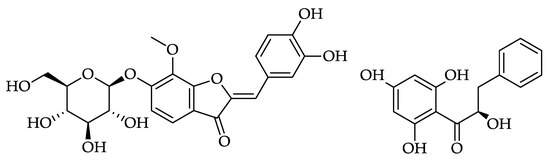
Abstract
The aim of this review is to provide a comprehensive overview of the large variety of phenolic compounds that have to date been identified in a wide range of monofloral honeys found globally. The collated information is structured along several themes, including the botanical family and genus of the monofloral honeys for which phenolic constituents have been reported, the chemical classes the phenolic compounds can be attributed to, and the analytical method employed in compound determination as well as countries with a particular research focus on phenolic honey constituents. This review covers 130 research papers that detail the phenolic constituents of a total of 556 monofloral honeys. Based on the findings of this review, it can be concluded that most of these honeys belong to the Myrtaceae and Fabaceae families and that Robinia (Robinia pseudoacacia, Fabaceae), Manuka (Leptospermum scoparium, Myrtaceae), and Chestnut (Castanea sp., Fagaceae) honeys are to date the most studied honeys for phenolic compound determination. China, Italy, and Turkey are the major honey phenolic research hubs. To date, 161 individual phenolic compounds belonging to five major compound groups have been reported, with caffeic acid, gallic acid, ferulic acid and quercetin being the most widely reported among them. HPLC with photodiode array detection appears to be the most popular method for chemical structure identification.
1. Introduction
Honey is a stored food of honeybees (Apis mellifera) that originates from plant nectar and is converted to honey with the aid of enzymes secreted from the glands of worker bees. Inside a colony, forager bees with full honey sacs transfer nectar into honeycombs and then flutter their wings to hasten the decrease in nectar moisture before worker bees seal the cells for storage [1]. Honeys are classified either as monofloral/unifloral or polyfloral/multifloral, the former being derived from a predominant botanical species, thus from mainly one type of nectar with only minor, if any, nectar contributions from other botanical sources. Polyfloral honeys, on the other hand, are linked to several botanical sources, none of which predominate [2].
Honey has been extensively used throughout history, not only as a food and food sweetener but also for medicinal purposes, which are associated, for example, with its antimicrobial and/or antioxidant properties [2,3,4,5]. However, honey’s potential health benefits can vary considerably due to the diversity of nectar collected by bees as they move from plant to plant [6]. Thus, different phytochemicals present in the nectar of melliferous plants contribute to the variability in honeys’ secondary metabolite profiles and this might also impact their bioactivity levels [3].
For bees, honey provides a rich source of carbohydrates, which is reflected in its chemical composition of at least 60% glucose and fructose combined, approximately 10% other sugar constituents and approximately 18% water. The remaining 2–3% of honey consists of a diverse mixture of more than 200 individual compounds including carotenoids, flavonoids and phenolics, along with several other minor components, such as proteins, free amino acids, minerals, vitamins and organic acids [7,8]. These minor constituents are considered to be very important in influencing not only the organoleptic characteristics of honeys but also their respective bioactivity profiles [3].
The term ‘phenolic’ or ‘polyphenol’ is chemically defined as a substance that possesses an aromatic ring bearing one or more hydroxyl substituents including functional derivatives such as esters and glycosides. These compounds, which can be further divided into subgroups such as phenolic acids and flavonoids, are extensively found across the plant kingdom and are closely linked to the sensory and nutritional quality of fresh and processed plant foods. Within the phenolic acid subgroup, hydroxybenzoic acid (such as methyl syringate, gallic acid, ellagic acid, protocatechuic acid, syringic acid, benzoic acid and 4-hydroxybenzoic acid), hydroxycinnamic acid (such as chlorogenic, vanillic, caffeic, p-coumaric and ferulic acids) and hydroxyphenylacetic acid (such as homogentisic and phenylacetic acids) derivatives have been detected in various honey samples around the world. Among the flavonoid groups, flavonols (such as myricetin, kaempferol, 8-methoxy kaempferol, quercetin, isorhamnetin, quercetin-3-methyl ether, quercetin-3,7-dimethyl ether, pinobanksin, rutin and galangin), flavones (such as genkwanin, luteolin, apigenin, tricetin and chrysin) and flavanones (such as pinocembrin and pinostrobin) have also been identified in some honeys [6].
Despite their relatively minor presence, phenolic compounds are one of the most studied honey constituents due to their well-known biological activities [3,9]. They are, furthermore, reported to influence the organoleptic characteristics of honeys [3] and can also potentially be used to identify or confirm the botanical origin of honeys [9]. Most of the floral markers in honey, which are derived from the nectar of melliferous plants, are flavonoids or phenolic acids. The identification of these compounds in honey can, thus, be an important tool for the recognition of its floral type [9]. Furthermore, phenolic compounds can also be used to monitor honey quality in order to choose the best processing practices [10].
A number of reviews have already been carried out on honey phenolics. These reviews can be categorized into honey phenolic analysis, determination and separation [11,12,13,14]; phenolics as authentication and marker compounds for the botanical origin of honey [2,15,16,17]; and honey phenolics and their associated health benefits [3,18,19,20,21,22]. A drawback of these reviews is that they tend to focus only on a few subsets of monofloral honeys found in particular regions. To our knowledge, a comprehensive review of all phenolic compounds isolated to date from honeys around the globe has not yet been published and is therefore the subject of this study. This paper, thus, presents a comprehensive survey of phenolic compounds reported across a very wide range of monofloral honeys from different geographical locations worldwide to provide an overview of their respective phenolic profiles and to assist with the identification of ubiquitous phenolics that are found across various floral sources and across different geographical locations. It also provides information on the botanical orgins of honeys for which phenolic constituents have to date been identified and allows determination of regional hotspots of research on phenolic honey constituents.
2. Materials and Methods
2.1. Literature Search
To ensure that a wide range of publications on phenolics and polyphenols present in honey are captured in this review, a thorough literature search was carried out by the Scopus database (published until January 2021) using combinations of the following sets of keywords: honey phenolic profile, monofloral honey and chemistry, honey and phytochemi*, honey and flavonoid* and honey phenol*. Only papers that were written in English and that reported on phenolic compounds in ripe (thus excluding nectar or unripe honey) monofloral honeys of nectar and honeydew origin produced by Apis mellifera bees were included in this review. Google Scholar was also used to further enhance the results of this initial literature search.
2.2. Data Tabulation and Representation
Information such as the common name, genus and/or species name, and the family of the monofloral honeys’ botanical source (verified using https://www.gbif.org accessed on 19 January–1 February 2022), country of origin, country where the research was performed, as well as the total number of analysed samples, the detected compounds, and the methods of detection/identification employed in compound determination were recorded and tabulated. The collated information was then grouped based on genus, botanical species and/or common name, and also based on the country where the research was performed.
Guided by pre-existing chemical classifications [23,24,25], the reported compounds were grouped into five classes, namely flavonoids, hydroxycinnamic acid derivatives (HCAD), hydroxybenzoic acid derivatives (HBAD), and miscellaneous/other phenolics as well as non-phenolic compounds. Furthermore, a CAS Registry Number (CAS No.) was assigned to each compound, and synonyms, molecular formula, molar mass, and Simplified Molecular Input Line Entry System (SMILES) information were also determined. Molecular structures were generated based on the SMILES information using ChemDraw version 20.0.0.41, PerkinElmer Informatics, Inc.
The prevalence of studies on phenolic constituents of honeys on a global level as well as in various geographical regions was visually represented using maps generated by ARCGIS Version 10.8. Redlands, CA, USA.
3. Results and Discussion
3.1. Botanical Classification
The literature search yielded a total of 130 original research articles that detail phenolic compounds identified in monofloral honeys in various countries around the globe. Since most studies analysed more than one monofloral honey, this review captures the published data for a total of 556 monofloral honeys. Their predominant botanical sources can be attributed to a total of 51 plant families; 90 of the reported monoflorals originated from members of the Fabaceae family, 88 from Myrtaceae, 56 from Lamiaceae, 41 from Ericaceae, 34 from Rutaceae, 33 from Fagaceae and 30 from Asteraceae. Thus, these seven plant families appear to be the most common botanical sources of honeys for which phenolic constituents have to date been reported in the literature.
The reviewed reports were further categorised into 159 monofloral groups taking into account not only the botanical family, but also the common name and/or the genus or species of the honeys’ botanical origin. It was found that 23 of these monofloral groups belonged to the Myrtaceae family, 17 were Fabaceae, 14 Lamiaceae, 12 Asteraceae and 9 belonged to the Rosaceae family.
It could also be determined from this grouping that Robinia honey (Robinia pseudoacacia, Fabaceae), which has been reported in 36 research papers, is the most studied honey with respect to phenolic constituents. This is followed by Manuka honey (Leptospermum scoparium, Myrtaceae) with 29 research papers, Chestnut honey (Castanea sp., Fagaceae) with 28, Linden honey (Tilia sp., Malvaceae) with 25, Rape honey (Brassica sp., Brassicaceae) and Heather honey (Calluna vulgaris and Erica sp. (L.) Hull, Ericaceae) with 24 each, Eucalyptus honey (Eucalyptus sp., Myrtaceae) with 21, Thyme honey (Thymus sp., Lamiaceae) and Buckwheat honey (Fagopyrum sp., Polygonaceae) with 16 reports each, and Sunflower honey (Helianthus sp., Asteraceae) with 15 reports on phenolic profiling. This grouping, based on the botanical origin of the honeys, was also used to structure the overview on phenolic compounds identified in the monofloral honeys (Table 1). The collated information was further used to create two groups of monofloral honeys—the first group containing high-frequency monoflorals (HFMs), where there are four or more studies reporting on their phenolic constituents (31 monofloral honey groups), and the second group, referred to as other monoflorals (OMs), having three or less studies dedicated to their phenolic composition (128 monofloral honey groups). To date, worldwide research efforts on phenolic constituents have mainly focused on the 31 monofloral honey groups referred to as HFMs in this review. The identification of the HFMs was also used in the construction of the regional maps of honey research shown in Section 3.3.

