-1%BHT-1%BHA (T4) | 69.85 ± 0.003 d | 109.03 ± 3.60 a |
| L * | A * | B * | ∆E | YI | WI | |
|---|---|---|---|---|---|---|
| Pure PP (control) (T1) | 63.7 ± 0.61 * a | −8.1 ± 0.99 a | −4.8 ± 1.25 a | 6.4 ± 1.04 a | −3.2 ± 0.86 a | 60.5 ± 0.58 a |
| PP-2%SA-1%BHT (T2) | 62.3 ± 1.46 a | −8.8 ± 1.31 a | −5.7 ± 2.72 a | 6.7 ± 2.64 a | −3.9 ± 1.88 a | 60.7 ± 1.45 a |
| PP-2%SA-3%BHA (T3) | 63.2 ± 0.60 a | −9.7 ± 0.31 a | −4.7 ± 0.23 a | 6.6 ± 0.16 a | −3.2 ± 0.18 a | 61.6 ± 0.53 a |
| PP-2%SA-1%BHT-1%BHA (T4) | 62.9 ± 0.50 a | −9.7 ± 1.75 a | −2.2 ± 1.59 a | 6.6 ± 1.72 a | −1.5 ± 1.11 a | 61.5 ± 0.36 a |
| Film Samples | DPPH-RSA (%) | Microbial Analysis | ||
|---|---|---|---|---|
| E. coli (Log cfu/mL) | S. aureus (Log cfu/mL) | A. niger (Inhibition Area, cm2) | ||
| Pure PP (control) (T1) | 0.83 ± 0.12 *,d | 8.70 ± 0.02 a | 8.61 ± 0.00 a | 0 |
| PP-2%SA-1%BHT (T2) | 81.84 ± 1.02 b | 8.41 ± 0.01 b | 8.40 ± 0.01 b | 0 |
| PP-2%SA-3%BHA (T3) | 62.47 ± 1.53 c | 8.38 ± 0.01 bc | 8.38 ± 0.00 b | 0 |
| PP-2%SA-1%BHT-1%BHA (T4) | 90.81 ± 2.04 a | 8.35 ± 0.01 c | 8.20 ± 0.01 c | 0 |
| Film Samples | Aqueous Foods (pH > 4.5) | Acidic Foods (pH ≤ 4.5) | Fatty Foods |
|---|---|---|---|
| Pure PP (control) (T1) | 0.78 ± 0.09 * b | 0.74 ± 0.06 d | 0.78 ± 0.09 g |
| PP-2% SA | 1.95 ± 0.69 a | 4.62 ± 0.33 a | 2.09 ± 0.39 f |
| PP-1% BHT | 1.45 ± 0.90 a | 1.99 ± 0.21 c | 4.21 ± 0.17 e |
| PP-2% BHT | 1.76 ± 0.59 a | 3.38 ± 0.45 b | 5.46 ± 0.27 d |
| PP-3% BHT | 1.96 ± 0.25 a | 4.96 ± 0.33 a | 7.96 ± 0.35 a |
| PP-1% BHA | 1.38 ± 0.12 a | 2.09 ± 0.21 c | 4.62 ± 0.33 e |
| PP-2% BHA | 1.96 ± 0.66 a | 3.98 ± 0.16 b | 5.84 ± 0.27 d |
| PP-3% BHA | 1.99 ± 0.63 a | 5.22 ± 0.26 a | 8.54 ± 0.26 a |
| PP-2%SA-1%BHT (T2) | 2.32 ± 0.39 a | 2.12 ± 0.23 c | 6.37 ± 0.30 c |
| PP-2%SA-3%BHA (T3) | 2.84 ± 0.44 a | 3.66 ± 0.36 b | 7.22 ± 0.17 b |
| PP-2%SA-1%BHT-1%BHA (T4) | 2.43 ± 0.50 a | 2.36 ± 0.28 c | 6.64 ± 0.16 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peighambardoust, S.H.; Fasihnia, S.H.; Peighambardoust, S.J.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization. Foods 2021, 10, 722. https://doi.org/10.3390/foods10040722
Peighambardoust SH, Fasihnia SH, Peighambardoust SJ, Pateiro M, Domínguez R, Lorenzo JM. Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization. Foods. 2021; 10(4):722. https://doi.org/10.3390/foods10040722
Chicago/Turabian StylePeighambardoust, Seyed Hadi, Seyedeh Homa Fasihnia, Seyed Jamaleddin Peighambardoust, Mirian Pateiro, Rubén Domínguez, and José M. Lorenzo. 2021. "Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization" Foods 10, no. 4: 722. https://doi.org/10.3390/foods10040722
APA StylePeighambardoust, S. H., Fasihnia, S. H., Peighambardoust, S. J., Pateiro, M., Domínguez, R., & Lorenzo, J. M. (2021). Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization. Foods, 10(4), 722. https://doi.org/10.3390/foods10040722