Development of Healthier and Functional Dry Fermented Sausages: Present and Future
Abstract
1. Introduction
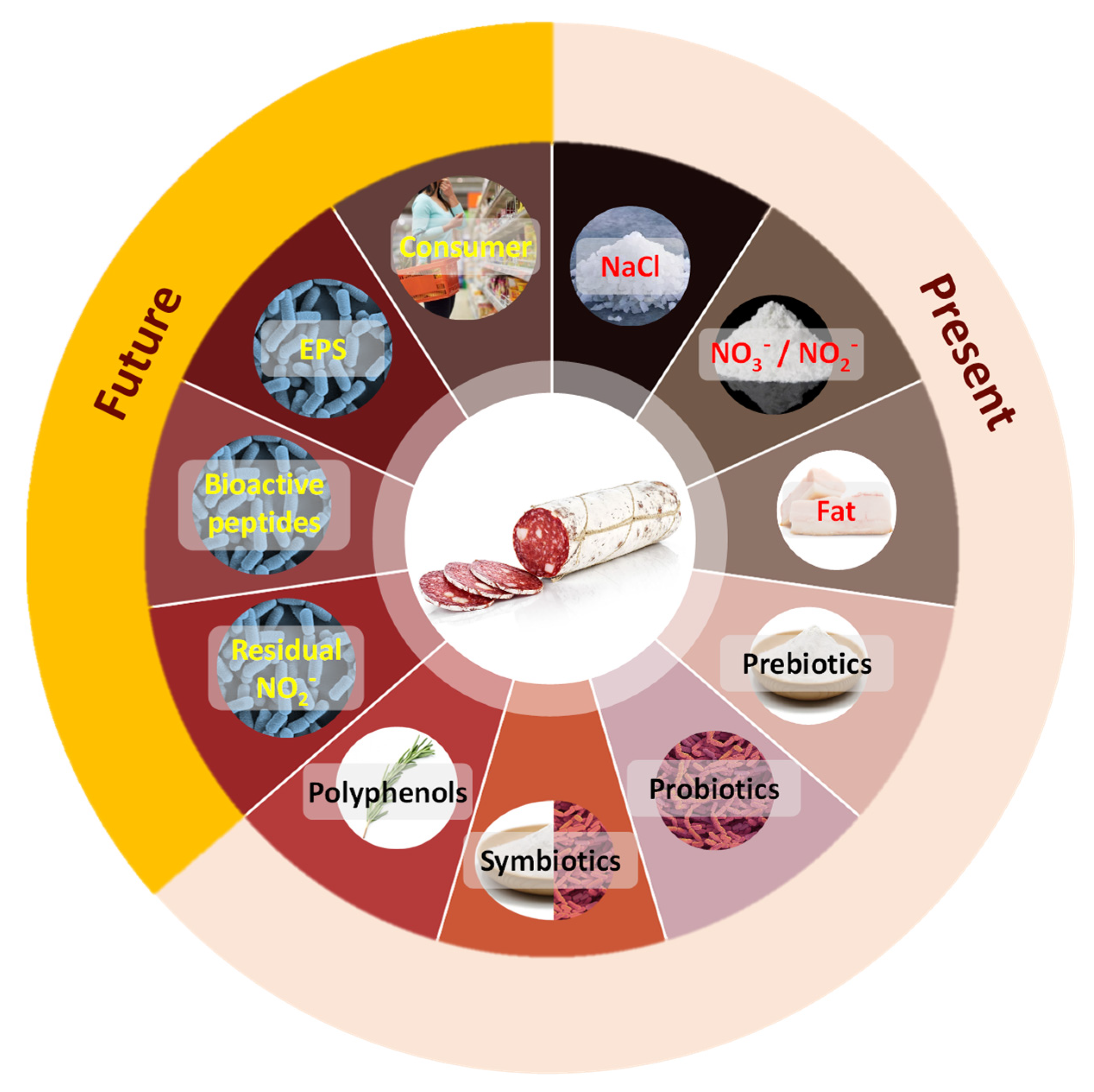
2. Present Healthier and Functional Fermented Meat Products
2.1. Reduction or Replacement of Ingredients of Major Concern for Consumers
2.1.1. Sodium Chloride
2.1.2. Nitrate and Nitrite Salts
2.1.3. Saturated Fat Replacement
2.2. Adding Functional Ingredients into Meat Products
2.2.1. Prebiotics
2.2.2. Probiotics
2.2.3. Symbiotic
2.2.4. Polyphenols
3. Future Healthier and Functional Fermented Meat Products: Tendencies
3.1. Fermented Meat Product Evolution
3.2. Residual Nitrite and Nitrosamine Accumulation and Microbial Synthesis of NO
3.3. Postbiotics
3.3.1. Bioactive Peptides
3.3.2. Exopolysaccharides
3.4. Consumer Perception about Healthier and Functinal Meat Products
3.5. Recommendations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshahrani, S.M.; Fraser, G.E.; Sabaté, J.; Knutsen, R.; Shavlik, D.; Mashchak, A.; Lloren, J.I.; Orlich, M.J. Red and Processed Meat and Mortality in a Low Meat Intake Population. Nutrients 2019, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.K.; Voutilainen, S.; Koskinen, T.T.; Mursu, J.; Kokko, P.; Ylilauri, M.P.T.; Tuomainen, T.-P.; Salonen, J.T.; Virtanen, J.K. Dietary proteins and protein sources and risk of death: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 109, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- GlobeNewswire. $228+ Billion Worldwide Functional Foods Industry to 2030-Identify Growth Segments for Investment. Available online: https://www.globenewswire.com/news-release/2021/12/07/2347213/28124/en/228-Billion-Worldwide-Functional-Foods-Industry-to-2030-Identify-Growth-Segments-for-Investment.html (accessed on 14 February 2022).