Table 1.
Summary of reported phenolic and other compounds in different monofloral honeys.
3.2. Global Hotspots of Honey Phenolics Research
Figure 1 presents the distribution of research on phenolic constituents in honeys across 37 countries. Countries that have yielded a high number of papers on phenolic honey constituents can be considered current ‘hot spots’ for this type of research. China leads the global research efforts with 76 reports on phenolic constituents in monofloral honeys, 76% of which were locally sourced. There were 74 papers originating from Italy with 89% of the reported samples being local honeys, and 45 reports from Turkey, all of which reported on Turkish honeys. Spain was also found to be a hotspot for research on phenolic honey constituents with 44 papers from this country, just under half of them (41%) reporting on locally sourced honeys. A total of 25 studies were carried out in Poland, with the vast majority (92%) investigating Polish honeys, 23 reports came from New Zealand (with 91% of the investigated honeys being local), 21 from Australia and 20 from Malaysia, both of which had 95% of the reported honeys sourced locally.
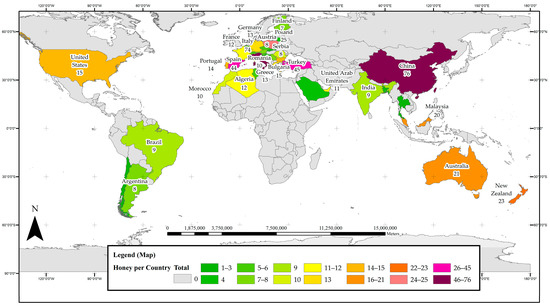
Figure 1.
Map of hotspots of honey phenolic research worldwide.
3.3. Regional Hotspots of Honey Phenolics Research
The distribution of studies on the phenolic constituents of honeys was further divided into four subregions to ascertain the most prevalent monoflorals studied in the respective geographical subregions of Australia and New Zealand; Asia; the Americas, and Africa and Europe. Figure 2, Figure 3, Figure 4 and Figure 5 detail the most frequently studied honeys in the four respective regions, with colour coding used for each region also conveying information on the respective popularity of honey phenolics research. The pie chart included in the maps allows assessing which monofloral honey species these regional research efforts were focused on.
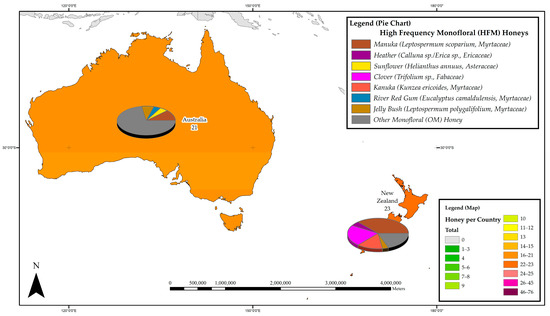
Figure 2.
Most frequently studied honeys with respect to their phenolic profile in Australia and New Zealand.
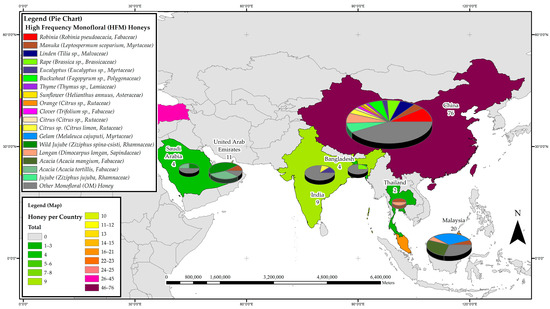
Figure 3.
Most frequently studied honeys with respect to their phenolic profile in Asia.
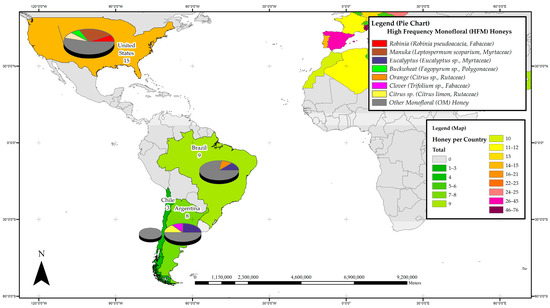
Figure 4.
Most frequently studied honeys with respect to their phenolic profile in the Americas.
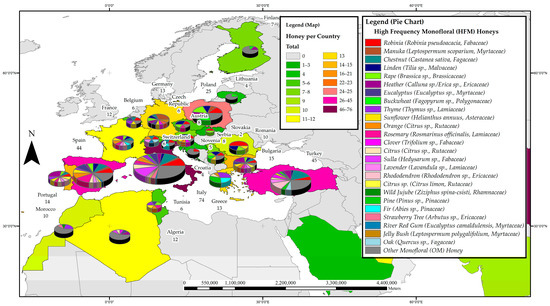
Figure 5.
Most frequently studied honeys with respect to their phenolic profile in Africa and Europe.
3.3.1. Australia and New Zealand
In Australia and New Zealand, the phenolic constituents of a total of 44 monofloral honeys belonging to 9 plant families have to date been reported, of which 31 belong to the Myrtaceae family, 4 are from the Fabaceae family and 2 from the Proteaceae family. New Zealand leads the region in honey phenolics research with 23 individual reports, 9 of which are attributed to phenolics research on Manuka honey. A total of 21 studies on honey phenolic constituents were carried out in Australia, 10 of which are focused on various Eucalyptus honeys (Myrtaceae). Within the region, Manuka (Leptospermum scoparium, Myrtaceae) honeys are the most studied, with 11 reports on their phenolic constituents, followed by Kanuka (Kunzea ericoides, Myrtaceae) and Clover honeys (Trifolium sp., Fabaceae), each with 4 reports, and Jelly bush honey (Leptospermum polygalifolium, Myrtaceae) and Rewarewa honey (Knightia excelsa, Proteaceae), with two reports each. Figure 2 details the most frequently studied honeys in the Australia and New Zealand region.
3.3.2. Asia
To date, research on honey phenolic constituents has been carried out in seven countries in Asia, namely Bangladesh, China, India, Malaysia, Saudi Arabia, Thailand and the United Arab Emirates. A total of 126 reports have been produced in the region, hotspots of research on honey phenolics being China with 76 studies, Malaysia with 20 and the United Arab Emirates with 11. Considering the botanical origin of the honeys studied in the region, 26 belong to the Fabaceae family, 17 are Myrtaceae, 10 Rhamnaceae and 8 each are Lamiaceae and Sapindaceae. The phenolic constituent profile of Gelam honey (Melaleuca cajuputi, Myrtaceae) appears to be the most studied in the region with seven reports, followed by that of New Zealand Manuka honey (Leptospermum scoparium), Rape honey (Brassica sp., Brasicaceae), Longan honey (Dimocarpus longan, Sapindaceae), Robinia honey (Robinia pseudoacacia L., Fabaceae) and Wild Jujube honey (Ziziphus spina-csisti, Rhamnaceae), which have been addressed in six reports each. Furthermore, the phenolic profile of Acacia honeys from Acacia mangium (Fabaceae) and Acacia tortilis (Fabaceae), Buckwheat honey (Fagopyrum esculentum, Polygonaceae) and Linden honey (Tilia sp., Malvaceae) have also been discussed in five studies each. Figure 3 details the most frequently studied honeys in the Asia region with respect to phenolic profile, and it visually conveys the importance of China for honey phenolic research in the region. Not only do the researchers in China report on a relatively large number of monofloral honeys, more than half of the honeys studied can be considered HFMs based on the reports generated. Thus, in many respects, China’s research efforts strongly influence what constitutes, seen through a global lense, a HFM honey.
3.3.3. The Americas
In the Americas region, Argentina, Brazil, Chile and the United States can be considered honey phenolics research hotspots with a total of 35 published studies reporting on the phenolic constituents of monofloral honeys from 16 plant families, 7 each from Fabaceae and Myrtacea. Most studies (15 reports) were carried out in the United States, 9 in Brazil and 8 in Argentina. Interestingly, within the Americas, the New Zealand Manuka honey (Leptospermum scoparium, Myrtaceae) was the most studied, with four individual reports, followed by three studies on the phenolic constituents of Eucalypt honey (Eucalyptus sp., Myrtaceae) and two each on Azara honey (Azara sp., Salicaceae), Schinus honey (Schinus terebinthifolius, Anacardiaceae) and Tupelo honey (Nyssa aquatica, Nyssaceae). Figure 4 details the most frequently studied honeys in the Americas and it can be seen that, with the exception of the United States, honey phenolic research in this vast region is mainly focused on regionally important honeys classified as OMs in this review, and have, on a global scale, not yet attracted considerable research attention.
3.3.4. Africa and Europe
Research on phenolic constituents in honey has been reported from 24 countries in the Africa and Europe region, totalling 351 reports on monofloral honeys from 31 families. Italy leads the region with 74 reports, followed by Turkey with 45, Spain with 44, and Poland with 25. Honeys of the Fabaceae family were researched the most (53 papers), followed by honeys from the Lamiaceae (47), Ericaceae (39), Myrtaceae (33), Fagaceae (32), Rutaceae (25), Asteraceae (23), Malvaceae (21), Brassicaceae (18) and Pinaceae (13) families. Of these, the Robinia honey (Robinia pseudoacacia L., Fabaceae) appears to be the most studied with 29 reports, followed by Chestnut honey (Castanea sativa Mill. Fagaceae) with 28, Heather honey (Calluna sp./Erica sp. Ericaceae) with 23, Linden honey (Tilia sp., Malvaceae) with 20, Rape honey (Brassica sp., Brasicaceae) with 18, and Eucalypt honey (Eucalyptus sp., Myrtaceae) and Thyme honey (Thymus sp. Lamiaceae) with 15 reports each. Additionally, attracting attention were the Rosemary honey (Rosmarinus officinalis L. Lamiaceae) and Sunflower honey (Helianthus sp., Asteraceae) with 13 studies each, as well as Orange honey (Citrus aurantium/sinensis, Rutaceae) with 12 and Buckwheat honey (Fagopyrum esculentum, Polygonaceae), Sulla honey (Hedysarum sp., Fabaceae) and Lavender honey (Lavandula sp. Lamiaceae) with 10 reports each. Figure 5 details the most frequently studied honeys in Africa and Europe. From the pie charts, it can be seen that most of the European countries appear to contribute research on HFMs and also tend to have a broader research focus than the African countries. It is also evident that Robinia (Robinia pseudoacacia L., Fabaceae) honey seems to attract a lot of research interest across Europe, reflected in the high number of individual research reports on this monofloral honey.
3.4. Phenolic Honey Constituents
Table 1 summarises the 170 compounds, 161 of them phenolic in nature, reported from the 159 monofloral honey groups covered by this literature review. Based on existing phenolic compound classifications with minor modifications, the compounds are grouped into five chemical classes, namely simple phenols (two groups), polyphenols, a miscellaneous and an ‘other phenolics’ group as well as non-phenolics [23,24,25,26,27,28]. Simple phenols include phenolic acids, which are chemically defined as carboxylic acid derivatives of phenols and are generally grouped into two subclasses, hydroxylcinnamic derivatives (HCAD group) and hydroxylbenzoic acids derivatives (HBAD group). A total of 20 HCADs and 21 HBADs were reported from honeys around the globe, making them the most common phenolic constituent class in honeys identified to date. Polyphenols, on the other hand, are a group of compounds which are characterized by the existence of more than one phenol unit or building block per molecule and can further be subdivided into two classes, tannins and flavonoids (flavonoid group) with the former being further grouped into hydrolysable and condensed tannins and the latter being divided, for example, into flavones, flavonols, flavanones, dihydroflavonols, chalcones, aurones, isoflavonoids, bioflavonoids [23,26].
This review has identified 89 honey constituents from the flavonoid class in total, 12 of them being flavones, 42 flavonols, 11 flavanones, 7 flavanonols, 7 isoflavonoids, 7 flavan-3-ols and 1 each of anthocyanidin, aurone and chalcone. The miscellaneous or ‘other phenolics group’ comprises all other phenolic compounds that do not fall into the above distinct subgroups. Thirty-one honey constituents reported to date belong in this category and include alkylmethoxyphenols (1), alkylphenols (3), hydroxybenzaldehydes (4), hydroxyacetophenones (3), other/miscellaneous phenolics (2), hydroxycoumarins (1), guaiacol (1), anthraquinones (1), naphtoquinones (1), hydroxyphenylacetic acids (6), hydroxyphenyllactic acids (3), hydroxyphenylpropanoic acids (2), hydroxyphenylpentanoic acids (1), benzyl oxalate esters (1), and stilbenes (1) [23,24]. Nine non-phenolic compounds, which were mostly reported as biomarkers for certain honeys, are also included in this review.
Among the reported compounds, caffeic acid (HCAD) is the most prevalent in honeys having been identified in 118 of the 159 investigated monofloral honeys. Gallic acid (HBAD) came in second with 106 reports, followed by p-coumaric acid (HCAD) with 104, ferulic acid (HCAD) with 103 and quercetin (flavonol) with 102 reports.
When analysing the reported honey constituents along the honeys’ respective botanical classification, it was found that 93, or 55%, of the identified, mostly phenolic compounds in honey have been found in Robinia honey (Robinia pseudoacacia, Fabaceae), 76 (45%) in Chestnut honey (Castanea sativa Mill., Fagaceae), 75 (44%) in Manuka honey (Leptospermum scoparium, Myrtaceae), 69 (41%), respectively, in various Eucalyptus honeys (Eucalyptus sp., Myrtaceae), Rape honey (Brassica sp. Brassicaceae) and Linden honey (Tilia sp., Malvaceae), and 63 (37%) in Sunflower honey (Helianthus annuus, Asteraceae).
3.4.1. Flavonoids
Chemically, flavonoids can be classified as polyphenols as they possess at least one hydroxyl substituent in their structure. They are made up of a flavane nucleus of 15 carbon atoms (C6-C3-C6) and are diphenyl-propanoids. The C6 and C3 moieties are arranged to form two fused rings in which the first is an oxygen-containing heterocycle and the second one is a benzene ring constituting a phenylchromane nucleus (2,3-dihydro-2-phenylchroman-4-one). To the base skeleton of the phenylchromane, a second phenyl substituent is linked and, according to the bond position (C2, C3, C4), flavanes, isoflavanes, and neoflavanes, respectively, can be distinguished [26]. These groups usually share a common chalcone precursor and are therefore biogenetically and structurally related [27]. On the other hand, as seen in Figure 6, on the basis of the substitution patterns of the three rings, several subclasses of flavonoids can be identified (e.g., flavones, flavonols, flavanones, flavanonols, isoflavanonoids, flavan-3-ols, and anthocyanidins) [26]. Other natural products such as chalcones and aurones also contain a C6–C3–C6 backbone and are thus considered minor flavonoids [27,28]. Flavonoids may exist as both aglycones and prenylated and methyl ethers, and in glycosylated forms incorporating sugar residues that can be linked to several positions of the three rings in form of both O- and C-glycosides [26].
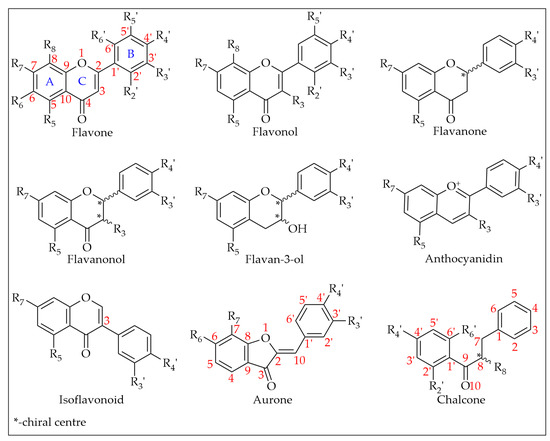
Figure 6.
Flavonoid subclasses reported in monofloral honeys.
Seventy percent of the reported flavonoids in honey were found as aglycones, probably due to the action of amylase in bee saliva, which can rapidly cleave glycosidic linkages to liberate flavonoid aglycones from the respective glycosides [29].
Flavonoids are synthesised in all parts of a plant and play an important role in providing color, fragrance and taste to fruits, flowers and seeds, making them attractants for insects, birds, and mammals, which aid in pollen and seed transmission [29,159,160,161,162]. However, plants also release numerous chemicals such as flavonoids to deter insects and other predators [159,160]. Aside from that, the strong light absorbance of flavonoids in the ultra-violet region also allows them to act as a protective screen against harmful UV-B radiation [29,162]. They also function as signal molecules, allopathic compounds, phytoalexins, detoxifying agents and antimicrobial defensive compounds [162]. Flavonoids, along with other phenolic compounds are responsible for the organoleptic characteristics of honey [3]. In honey, they originate not only from the nectar but, to an extent, also from plant pollen and plant resins collected by bees. Flavonoids can thus be considered as markers for the botanical and geographical origins of honeys [163] and have associated biological and pharmacological activities such as antioxidant [27,162,164,165], antimicrobial [164], anticancer [164,166,167], anti-inflammatory [162,164], antiallergic [164], antithrombotic [164], cardioprotective [164], hepatoprotective [164,168] neuroprotective [164], antimalarial [161], antileishmanial [161], anticholinesterase [162], anti-Alzheimer’s disease [169], antiulcer [164], antiatherosclerotic [164], antidiabetic [164], estrogenic effect [27], steroid-genesis modulators [162], vasorelaxant effect [164], improved blood flow [170], the inhibition of cholesterol absorption [171], countering antibiotic resistance [162], and protection from damage by ultraviolet B radiation [172].
Based on the findings of this study, the vast majority of monofloral honeys included in this review (82%) were reported to contain flavonoids, 89 different types in total. Robinia honey (Robinia pseudoacacia, Fabaceae) was found to contain 53 of the identified flavonoids in honey. 35 flavonoids, respectively, have to date been identified in Eucalyptus honey (Eucalyptus sp., Myrtaceae) and Linden honey (Tilia sp., Malvaceae), 34 in Chestnut honey (Castanea sativa Mill., Fagaceae) and 32 each in Manuka (Leptospermum scoparium, Myrtaceae) and Rape (Brassica sp. Brassicaceae) honeys.
Flavones
Flavones are a subclass of flavonoids that contain a double bond between C2 and C3 in the flavonoid skeleton, no substituent on the C3 position and the C4 position is oxidised (Figure 7). Along with flavonols, flavones are the primary pigments in white- and cream-colored flowers and act as copigments with anthocyanins in blue flowers. They also act as UV-B protectants in plants as they absorb in the 280–315 nm range [173].
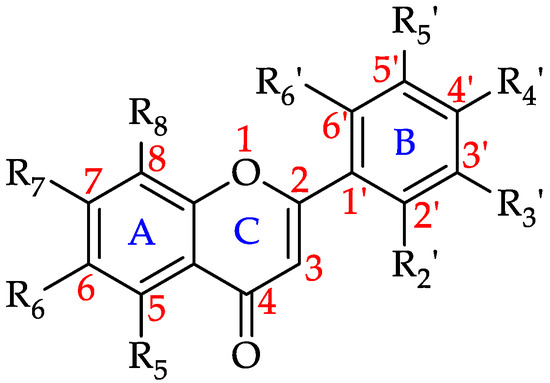
Figure 7.
Basic flavone structure (see 1–12 in Table 2).
Based on the findings of this comprehensive review, at least one flavone has to date been reported to be present in 64% of the monofloral honeys, with Robinia honey (Robinia pseudoacacia, Fabaceae) containing nine different flavones. Chrysin has been found to be the most common flavone, reported to be present in 83 monofloral honeys, followed by apigenin in 74, luteolin in 69, tectochrysin in 16 and acacetin in 15 honeys. Table 2 shows all the flavones that have to date been identified in monofloral honeys and the number of honeys in which they were identified.