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Beriain, M.J.; Gómez, I.; Ibáñez, F.C.; Sarriés, M.V.; Ordóñez, A.I. Improvement of the Functional and Healthy Properties of Meat Products. Food Qual. Balanc. Health Dis. 2018, 13, 1–74. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Consumer perceptions towards healthier meat products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Agüero, N.D.L.; Frizzo, L.S.; Ouwehand, A.C.; Aleu, G.; Rosmini, M.R. Technological Characterisation of Probiotic Lactic Acid Bacteria as Starter Cultures for Dry Fermented Sausages. Foods 2020, 9, 596. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and chia products as ingredients for healthier processed meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Ruusunen, M.; Puolanne, E. Reducing sodium intake from meat products. Meat Sci. 2005, 70, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.A.; Munekata, P.E.S.; Baldin, J.C.; Rocha, Y.J.P.; Carvalho, L.T.; dos Santos, I.R.; Barros, J.C.; Trindade, M.A. The effect of sodium reduction on the microstructure, texture and sensory acceptance of Bologna sausage. Food Struct. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Cittadini, A.; Domínguez, R.; Lorenzo, J.M. Metallic-based salt substitutes to reduce sodium content in meat products. Curr. Opin. Food Sci. 2021, 38, 21–31. [Google Scholar] [CrossRef]
- Rosmini, M.R.; Frizzo, L.S.; Zogbi, A.P. Meat Products with Low Sodium Content: Processing and Properties. In Technological Strategies for Functional Meat Products Development; Fernández-López, J., Ed.; Transworld Research Network: Trivandrum, KA, India, 2008; pp. 87–108. [Google Scholar]
- Appel, L.J.; Anderson, C.A.M. Compelling evidence for public health action to reduce salt intake. N. Engl. J. Med. 2010, 362, 650–652. [Google Scholar] [CrossRef] [PubMed]
- WHO. European Food and Nutrition Action Plan 2015–2020. Regional Office for Europe. Available online: https://apps.who.int/iris/handle/10665/329405 (accessed on 14 February 2022).
- Vidal, V.A.S.; Paglarini, C.S.; Lorenzo, J.M.; Munekata, P.E.S.; Pollonio, M.A.R. Salted Meat Products: Nutritional Characteristics, Processing and Strategies for Sodium Reduction. Food Rev. Int. 2021; in press. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Pérez-Santaescolástica, C.; Munekata, P.E.S.; Lorenzo, J.M. Salt Reduction Strategies in Meat Products Made from Whole Pieces. In Strategies for Obtaining Healthier Foods; Lorenzo, J.M., Carballo, F.J., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 267–289. ISBN 978-1-53612-159-9. [Google Scholar]
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Flores, M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci. 2013, 93, 776–785. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhang, H.; Wang, Y.; Chen, Q.; Kong, B. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT 2020, 124, 109061. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Munekata, P.E.S.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2021, 171, 108275. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.M.; Baltic, M.; Glisic, M.M.; Trbovic, D.; Jokanovic, M.; Parunovic, N.; Dimitrijevic, M.; Suvajdzic, B.; Boskovic, M.; Vasilev, D. Inulin-based emulsion-filled gel as a fat replacer in prebiotic- and PUFA-enriched dry fermented sausages. Int. J. Food Sci. Technol. 2019, 54, 787–797. [Google Scholar] [CrossRef]
- Öztürk-Kerimoğlu, B.; Kavuşan, H.S.; Benzer Gürel, D.; Çağındı, Ö.; Serdaroğlu, M. Cold-set or hot-set emulsion gels consisted of a healthy oil blend to replace beef fat in heat-treated fermented sausages. Meat Sci. 2021, 176, 108461. [Google Scholar] [CrossRef]
- Alejandre, M.; Poyato, C.; Ansorena, D.; Astiasarán, I. Linseed oil gelled emulsion: A successful fat replacer in dry fermented sausages. Meat Sci. 2016, 121, 107–113. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; dos Santos, B.A.; Morgano, M.A.; Terra, N.N.; Pollonio, M.A.R. Application of lysine, taurine, disodium inosinate and disodium guanylate in fermented cooked sausages with 50% replacement of NaCl by KCl. Meat Sci. 2011, 87, 239–243. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.A.; Campagnol, P.C.B.; Morgano, M.A.Ô.; Pollonio, M.A.R. Monosodium glutamate, disodium inosinate, disodium guanylate, lysine and taurine improve the sensory quality of fermented cooked sausages with 50% and 75% replacement of NaCl with KCl. Meat Sci. 2014, 96, 509–513. [Google Scholar] [CrossRef]
- van Buren, L.; Dötsch-Klerk, M.; Seewi, G.; Newson, R. Dietary Impact of Adding Potassium Chloride to Foods as a Sodium Reduction Technique. Nutrients 2016, 8, 235. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Human Safety Controversies Surrounding Nitrate and Nitrite in the Diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Lu, J.; Li, M.; Huang, Y.; Xie, J.; Shen, M.; Xie, M. A comprehensive review of advanced glycosylation end products and N-Nitrosamines in thermally processed meat products. Food Control 2022, 131, 108449. [Google Scholar] [CrossRef]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Parveen, S. All Natural and Clean-Label Preservatives and Antimicrobial Agents Used during Poultry Processing and Packaging. J. Food Prot. 2017, 80, 540–544. [Google Scholar] [CrossRef]
- Ko, Y.M.; Park, J.H.; Yoon, K.S. Nitrite formation from vegetable sources and its use as a preservative in cooked sausage. J. Sci. Food Agric. 2017, 97, 1774–1783. [Google Scholar] [CrossRef]
- Oliveira, W.A.; Rodrigues, A.R.P.; Oliveira, F.A.; Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; Lemos Junior, W.J.F.; Paula, B.P.; Esmerino, E.A.; Corich, V.; et al. Potentially probiotic or postbiotic pre-converted nitrite from celery produced by an axenic culture system with probiotic lacticaseibacilli strain. Meat Sci. 2021, 174, 108408. [Google Scholar] [CrossRef]