Table 2.
Flavones reported in monofloral honeys (see Figure 7 for general structure).
Flavonols
Flavonols are naturally yellow in color (flavus is Latin for yellow) and are present in plant and fungi [174]. They are also known as 3-hydroxyflavones, the only difference to flavones being the hydroxyl group at C3 position. Flavonols are frequently found as O-glycosides, with glycosidation occuring mainly at the 3-position of the C-ring (Figure 8) [175]. Flavonols are primarily accrued in the epidermal cells of plant tissues and serve as a protection against solar radiation, especially UV-B. They also play an important role, along with xanthophylls, in protecting the photosynthetic apparatus in situ from excess solar radiation and are known to moderate drought-related oxidative damage because of their strong radical scavenging activity [176].
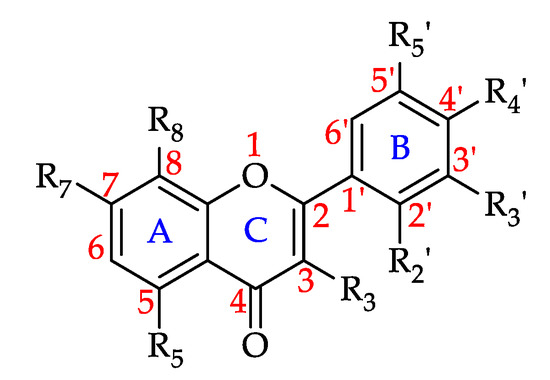
Figure 8.
Basic flavonol structure (see 13–54 in Table 3).
Based on the findings of this review, more than 74% of the honeys were found to contain at least one of the 42 reported flavonols. Acacia honey (Robinia pseudoacacia, Fabaceae) has the highest number of published studies reporting on its flavonols, followed by Eucalyptus honey (Eucalyptus sp., Myrtaceae) with 17 studies. Quercetin is the most commonly isolated flavonol reported to be present in 102 monofloral honey groups, followed by kaempferol in 89 honeys, galangin in 66, rutin in 58, myricetin in 54, and isorhamnetin in 43 monofloral honeys. Table 3 shows all the flavonols that have to date been identified in monofloral honeys and the number of monofloral honey groups for which they were reported.

Table 3.
Flavonols reported in monofloral honeys (see Figure 8 for general structure).
Flavanones
Flavanones, also referred to as dihydroxyflavones, are characterised by the lack of a double bond between C2 and C3 in the C-ring of the flavonoid skeleton, resulting in a chiral center at C2 (Figure 9) [177]. The chirality creates an angle between the B-ring relative to the A–C rings. This variation in the molecule’s structural orientation impacts flavanones’ interactions with biological receptors, in turn influencing their bioactivities [178,179].
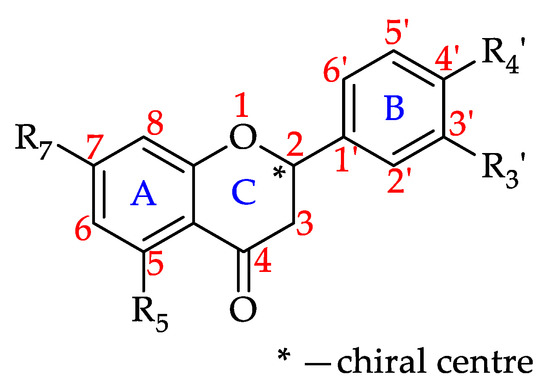
Figure 9.
Basic flavanone structure (see 55–65 in Table 4).
Based on the findings of this review, 62% of the honeys were reported to contain at least one of the 11 flavanones that to date have been isolated from honeys. Robinia honey (Robinia pseudoacacia, Fabaceae) has been found to contain nine of these flavanones. Pinocembrin has been identified in 64 monofloral honeys, followed by naringenin found in 54 and hesperitin in 49 honeys, respectively. Table 4 shows all the flavanones that have to date been identified in monofloral honeys and the number of honeys they have been reported to be present in.

Table 4.
Flavanones reported in monofloral honeys (see Figure 9 for general structure).
Flavanonols
Flavanonols, which are also known as dihydroflavonols, are 3-hydroxy derivatives of flavanones (Figure 10) [180]. This review found that the presence of at least one of the seven flavanonols that have to date been isolated in honey, was reported for 32% of the monofloral honeys. Four of these seven flavanonols were identified in Robinia honey (Robinia pseudoacacia, Fabaceae). Pinobanksin is the most prevalent flavanonol, reported to be present in 49 honeys. Table 5 shows all the flavanonols that that have to date been identified in monofloral honeys and the number of monofloral honeys in which they were found.
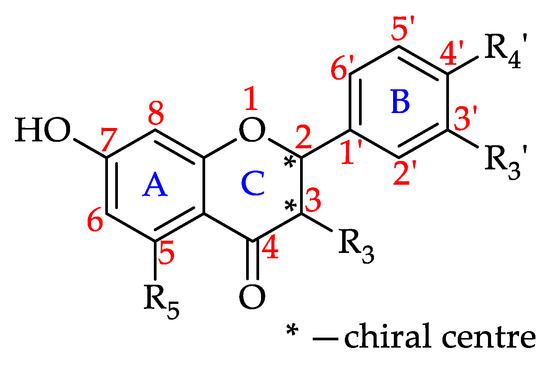
Figure 10.
Basic flavononol structure (see 66–72 in Table 5).

Table 5.
Flavanonols reported in monofloral honeys (see Figure 10 for general structure).
Flavan-3-ols
Flavan-3-ols or flavanols are also known as catechins. They are characterised by the absence of a double bond between C2 and C3 as well as the absence of a carbonyl on C4 of ring C. As a result, flavan-3-ols feature two chiral carbons and can form four possible diastereomers [181,182]. They exist in both monomeric (catechins) and in polymeric (proanthocyanidins) forms. The monomeric form can vary in its degree of hydroxylation at position 5 and 7 on ring A and at positions 3′, 4′ and 5′ on ring B. C3 of ring C usually carries a hydroxyl group or is esterified with gallic acid (gallate) (Figure 11) [183]. The polymeric form, also known as condensed tannin, features dimers, trimers, oligomers and polymers of flavan-3-ol units linked by C–C bonds either at 4–6 (A-type proanthocyanidins) or 4–8 (B-type proanthocyanidins). They are also classified as procyanidins when derived from catechin, epicatechin and their gallic esters [183].
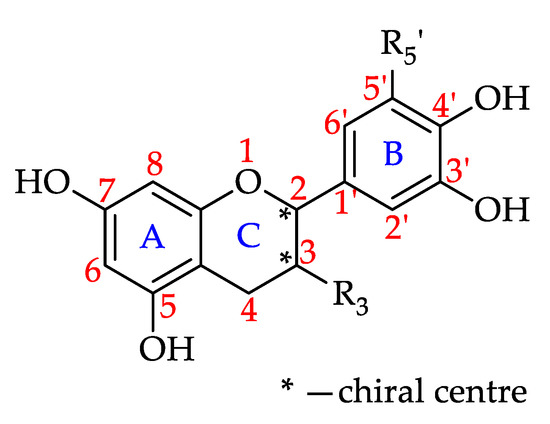
Figure 11.
Basic flavan-3-ol structure (see 73–80 in Table 6).
Seven flavan-3-ols have to date been identified in honeys with at least one flavan-3-ol reported to be present in just over a third (34.6%) of the monofloral honey groups. Five different flavan-3-ols have been identified in Sage honey (Salvia officinalis L., Lamiaceae), making it the honey with the highest number of reported flavan-3-ols. Catechin and epicatechin are the most prevalent flavan-3-ols in honeys, being present in 29 and 24 honeys, respectively. Table 6 shows all the flavan-3-ols that have to date been identified in monofloral honeys and the number of honeys for which their presence has been reported.

Table 6.
Flavan-3-ols reported in monofloral honeys (see Figure 1 for general structure).
Isoflavonoids
The structure of isoflavonoids is somewhat different to that of other flavonoids in so far that ring B is connected to C3 of ring C instead of C2 (Figure 12) [184]. Seven isoflavonoids have to date been identified in honey. They do not appear to be a particularly common honey constituent class as only 17% of the monofloral honeys covered by this review were found to contain them. Amonst them, Robinia honey (Robinia pseudoacacia, Fabaceae) was reported to contain six different isoflavonoids. Genistein is the most common identified isoflavonoid in honeys with 23 reports, followed by formononetin with 8 reports. Table 7 shows all the isoflavonoids that have to date been identified in monofloral honeys and the number of honeys they have been found in.
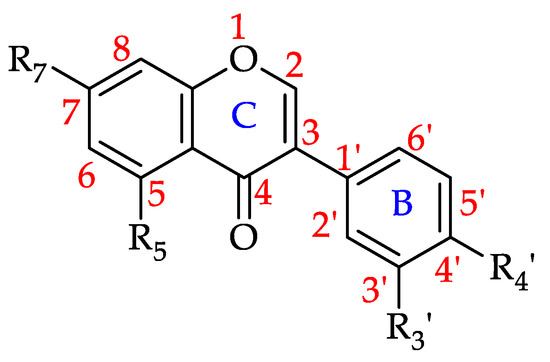
Figure 12.
Basic isoflavonoid structure (see 81–87 in Table 7).

Table 7.
Isoflavonoids reported in honeys (see Figure 12 for general structure).
Aurones and Chalcones
Due to their bright yellow color, the word aurones is derived from the Latin word aurum for gold. Aurones are considered a minor class of flavonoids. They also contain 15 carbon atoms, arranged in the general structure C6–C3–C6 (Figure 13). They occur in hydroxylated, methoxylated or glycosylated forms [185]. The word chalcone, on the other hand, is derived from the Greek word chalcos, meaning bronze, reflecting the typical colour of most natural chalcones [186]. Chalcones are α,β-unsaturated ketones (trans-1,3-diaryl-2-propen-1-ones) consisting of two aromatic rings attached to an α,β-unsaturated carbonyl system with a variety of substituents (Figure 8) [187]. Aurones and chalcones were only identified in 3% and 4%, respectively, of the monfloral honeys covered by this review. Table 8 details these compounds and the number of monofloral honey groups that were found to contain them.