- Jin, S.-K.K.; Choi, J.S.; Yang, H.-S.S.; Park, T.-S.S.; Yim, D.-G.G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Palamutoğlu, R.; Fidan, A.; Kasnak, C. Spinach powder addition to sucuk for alternative to nitrite addition. Bull. Transilv. Univ. Brasov. For. Wood Ind. Agric. Food Eng. Ser. II 2018, 11, 155–162. [Google Scholar]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Barone, A.M.; Banovic, M.; Asioli, D.; Wallace, E.; Ruiz-Capillas, C.; Grasso, S. The usual suspect: How to co-create healthier meat products. Food Res. Int. 2021, 143, 110304. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.C.; De Brún, A.; Henchion, M.; Li, C.; Murrin, C.; Wall, P.G.; Monahan, F.J. Consumer evaluations of processed meat products reformulated to be healthier–A conjoint analysis study. Meat Sci. 2017, 131, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Urgu-Öztürk, M.; Öztürk-Kerimoğlu, B.; Serdaroğlu, M. Design of healthier beef sausage formulations by hazelnut-based pre-emulsion systems as fat substitutes. Meat Sci. 2020, 167, 108162. [Google Scholar] [CrossRef] [PubMed]
- Saygi, D.; Ercoşkun, H.; Şahin, E. Hazelnut as functional food component and fat replacer in fermented sausage. J. Food Sci. Technol. 2018, 55, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- de Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Delles, R.M.; Xiong, Y.L.; True, A.D.; Ao, T.; Dawson, K.A. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult. Sci. 2014, 93, 1561–1570. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Rubio, B.; Martínez, B.; Sánchez, M.J.; Dolores García-Cachán, M.; Rovira, J.; Jaime, I. Study of the shelf life of a dry fermented sausage “salchichon” made from raw material enriched in monounsaturated and polyunsaturated fatty acids and stored under modified atmospheres. Meat Sci. 2007, 76, 128–137. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Talukder, S. Effect of Dietary Fiber on Properties and Acceptance of Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Kim, J.H.; Ju, M.G.; Hong, G.E.; Yeon, S.J.; Seo, H.G.; Lee, C.H. Enhancing quality characteristics of salami sausages formulated with whole buckwheat flour during storage. J. Food Sci. Technol. 2017, 54, 326–332. [Google Scholar] [CrossRef]
- Mikami, N.; Tsukada, Y.; Pelpolage, S.W.; Han, K.H.; Fukushima, M.; Shimada, K. Effects of Sake lees (Sake-kasu) supplementation on the quality characteristics of fermented dry sausages. Heliyon 2020, 6, e03379. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T. Dry-fermented sausages inoculated with Enterococcus faecium CECT 410 as free cells or in alginate beads. LWT 2021, 139, 110561. [Google Scholar] [CrossRef]
- Vasconcelos, L.I.M.; da Silva-Buzanello, R.A.; Kalschne, D.L.; Scremin, F.R.; Bittencourt, P.R.S.; Dias, J.T.G.; Canan, C.; Corso, M.P. Functional fermented sausages incorporated with microencapsulated Lactobacillus plantarum BG 112 in Acrycoat S100. LWT 2021, 148, 111596. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B. Utilization of optimized processing conditions for high yield synthesis of conjugated linoleic acid by L. plantarum AB20–961 and L. plantarum DSM2601 in semi-dry fermented sausage. Meat Sci. 2020, 169, 108218. [Google Scholar] [CrossRef]
- Coelho, S.R.; Lima, Í.A.; Martins, M.L.; Benevenuto Júnior, A.A.; Torres Filho, R.d.A.; Souza Ramos, A.d.L.; Ramos, E.M. Application of Lactobacillus paracasei LPC02 and lactulose as a potential symbiotic system in the manufacture of dry-fermented sausage. LWT 2019, 102, 254–259. [Google Scholar] [CrossRef]