Table 8.
Aurones and chalcones reported in monofloral honeys.
3.4.2. Hydroxycinnamic Acid and Its Derivatives
Hydroxycinnamic acid and its derivatives (HCADs) are phenolic acids that are prevalent in plants [188]. They can be considered hydroxy metabolites of cinnamic acid featuring a C6–C3 backbone (Figure 14) [189,190].
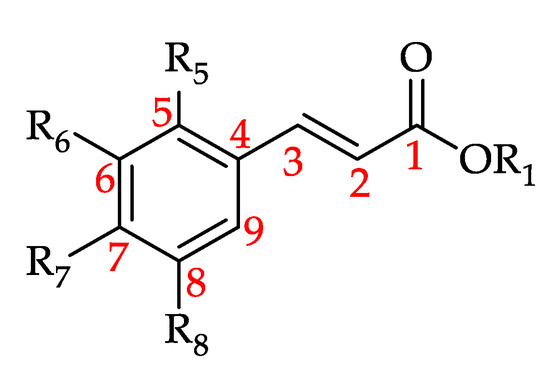
Figure 14.
Basic structure of hydroxycinnamic acid and its derivatives (HCADs) (see 90–109 in Table 9).
A high proportion, 88%, of the monfloral honey groups covered by this review were reported to contain at least 1 of the 20 HCADs that have to date been identified in honeys. Robinia honey (Robinia pseudoacacia, Fabaceae) had the highest number of HCADs, 15 in total, while 12 HCADs each were reported for Rape (Brassica sp., Brassicaceae) and Sunflower (Helianthus annuus, Asteraceae) honeys. Among the HCADs, caffeic acid appears to be the most prevalent, having been reported in 117 of the honeys, followed by p-coumaric acid in 103, ferulic acid in 102, chlorogenic acid in 85 and t-cinnamic acid in 57 honeys. Table 9 shows all the HCADs that have to date been identified in monofloral honeys and the number of honeys in which they were found to be present.

Table 9.
Hydroxycinnamic acid and its derivatives reported in monofloral honeys (see Figure 14 for general structure).
3.4.3. Hydroxybenzoic Acid and Its Derivatives
Hydroxybenzoic acid and its derivatives (HBADs) are phenolic metabolites featuring the general structure C6 ± C1 (Figure 15 and Figure 16) [191,192]. Of the monofloral honey groups covered by this review, 90% have been reported to contain at least one of the 21 HBADs that have to date been identified in honeys. Chestnut honey (Castanea sativa Mill., Fagaceae) and Manuka honey (Leptospermum scoparium, Myrtaceae) are reported to contain 16 of the HBADs, while Rape honey (Brassica sp., Brassicaceae) and Clover honey (Trifolium sp., Fabaceae) contain 15 each. Gallic Acid is the most prevalent HBAD with 105 reports, followed by syringic acid with 85, p-hydroxybenzoic acid with 79, vanillic acid with 66 and protocatechuic acid with 57. Table 10 shows all the HBADs that have to date been identified in monofloral honeys and the number of honeys which were found to contain them.
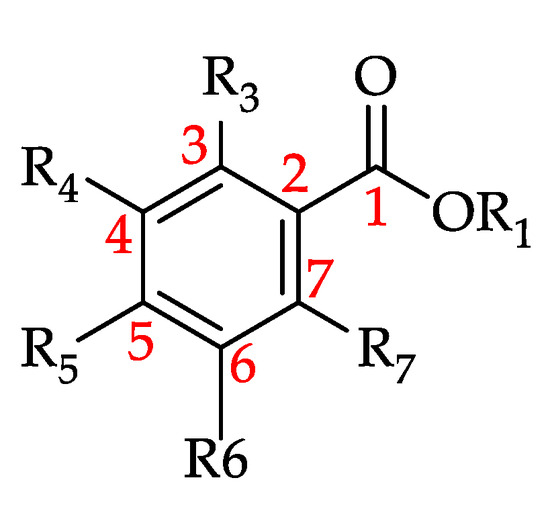
Figure 15.
Basic structure of hydroxybenzoic acid and its derivatives (HBADs) (see 110–130 in Table 10).
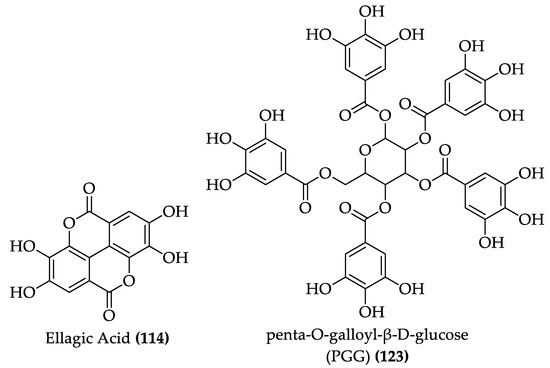
Figure 16.
Structure of ellagic acid and penta-O-galloyl-β-D-glucose (PGG) (122–123).
3.4.4. Miscellaneous and ‘Other’ Phenolics
Some uncommon phenolic compounds were also identified in the monofloral honeys. This miscellaneous or ‘other’ phenolics group comprises 31 phenolic compounds that do not fall into the subgroups discussed previously. They include six hydroxyphenylacetic acids (HPAAD) (Figure 17 and Table 11); three hydroxyphenyllactic acids (HPLAD) and two hydroxyphenylpropanoic acids (HPPAAD) (Figure 18 and Table 12); one hydroxyphenylpentanoic acid (Figure 19 and Table 13); one alkylmethoxyphenol, three alkylphenols, four hydroxybenzaldehydes and three hydroxyacethophenones, one guaiacol, and two other/miscellaneous phenolic compounds (Figure 20 and Table 14), one hydroxycoumarin, anthraquinone, naphtoquinone, benzyl oxalate ester, and stilbene, respectively (Figure 21 and Table 15).
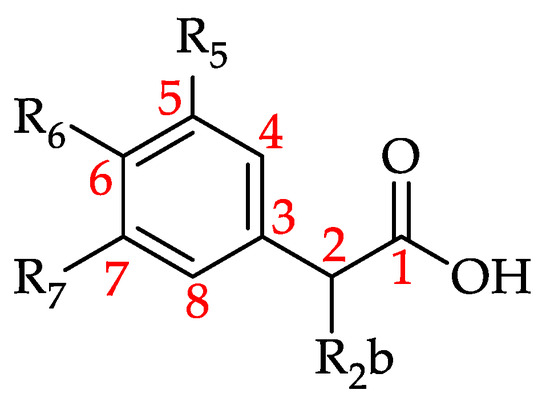
Figure 17.
Basic structure of hydroxyphenylacetic acid and derivatives (HPAAD) (see 131–136 in Table 11).

Table 11.
Hydroxyphenylacetic acid and derivatives (HPAAD) reported in monofloral honeys (see Figure 17 for general structure).
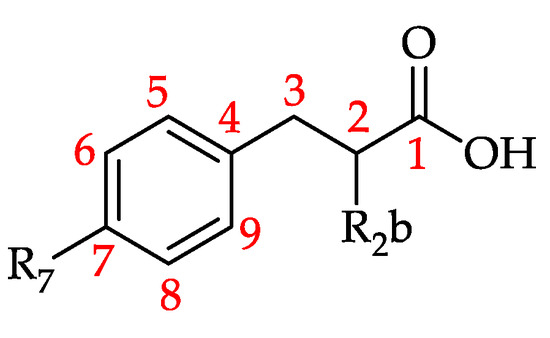

Table 12.
Hydroxyphenyllactic acid and derivatives (HPLAD) and hydroxyphenylpropanoic acid and derivatives (HPPAD) reported in monofloral honeys (see Figure 18 for general structure).
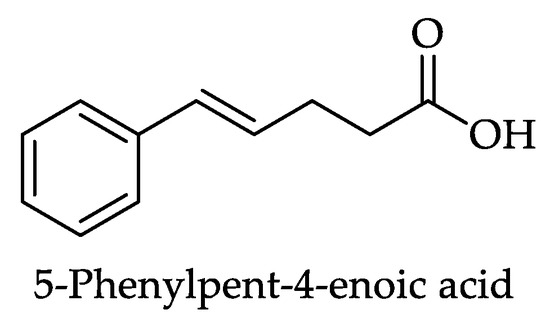
Figure 19.
Structure of 5-phenylpent-4-enoic acid, a hydroxyphenylpentanoic acid (HPPeA) (142, Table 13).

Table 13.
Hydroxyphenylpentanoic acid (HPPeA) reported in monofloral honeys.
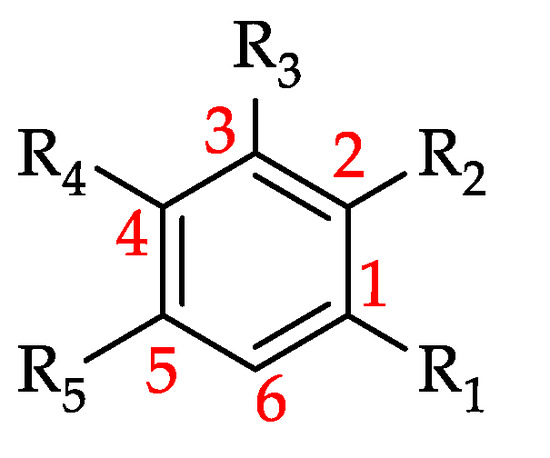
Figure 20.
Basic structure of other phenolic compounds (see 143–156 in Table 14).

Table 14.
Alkylmethoxyphenol, alkylphenols, hydroxybenzaldehydes, hydroxybenzoketones and hydroxyphenylketone reported in monofloral honeys (see Figure 20 for general structure).
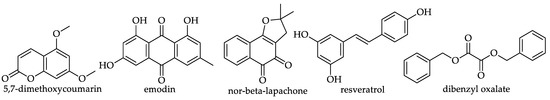

Table 15.
Hydroxycoumarin, anthraquinone, naphthoquinone, stilbenes and benzyl oxalate ester reported in monofloral honeys.
Slightly over one-quarter (27%) of the monofloral honey groups covered by this review were reported to contain one or more of the miscellaneous or ‘other’ phenolic compounds. A total of 20 of them were identified in Chestnut honey (Castanea sativa Mill., Fagaceae), 19 in Robinia honey (Robinia pseudoacacia, Fabaceae), 17 in Lavender honey (Lavandula sp., Lamiaceae) and 15 in Clover honey (Trifolium sp., Fabaceae). Protocatechualdehyde (hydroxybenzaldehyde) was reported in 40 studies, homogentisic acid (hydroxyphenylacetic acid-HPAAD) in 24, DL-β-phenyllactic acid (hydroxyphenyllactic acid HPLAD) in 23, vanillin (hydroxybenzaldehyde) in 18, phenylacetic acid (HPAAD) and p-hydroxyphenylacetic acid (HPAAD) in 17 each and 3-phenyl propionic acid (hydroxyphenylpropanoic acids-HPPAD) was identified in 13 reports on honey constituents. Table 11, Table 12, Table 13, Table 14 and Table 15 show all the miscellaneous/‘other’ phenolic constituents that that have to date been identified in monofloral honeys and the number of honeys for which their presence has been reported.
3.4.5. Non-Phenolic Compounds
Nine non-phenolic compounds were also reported in 26.7% of the monofloral honey groups covered by this review. Manuka honey (Leptospermum scoparium, Myrtaceae) was reported to contain 6 of the 9 non-phenolic compounds, 5 were identified in Kanuka honey (Kunzea ericoides, Myrtaceae) and 4 in Eucalyptus honey (Eucalyptus sp., Myrtaceae). Absiscic acid, which has been detected in 36 honeys, is the most commonly reported non-phenolic honey constituent. Figure 22 and Table 16 detail the different non-phenolic compounds identified to date in the monofloral honeys.
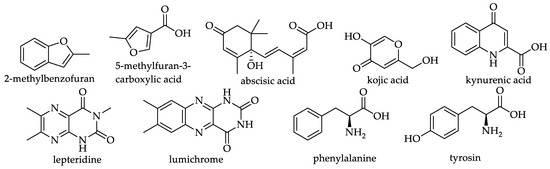
Figure 22.
Structures of non-phenolic compounds (see 162–170 in Table 16).

Table 16.
Non-phenolic compounds reported in monofloral honeys.
3.5. Analytical Methods Used in Compound Detection
Table 17 details the different analytical methods found in this review for the detection of phenolic compounds in the monofloral honeys. It is evident that the phenolic compounds were mostly identified by high-performance liquid chromatography (HPLC) (67%) using either UV, UV–Vis, UV–UV, photodiode array (DAD or PDA), DAD–UV, electron capture (ECD), or EDC–UV as detectors. Almost one-quarter (24%) of the reports indicated the use of liquid chromatography coupled with mass spectrometry (LC–MS), 5% used a combination of HPLC, LC–MS and/or gas chromatography coupled with mass spectrometry (GC–MS), 1% of the analyses used gas chromatography coupled with mass spectrometry (GC–MS) and high-performance thin-layer chromatography (HPTLC), respectively, and finally, less than 1% used fluorescence spectroscopy to identify the phenolic compounds.