- Sirini, N.; Roldán, A.; Lucas-González, R.; Fernández-López, J.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Frizzo, L.S.; Rosmini, M.R. Effect of chestnut flour and probiotic microorganism on the functionality of dry-cured meat sausages. LWT 2020, 134, 110197. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Domínguez, R.; Penna, A.L.B.; Lorenzo, J.M.; Silva Barretto, A.C. Impact of fructooligosaccharides and probiotic strains on the quality parameters of low-fat Spanish Salchichón. Meat Sci. 2020, 159, 107936. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.L.; Paliy, O.; Rufián-Henares, J.Á. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Pilar Francino, M.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Dos Santos Cruxen, C.E.; Funck, G.D.; Haubert, L.; da Silva Dannenberg, G.; de Lima Marques, J.; Chaves, F.C.; da Silva, W.P.; Fiorentini, Â.M. Selection of native bacterial starter culture in the production of fermented meat sausages: Application potential, safety aspects, and emerging technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, A.; de la Foye, A.; Loux, V.; Talon, R.; Leroy, S. Transcriptomic analysis of Staphylococcus xylosus in the presence of nitrate and nitrite in meat reveals its response to nitrosative stress. Front. Microbiol. 2014, 5, 691. [Google Scholar] [CrossRef]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; FAO; WHO: London, ON, Canada, 2002. [Google Scholar]
- Cocolin, L.; Dolci, P.; Rantsiou, K. Biodiversity and dynamics of meat fermentations: The contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011, 89, 296–302. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Sirini, N.; Frizzo, L.S.; Aleu, G.; Soto, L.P.; Rosmini, M.R. Use of probiotic microorganisms in the formulation of healthy meat products. Curr. Opin. Food Sci. 2021, 38, 141–146. [Google Scholar] [CrossRef]
- Khan, M.I.; Arshad, M.S.; Anjum, F.M.; Sameen, A.; Rehman, A.-U.; Gill, W.T. Meat as a functional food with special reference to probiotic sausages. Food Res. Int. 2011, 44, 3125–3133. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; de Almeida, C.A.M.; Mattarugo, N.M.D.S.; Ferri, E.A.V.; Bittencourt, P.R.S.; Colla, E.; Drunkler, D.A. Soy extract and maltodextrin as microencapsulating agents for Lactobacillus acidophilus: A model approach. J. Microencapsul. 2018, 35, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; de Souza, S.; Monsan, P.; Ouarné, F.; Theodorou, V.; Roques, C. In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int. J. Food Microbiol. 2010, 144, 42–50. [Google Scholar] [CrossRef]
- Brahma, S.; Sadiq, M.B.; Ahmad, I. Probiotics in Functional Foods. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for Improved Human Health: Recent Developments, Challenges, and Opportunities. Annu. Rev. Food Sci. Technol. 2018, 9, 451–479. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jimenez-Colmenero, F.; de Menezes, C.R.; Fries, L.L.M. Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 2015, 46, 120–131. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Bellucci, E.R.B.; Domínguez, R.; da Silva Barretto, A.C.; Lorenzo, J.M. Strategies to Increase the Shelf Life of Meat and Meat Products with Phenolic Compounds. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2021. [Google Scholar]
- Giada, M.D.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; IntechOpen: London, UK, 2013; pp. 87–112. ISBN 978-953-51-1123-8. [Google Scholar]
- Meira, N.V.B.; Holley, R.A.; Bordin, K.; de Macedo, R.E.F.; Luciano, F.B. Combination of essential oil compounds and phenolic acids against Escherichia coli O157:H7 in vitro and in dry-fermented sausage production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef]
- Beck, P.H.B.; Matiucci, M.A.; Neto, A.A.M.; Feihrmann, A.C. Sodium chloride reduction in fresh sausages using salt encapsulated in carnauba wax. Meat Sci. 2021, 175, 108462. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Zhou, T.; Li, J.; Yang, J.; Chen, W.; Xiong, Y.L. Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (Nanx Wudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Sci. 2016, 121, 302–309. [Google Scholar] [CrossRef]