Table 17.
Analytical methods used in phenolic compound analysis for monofloral honeys.
4. Conclusions
This review investigated 130 original research articles that detailed the phenolic compounds identified in 556 monofloral honeys. The honeys from 51 botanical families were grouped into 159 monofloral groups. Most of the monofloral honeys belonged to the Myrtaceae and Fabaceae families. The Robinia honey (Robinia pseudoacacia, Fabaceae), Manuka honey (Leptospermum scoparium, Myrtaceae) and Chestnut honey (Castanea sp., Fagaceae) were the most studied monofloral honeys for their phenolic constituents. China, Italy and Turkey were the major hubs the honey phenolic research. A total of 161 phenolic compounds were reported in the honeys and these were classified in this review into five major compound groups, namely flavonoids, hydroxycinnamic acid and its derivatives (HCAD), hydroxybenzoic acid and its derivatives (HBAD), miscellaneous or ‘other phenolics’, as well as nine non-phenolics which were mainly used as marker compounds for specific monofloral honeys. Hydroxycinnamic acid derivatives (HCAD) and hydroxybenzoic acid derivatives (HBAD) were the most prevalent phenolic constituents in the monofloral honeys, with caffeic acid, gallic acid, ferulic acid, and quercetin being the most reported phenolic compounds. Robinia honey (Robinia pseudoacacia, Fabaceae), Chestnut honey (Castanea sativa Mill., Fagaceae), and Manuka honey (Leptospermum scoparium, Myrtaceae) were the monofloral honeys for which the highest number of phenolic compounds has to date been identified. Most of these phenolic compounds were detected and structurally identified using HPLC.
The information compiled in this review can serve as a guide for future research into the identification of phenolic compounds in honey. It illustrates which geographical locations are very active in phenolics research in honey. It also provides information for which monofloral honeys worldwide phenolic compounds have already been determined. Moreover, it also details the specific phenolic constituents that have to date been detected in monofloral honeys and the analytical methods used to identify them. In doing so, it assists with the identification of common or ubiquitous phenolic honey constituents and those that to date have only been found in specific monofloral honeys or honeys derived from particular botanical families.
Author Contributions
Conceptualisation, I.L.L. and C.L.; methodology, I.L.L. and C.L.; formal analysis, I.L.L.; software, I.L.L. and R.J.; writing—original draft preparation, I.L.L.; writing—review and editing, C.L. and L.-Y.L.; supervision, C.L., L.-Y.L. and K.A.H.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cooperative Research Centre for Honey Bee Products (CRC HBP) Project 33.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pattamayutanon, P.; Angeli, S.; Thakeow, P.; Abraham, J.; Disayathanoowat, T.; Chantawannakul, P. Biomedical Activity and Related Volatile Compounds of Thai Honeys from 3 Different Honeybee Species. J. Food Sci. 2015, 80, M2228–M2240. [Google Scholar] [CrossRef]
- Shen, Y.-B.; Tian, H.-X.; Chen, C. Research on identification methods of varieties of honey. Shipin Gongye 2016, 37, 251–254. [Google Scholar]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; Silva, W.P.D.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT—Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef] [Green Version]
- Sant’ana, L.D.O.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. Int. J. Food Prop. 2014, 17, 65–76. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Tulipani, S.; Romandini, S.; Vidal, A.; Battino, M. Methodological Aspects about Determination of Phenolic Compounds and In Vitro Evaluation of Antioxidant Capacity in the Honey: A Review. Curr. Anal. Chem. 2009, 5, 293–302. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Gomez-Romero, M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T. Analysis of Polyphenols in Honey: Extraction, Separation and Quantification Procedures. Sep. Purif. Rev. 2017, 47, 142–158. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gasic, U.M.; Milojkovic-Opsenica, D.M.; Tesic, Z.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Kaskoniene, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Suen, A.B. A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. Int. J. Mol. Sci. 2012, 13, 15054–15073. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E. Honey and its Phytochemicals: Plausible Agents in Combating Colon Cancer through its Diversified Actions. J. Food Biochem. 2016, 40, 613–629. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Abbas, N. Chapter 4—Chemistry of Himalayan Phytochemicals. In Himalayan Phytochemicals; Jan, S., Abbas, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–166. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzand, K. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res. 2012, 5, 37. [Google Scholar]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The Stereochemistry of Flavonoids. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Deadman, B.J. The Flavonoid Profile of New Zealand Manuka Honey. Master of Science (MSc) Thesis, The University of Waikato, Hamilton, New Zealand, 2009; pp. 1–270. Available online: https://hdl.handle.net/10289/5443 (accessed on 1 March 2022).
- Alvarez-Suarez, J.M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant Characterization of Native Monofloral Cuban Honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar] [CrossRef]
- Souza do Nascimento, K.; Sattler, J.A.G.; Macedo, L.F.L.; Gonzalez, C.V.S.; Pereira de Melo, I.L.; Araujo, E.D.S.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT—Food Sci. Technol. 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT—Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Evid. Based Complement. Altern. Med. 2019, 2019, 8768210. [Google Scholar] [CrossRef]
- Istasse, T.; Jacquet, N.; Berchem, T.; Haubruge, E.; Nguyen, B.K.; Richel, A. Extraction of Honey Polyphenols: Method Development and Evidence of Cis Isomerization. Anal. Chem. Insights 2016, 11, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Chen, X.; Liu, T.; Zhuo, Q.; Zhang, S.-Q. Evaluation of cellular antioxidant components of honeys using UPLC-MS/MS and HPLC-FLD based on the quantitative composition-activity relationship. Food Chem. 2019, 293, 169–177. [Google Scholar] [CrossRef]
- Marshall, S.M.; Schneider, K.R.; Cisneros, K.V.; Gu, L. Determination of antioxidant capacities, α-dicarbonyls, and phenolic phytochemicals in florida varietal honeys using HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2014, 62, 8623–8631. [Google Scholar] [CrossRef]
- Aljadi, A.; Mohd Yusoff, K. Isolation and Identification of Phenolic Acids in Malaysian Honey with Antibacterial Activities. Turk. J. Med. Sci. 2003, 33, 229–236. [Google Scholar]
- Petretto, G.L.; Cossu, M.; Alamanni, M.C. Phenolic content, antioxidant and physico-chemical properties of Sardinian monofloral honeys. Int. J. Food Sci. Technol. 2015, 50, 482–491. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Ahmed, Y.B.H.; Alothman, Z.A.; Obbed, M.S.; Al-Harbi, N.M.; Al-Turki, T.M. Ultra high performance liquid chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multiwalled carbon nanotubes as extraction sorbents. J. Sep. Sci. 2015, 38, 2597–2606. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Santos-Buelga, C.; Era, B.; González-Paramás, A.M.; Tuberoso, C.I.G.; Medda, R.; Pintus, F.; Fais, A. Sardinian honeys as sources of xanthine oxidase and tyrosinase inhibitors. Food Sci. Biotechnol. 2018, 27, 139–146. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersive liquid–liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, F.B.; Lira, A.F.; Rumjanek, V.M.; Castro, R.N. Phenolic Composition and Antioxidant Properties of Brazilian Honeys. Quim. Nova 2014, 37, 821–826. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Rel. Technol. 1997, 20, 2281–2288. [Google Scholar] [CrossRef]
- Soler, C.; Gil, M.I.; García-Viguera, C.; Tomás-Barberán, F.A. Flavonoid patterns of French honeys with different floral origin. Apidologie 1995, 26, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F.A. Liquid chromatography-tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomas-Barberan, F.A. Flavonoid Composition of Tunisian Honeys and Propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2016, 20, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Zhang, J.; Li, Y.; Chen, L.; Zhao, W.; Zhou, J.; Jin, Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules 2017, 22, 735. [Google Scholar] [CrossRef] [Green Version]
- Mattonai, M.; Parri, E.; Querci, D.; Degano, I.; Ribechini, E. Development and validation of an HPLC-DAD and HPLC/ESI-MS 2 method for the determination of polyphenols in monofloral honeys from Tuscany (Italy). Microchem. J. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Joerg, E.; Sontag, G. Multichannel coulometric detection coupled with liquid chromatography for determination of phenolic esters in honey. J. Chromatogr. 1993, 635, 137–142. [Google Scholar] [CrossRef]
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Gruyaert, S.; Poliwoda, A.; Kafarski, P. Chemical Profiling of Polyfloral Belgian Honey: Ellagic Acid and Pinocembrin as Antioxidants and Chemical Markers. J. Chem. 2017, 2017, 5393158. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Zhuo, Q.; Chen, X.; Liu, T.; Zhang, S.Q. Quantitative and discriminative evaluation of contents of phenolic and flavonoid and antioxidant competence for chinese honeys from different botanical origins. Molecules 2018, 23, 1110. [Google Scholar] [CrossRef] [Green Version]
- Ciucure, C.T.; Geana, E.-I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, J.; Li, X.; Wang, W.; Huang, Z. Sugaring-out assisted liquid-liquid extraction coupled with high performance liquid chromatography-electrochemical detection for the determination of 17 phenolic compounds in honey. J. Chromatogr. A 2019, 1601, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT—Food Sci. Technol. 2010, 43, 52–58. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Kovacik, J.; Gruz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Kosice (eastern Slovakia). Environ. Sci. Pollut. Res. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, N.; Xue, X.; Wu, L.; Zhu, X.; Cao, W. Chromatographic ECD fingerprints combined with a chemometric method used for the identification of three light-coloured unifloral honeys. Anal. Methods 2015, 7, 8393–8401. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yung, A.C.; Azlan, S.A.B.M.; Sulaiman, S.A.; Rao, P.V.; Hawlader, M.N.I.; Gan, S.H. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: Determination of antioxidant capacity. Biomed. Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Du, X.; Cheng, N.; Chen, L.; Zhao, J.; Zheng, J.; Cao, W. Identification of Acacia Honey Adulteration with Rape Honey Using Liquid Chromatography–Electrochemical Detection and Chemometrics. Food Anal. Methods 2014, 7, 2003–2012. [Google Scholar] [CrossRef]
- Scripca, L.A.; Norocel, L.; Amariei, S. Comparison of Physicochemical, Microbiological Properties and Bioactive Compounds Content of Grassland Honey and other Floral Origin Honeys. Molecules 2019, 24, 2932. [Google Scholar] [CrossRef] [Green Version]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT—Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Truchado, P.; Tourn, E.; Gallez, L.M.; Moreno, D.A.; Ferreres, F.; Tomás-Barberán, F.A. Identification of botanical biomarkers in argentinean diplotaxis honeys: Flavonoids and glucosinolates. J. Agric. Food Chem. 2010, 58, 12678–12685. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Mohd Yusof, Y.A. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378–6395. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdul Kadir, M.R.; Mahmood, N.H.; Singh, O.P.; Iqbal, N.; Zulkifli, R.M. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J. Apic. Res. 2016, 55, 137–150. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Amrah Sulaiman, S.; Gan, S.H. Phenolic Acid and Flavonoid Composition of Malaysian Honeys. J. Food Biochem. 2016, 41, e12282. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Novellino, E. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula). Food Chem. Toxicol. 2012, 50, 1955–1961. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gonzalez-Paramas, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Leyton, F.; Ascar, L.; Ramirez, O.; Giordano, A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CyTA—J. Food 2020, 18, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Daniels, B.J.; Middleditch, M.J.; Furkert, D.P.; Brimble, M.A.; Bong, J.; Stephens, J.M.; Loomes, K.M. Utility of the Leptospermum scoparium Compound Lepteridine as a Chemical Marker for Manuka Honey Authenticity. ACS Omega 2020, 5, 8858–8866. [Google Scholar] [CrossRef] [Green Version]
- Weston, R.J.; Brocklebank, L.K.; Lu, Y. Identification and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 2000, 70, 427–435. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 11, 2979–2989. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Canini, A.; Pichichero, E.; Alesian, D.; Canuti, L.; Leonardi, D. Nutritional and botanical interest of honey collected from protected natural areas. Plant Biosyst. Int. J. Deal. All. Asp. Plant Biol. 2009, 143, 62–70. [Google Scholar] [CrossRef]
- Aygul, I.; Yaylaci Karahalil, F.; Supuran, C.T. Investigation of the inhibitory properties of some phenolic standards and bee products against human carbonic anhydrase I and II. J. Enzyme Inhib. Med. Chem. 2016, 31, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Can, Z. Determination of in-vitro antioxidant, anti-urease, anti-hyaluronidase activities by phenolic rich bee products from different region of Turkey. Fresenius Environ. Bull. 2018, 27, 6858–6866. [Google Scholar]
- Huttunen, S.; Riihinen, K.; Kauhanen, J.; Tikkanen-Kaukanen, C. Antimicrobial activity of different Finnish monofloral honeys against human pathogenic bacteria. APMIS 2013, 121, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Oelschlaegel, S.; Koelling-Speer, I.; Speer, K. Determination of the floral origin of honey by secondary plant metabolites. Dtsch. Lebensm.-Rundsch. 2012, 108, 415–418. [Google Scholar]
- Silici, S.; Sarioglu, K.; Dogan, M.; Karaman, K. HPLC-DAD Analysis to Identify the Phenolic Profile of Rhododendron Honeys Collected from Different Regions in Turkey. Int. J. Food Prop. 2014, 17, 1126–1135. [Google Scholar] [CrossRef]
- Salonen, A.; Julkunen-Tiitto, R. Characterisation of two unique unifloral honeys from the boreal coniferous zone: Lingonberry and mire honeys. Agric. Food Sci. 2012, 21, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Jakštas, V. The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 2012, 132, 1544–1548. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tan, T.T.E. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 9, 313798. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem. 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Guo, P.; Deng, Q.; Lu, Q. Anti-alcoholic effects of honeys from different floral origins and their correlation with honey chemical compositions. Food Chem. 2019, 286, 608–615. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Gambacorta, E.; Simonetti, A.; Garrisi, N.; Intaglietta, I.; Perna, A. Antioxidant properties and phenolic content of sulla (Hedysarum spp.) honeys from Southern Italy. Int. J. Food Sci. Technol. 2014, 49, 2260–2268. [Google Scholar] [CrossRef]
- Iurlina, M.; Saiz, A.; Fritz, R.; Manrique, G. Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chem. Food Chem. 2009, 115, 1141–1149. [Google Scholar] [CrossRef]
- Ciappini, M.C. Polyhenolic profile of floral honeys in correlation with their pollen spectrum. J. Apic. Res. 2019, 58, 772–779. [Google Scholar] [CrossRef]
- Isla, M.I.; Craig, A.; Ordonez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomon, V.; Maldonado, L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT—Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y.; Zhang, H. Phenolics and abscisic acid identified in acacia honey comparing different SPE cartridges coupled with HPLC-PDA. J. Food Compost. Anal. 2016, 53, 91–101. [Google Scholar] [CrossRef]
- Daher, S.; Gülaçar, F.O. Analysis of Phenolic and Other Aromatic Compounds in Honeys by Solid-Phase Microextraction Followed by Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Marghitas, L.A.; Dezmirean, D.S.; Pocol, C.B.; Ilea, M.; Bobis, O.; Gergen, I. The development of a biochemical profile of acacia honey by identifying biochemical determinants of its quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 84–90. [Google Scholar]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Rostislav, H.; Petr, T.; Sanja Ćavar, Z. Characterisation of phenolics and other quality parameters of different types of honey. Czech J. Food Sci. 2016, 34, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoacacia L. nectar to Acacia monofloral honey: Biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Polak, T.; Kropf, U.; Korosec, M.; Golob, T. LC-DAD-ESI/MS analysis of flavonoids and abscisic acid with chemometric approach for the classification of Slovenian honey. Food Chem. 2011, 127, 296–302. [Google Scholar] [CrossRef]
- Stanek, N.; Kafarski, P.; Jasicka-Misiak, I. Development of a high performance thin layer chromatography method for the rapid qualification and quantification of phenolic compounds and abscisic acid in honeys. J. Chromatogr. A 2019, 1598, 209–215. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Lin, B.; Loomes, K.M.; Prijic, G.; Schlothauer, R.; Stephens, J.M. Lepteridine as a unique fluorescent marker for the authentication of manuka honey. Food Chem. 2017, 225, 175–180. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Yildiz, O.; Sahin, H.; Karaoglu, S.A. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill.) honeys. J. Enzyme Inhib. Med. Chem. 2016, 31, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93, 1169–1175. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio Petronio, G.; Straquadanio, C.; Crasci, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2019, 33, 843–850. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2017, 41, e12345. [Google Scholar] [CrossRef]
- Çol Ayvaz, M.; Ömür, B.; Ertürk, Ö.; Kabakçi, D. Phenolic profiles, antioxidant, antimicrobial, and DNA damage inhibitory activities of chestnut honeys from Black Sea Region of Turkey. J. Food Biochem. 2018, 42, e12502. [Google Scholar] [CrossRef]
- Sarikaya, A.L.I.; Ulusoy, E.; Öztürk, N.; Tuncel, M.; Kolayli, S. Antioxidant activity and phenolic acid constituents of chestnut (Castania Sativa Mill.) Honey and Propolis. J. Food Biochem. 2009, 33, 470–481. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Cakir, H.E.; Okan, O.T.; Yildiz, O. An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: Their physico-chemical, antioxidant and phenolic compounds properties. Turk. J. Biochem. 2018, 43, 362–374. [Google Scholar] [CrossRef]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT—Food Sci. Technol. 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT 2016, 74, 504–513. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Gyergyák, K.; Boros, B.; Marton, K.; Felinger, A.; Papp, N.; Farkas, Á. Bioactive constituents and antioxidant activity of some carpathian basin honeys. Nat. Prod. Commun. 2016, 11, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Gómez-Caravaca, A.M.; Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds in rosemary honey using solid-phase extraction by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2006, 41, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Ferreres, F.; Ortiz, A.; Subra, E.; Tomas-Barberan, F.A. Plant Phenolic Metabolites and Floral Origin of Rosemary Honey. J. Agric. Food Chem. 1995, 43, 2833–2838. [Google Scholar] [CrossRef]
- Gašić, U.M.; Natić, M.M.; Mišić, D.M.; Lušić, D.V.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compost. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Čačić, F. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem. 2008, 110, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Zekić, M.; Radonić, A.; Tuberoso, C.I.G. Screening of Satureja subspicata Vis. honey by HPLC-DAD, GC-FID/MS and UV/VIS: Prephenate derivatives as biomarkers. Molecules 2016, 21, 377. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Vavoura, M.V.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Differentiation of Greek Thyme Honeys According to Geographical Origin Based on the Combination of Phenolic Compounds and Conventional Quality Parameters Using Chemometrics. Food Anal. Methods 2014, 7, 2113–2121. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Dimitriou, E.; Kontakos, S.; Kontominas, M.G. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur. Food Res. Technol. 2016, 242, 1201–1210. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef] [Green Version]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Zhao, J.; Du, X.; Cheng, N.; Chen, L.; Xue, X.; Zhao, J.; Wu, L.; Cao, W. Identification of monofloral honeys using HPLC-ECD and chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef]
- Gašić, U.; Šikoparija, B.; Tosti, T.; Trifković, J.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. Phytochemical fingerprints of lime honey collected in Serbia. J. AOAC Int. 2014, 97, 1259–1267. [Google Scholar] [CrossRef]
- Devi, A.; Jangir, J.; Anu-Appaiah, K.A. Chemical characterization complemented with chemometrics for the botanical origin identification of unifloral and multifloral honeys from India. Food Res. Int. 2018, 107, 216–226. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomas-Barberan, F.A. Flavonoids in Monospecific Eucalyptus Honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids and abscisic acid in Australian Eucalyptus honeys and their potential for floral authentication. Food Chem. 2004, 86, 169–177. [Google Scholar] [CrossRef]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New approach: Chemical and fluorescence profiling of NZ honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef]
- Beitlich, N.; Koelling-Speer, I.; Oelschlaegel, S.; Speer, K. Differentiation of manuka honey from kanuka honey and from jelly bush honey using HS-SPME-GC/MS and UHPLC-PDA-MS/MS. J. Agric. Food Chem. 2014, 62, 6435–6444. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomas-Barberan, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Hempattarasuwan, P.; Settachaimongkon, S.; Duangmal, K. Impact of botanical source and processing conditions on physicochemical properties and antioxidant activity of honey in the northern part of Thailand. Int. J. Food Sci. Technol. 2019, 54, 3185–3195. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Rückriemen, J.; Henle, T. Pilot study on the discrimination of commercial Leptospermum honeys from New Zealand and Australia by HPLC–MS/MS analysis. Eur. Food Res. Technol. 2018, 244, 1203–1209. [Google Scholar] [CrossRef]
- Stephens, J.M.; Loomes, K.M.; Braggins, T.J.; Bong, J.; Lin, B.; Prijic, G. Fluorescence: A Novel Method for Determining Manuka Honey Floral Purity. In Honey Analysis; IntechOpen: London, UK, 2017. [Google Scholar]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Gardini, S.; Marcazzan, G.L.; Caboni, M.F. Buckwheat honeys: Screening of composition and properties. Food Chem. 2013, 141, 2802–2811. [Google Scholar] [CrossRef]
- Cheng, N.; Wu, L.; Zheng, J.; Cao, W. Buckwheat Honey Attenuates Carbon Tetrachloride-Induced Liver and DNA Damage in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 987385. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, P.; Cheng, N.; Gao, H.; Wang, B.; Wei, Y.; Cao, W. Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem. Toxicol. 2012, 50, 2766–2773. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, W.; Chen, W.; Xiao, X.-H.; Zheng, J.-B. Simultaneous determination of four phenolic components in citrus honey by high performance liquid chromatography using electrochemical detection. Food Chem. 2009, 114, 1537–1541. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Lianda, R.; Sant’Ana, L.; Echevarria, A.; Nora Castro, R. Antioxidant Activity and Phenolic Composition of Brazilian Honeys and their Extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Retamal, M.; Leyton, F.; Martinez, P.; Bridi, R.; Velasquez, P.; Montenegro, G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia Honeys. CyTA—J. Food 2018, 16, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Kuś, P.M.; Tuberoso, C.I.G.; Šarolić, M. Phytochemical and physical-chemical analysis of Polish willow (Salix spp.) honey: Identification of the marker compounds. Food Chem. 2014, 145, 8–14. [Google Scholar] [CrossRef]
- Mesbahi, M.A.; Ouahrani, M.R.; Rebiai, A.; Amara, D.G.; Chouikh, A. Characterization of Zygophyllum album L Monofloral Honey from El-Oued, Algeria. Curr. Nutr. Food Sci. 2019, 15, 476–483. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. Bioessays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids—From Biosynthesis to Human Health; IntechOpen: London, UK, 2017. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Truchado, P.; Ferreres, F. Flavonoids in Stingless-Bee and Honey-Bee Honeys. In Pot-Honey: A Legacy of Stingless Bees; Vit, P., Pedro, S.R.M., Roubik, D., Eds.; Springer New York: New York, NY, USA, 2013; pp. 461–474. [Google Scholar]
- Tapas, D.A.; Sakarkar, D.M.; Kakde, R. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Jankun, J.; Selman, S.H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar] [CrossRef]
- Garbisa, S.; Biggin, S.; Cavallarin, N.; Sartor, L.; Benelli, R.; Albini, A. Tumor invasion: Molecular shears blunted by green tea. Nat. Med. 1999, 5, 1216. [Google Scholar] [CrossRef] [PubMed]
- Afroz, R.; Tanvir, E.M.; Paul, S.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.D.I. DNA Damage Inhibition Properties of Sundarban Honey and its Phenolic Composition. J. Food Biochem. 2016, 40, 436–445. [Google Scholar] [CrossRef]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Kurhekar, J.V. Chapter 17—Antimicrobial lead compounds from marine plants. In Phytochemicals as Lead Compounds for New Drug Discovery; Egbuna, C., Kumar, S., Ifemeje, J.C., Ezzat, S.M., Kaliyaperumal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–274. [Google Scholar]
- Zuiter, A.S. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Das, A.B.; Goud, V.V.; Das, C. 9—Phenolic Compounds as Functional Ingredients in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 285–323. [Google Scholar]
- Duodu, K.G.; Awika, J.M. Chapter 8—Phytochemical-Related Health-Promoting Attributes of Sorghum and Millets. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Sawston, UK, 2019; pp. 225–258. [Google Scholar]
- Awika, J.M. Chapter 3—Sorghum: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 21–54. [Google Scholar]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2—Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Studies in Natural Products Chemistry; Atta, U.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78. [Google Scholar]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic Compounds: Occurrence, Classes, and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 346–351. [Google Scholar]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. 4.08—Extraction Techniques for the Determination of Phenolic Compounds in Food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 159–180. [Google Scholar]
- Shrinet, K.; Singh, R.K.; Chaurasia, A.K.; Tripathi, A.; Kumar, A. Chapter 17—Bioactive compounds and their future therapeutic applications. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 337–362. [Google Scholar]
- Popova, A.V.; Bondarenko, S.P.; Frasinyuk, M.S. Aurones: Synthesis and Properties. Chem. Heterocycl. Compd. 2019, 55, 285–299. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Raut, N.A.; Dhore, P.W.; Saoji, S.D.; Kokare, D.M. Chapter 9—Selected Bioactive Natural Products for Diabetes Mellitus. In Studies in Natural Products Chemistry; Atta, U.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 287–322. [Google Scholar]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Chapter 15—Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 315–336. [Google Scholar]
- Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M.; Wawer, I. Effects of structural differences on the NMR chemical shifts in cinnamic acid derivatives: Comparison of GIAO and GIPAW calculations. Chem. Phys. Lett. 2016, 653, 35–41. [Google Scholar] [CrossRef]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Chapter 18—Polyphenols and Intestinal Health. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 191–210. [Google Scholar]
- Dileep, K.V.; Remya, C.; Cerezo, J.; Fassihi, A.; Pérez-Sánchez, H.; Sadasivan, C. Comparative studies on the inhibitory activities of selected benzoic acid derivatives against secretory phospholipase A2, a key enzyme involved in the inflammatory pathway. Mol. Biosyst. 2015, 11, 1973–1979. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivative—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).