- Cheng, J.R.; Liu, X.M.; Zhang, Y.S. Characterization of Cantonese sausage fermented by a mixed starter culture. J. Food Process. Preserv. 2018, 42, e13623. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Sun, J.; Pan, P.; Liu, Y.; Tian, T. Effects of starter culture inoculation on microbial community diversity and food safety of Chinese Cantonese sausages by high-throughput sequencing. J. Food Sci. Technol. 2021, 58, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Şişik Oğraş, Ş.; Çelik, M.; Kaya, M. Nitrosamine formation in a semi-dry fermented sausage: Effects of nitrite, ascorbate and starter culture and role of cooking. Meat Sci. 2020, 159, 107917. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hur, S.J. Effect of six different starter cultures on the concentration of residual nitrite in fermented sausages during in vitro human digestion. Food Chem. 2018, 239, 556–560. [Google Scholar] [CrossRef]
- Thøgersen, R.; Gray, N.; Kuhnle, G.; Van Hecke, T.; De Smet, S.; Young, J.F.; Sundekilde, U.K.; Hansen, A.K.; Bertram, H.C. Inulin-fortification of a processed meat product attenuates formation of nitroso compounds in the gut of healthy rats. Food Chem. 2020, 302, 125339. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Staccini, B.; Ranucci, D.; Miraglia, D.; Altissimi, M.S.; Mercuri, M.L.; Haouet, N.M. Contribution of vegetables and cured meat to dietary nitrate and nitrite intake in italian population: Safe level for cured meat and controversial role of vegetables. Ital. J. Food Saf. 2018, 7, 168–173. [Google Scholar] [CrossRef]
- Merino, L.; Darnerud, P.O.; Toldrá, F.; Ilbäck, N.-G. Time-dependent depletion of nitrite in pork/beef and chicken meat products and its effect on nitrite intake estimation. Food Addit. Contam. Part A 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Santos, E.M.; Lorenzo, J.M. Cruciferous vegetables as sources of nitrate in meat products. Curr. Opin. Biotechnol. 2021, 38, 1–7. [Google Scholar] [CrossRef]
- Luo, H.; Li, P.; Zhang, H.; Diao, X.; Kong, B. Nitrosylmyoglobin formation in meat by Lactobacillus fermentum AS1.1880 is due to its nitric oxide synthase activity. Meat Sci. 2020, 166, 108122. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mi, R.; Qi, B.; Xiong, S.; Li, J.; Qu, C.; Qiao, X.; Chen, W.; Wang, S. Effect of proteolytic starter culture isolated from Chinese Dong fermented pork (Nanx Wudl) on microbiological, biochemical and organoleptic attributes in dry fermented sausages. Food Sci. Hum. Wellness 2021, 10, 13–22. [Google Scholar] [CrossRef]
- Mejri, L.; Vásquez-Villanueva, R.; Hassouna, M.; Marina, M.L.; García, M.C. Identification of peptides with antioxidant and antihypertensive capacities by RP-HPLC-Q-TOF-MS in dry fermented camel sausages inoculated with different starter cultures and ripening times. Food Res. Int. 2017, 100, 708–716. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.Q.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT 2019, 99, 346–354. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Velasco, L.; Weiss, J.; Loeffler, M. Influence of microbial in-situ heteropolysaccharide production on textural properties of raw fermented sausages (salami). J. Food Sci. Technol. 2021, 58, 562–570. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef]
- Grasso, S.; Brunton, N.P.; Lyng, J.G.; Lalor, F.; Monahan, F.J. Healthy processed meat products-Regulatory, reformulation and consumer challenges. Trends Food Sci. Technol. 2014, 39, 4–17. [Google Scholar] [CrossRef]
- Shan, L.C.; Henchion, M.; De Brún, A.; Murrin, C.; Wall, P.G.; Monahan, F.J. Factors that predict consumer acceptance of enriched processed meats. Meat Sci. 2017, 133, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Verbeke, W.; de Kok, T.M. Stakeholder and consumer reactions towards innovative processed meat products: Insights from a qualitative study about nitrite reduction and phytochemical addition. Food Control 2016, 60, 690–698. [Google Scholar] [CrossRef]
- Shan, L.C.; Regan, Á.; Monahan, F.J.; Li, C.; Lalor, F.; Murrin, C.; Wall, P.G.; McConnon, Á. Consumer preferences towards healthier reformulation of a range of processed meat products: A qualitative exploratory study. Br. Food J. 2017, 119, 2013–2026. [Google Scholar] [CrossRef][Green Version]
- European Union. EC Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
| Type of Fermented Sausage | Reformulation | Recommendation | Ref. |
|---|---|---|---|
| Chinese dry sausage | Sodium chloride reduction | 2% and 4% NaCl | [22] |
| Dry fermented sausages | NaCl reduction from 2.5% to 1.0% | [24] | |
| Dry fermented sausages | Sodium chloride replacement | Substitution of 30% of NaCl with KCl and flavor enhancers | [25] |
| Dry fermented sausages | Nitrate–rich extract addition | Beetroot and radish powders | [26] |
| Dry fermented sausages | Natural extract addition | Grape seed extract and olive pomace hydroxytyrosol and chestnut extract with olive pomace hydroxytyrosol | [27] |
| Fermented sausages | Fat replacement | Inulin gelled suspension and inulin linseed oil gelled emulsion | [28] |
| Fermented beef sausages | Gelled emulsion systems of peanut and linseed oils | [29] | |
| Dry fermented sausages | Gelled emulsion of high omega-3 and carrageenan content | [30] |
| Fermented Sausage | Category | Functional Ingredient | Ref. |
|---|---|---|---|
| Salami | Prebiotics | Whole buckwheat flour | [59] |
| Dry fermented sausages | Sake lees (Sake-kasu) | [60] | |
| Dry fermented sausages | Probiotic | Enterococcus faecium CECT 410 as free cells or encapsulated in alginate beads | [61] |
| Milano-type salami | Microencapsulated Lactiplantibacillus plantarum BG 112 in Acrycoat S100 | [62] | |
| Dry fermented sausages | Lactiplantibacillus plantarum L125 | [63] | |
| Semi-dry fermented sausage | Lactiplantibacillus plantarum AB20–961 and Lactiplantibacillus plantarum DSM2601 | [64] | |
| Dry fermented sausage | Symbiotic | Lacticaseibacillusparacasei LPC02 and lactulose | [65] |
| Longaniza de Pascua | Lactiplantibacillus plantarum and chestnut flour | [66] | |
| Dry fermented sausage | Lacticaseibacillusparacasei BGP1 or Lactobacillus rhmanosus GG with fructooligosaccharides | [67] | |
| Salami | Lacticaseibacillus rhamnosus with different fibers (citrus fiber, arabinogalactan, and inulin) | [68] | |
| Salami | Lacticaseibacillus rhamnosus HN001 with citrus fiber | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirini, N.; Munekata, P.E.S.; Lorenzo, J.M.; Stegmayer, M.Á.; Pateiro, M.; Pérez-Álvarez, J.Á.; Sepúlveda, N.; Sosa-Morales, M.E.; Teixeira, A.; Fernández-López, J.; et al. Development of Healthier and Functional Dry Fermented Sausages: Present and Future. Foods 2022, 11, 1128. https://doi.org/10.3390/foods11081128
Sirini N, Munekata PES, Lorenzo JM, Stegmayer MÁ, Pateiro M, Pérez-Álvarez JÁ, Sepúlveda N, Sosa-Morales ME, Teixeira A, Fernández-López J, et al. Development of Healthier and Functional Dry Fermented Sausages: Present and Future. Foods. 2022; 11(8):1128. https://doi.org/10.3390/foods11081128
Chicago/Turabian StyleSirini, Noelí, Paulo E. S. Munekata, José M. Lorenzo, María Ángeles Stegmayer, Mirian Pateiro, José Ángel Pérez-Álvarez, Néstor Sepúlveda, María Elena Sosa-Morales, Alfredo Teixeira, Juana Fernández-López, and et al. 2022. "Development of Healthier and Functional Dry Fermented Sausages: Present and Future" Foods 11, no. 8: 1128. https://doi.org/10.3390/foods11081128
APA StyleSirini, N., Munekata, P. E. S., Lorenzo, J. M., Stegmayer, M. Á., Pateiro, M., Pérez-Álvarez, J. Á., Sepúlveda, N., Sosa-Morales, M. E., Teixeira, A., Fernández-López, J., Frizzo, L., & Rosmini, M. (2022). Development of Healthier and Functional Dry Fermented Sausages: Present and Future. Foods, 11(8), 1128. https://doi.org/10.3390/foods11081128











