Animal Species Authentication in Dairy Products
Abstract
1. Introduction
2. Protein-Based Methods
2.1. Electrophoretic Techniques
2.2. Immunochemical Techniques
| Method | Target Species | Target Molecule | Type of Product | Sensitivity | Reference |
|---|---|---|---|---|---|
| Native PAGE | Cow | Bovine β-lactoglobulin and α-lactalbumin | Milk mixtures | 3% in caprine/bovine 5% in ovine/bovine | [11] |
| IEF | Cow | γ2-and γ3-caseins | Ewe’s and goat’s cheeses | - a | [12] |
| Cow | Bovine αs1-casein | Donkey’s milk | 5% of cow’s milk in donkey’s milk | [25] | |
| IEF and immunoblot analysis | Cow | Bovine γ2-casein | Water buffalo milk and derived mozzarella cheese | 0.25% bovine milk in water buffalo mozzarella cheese | [15] |
| CE | Cow, sheep, goat | Casein fractions and their breakdown products | Iberico-type cheeses made from cow, sheep or goat’s milk | - | [20] |
| Sheep and cow | Ovine and bovine proteins | Sheep’s/cow’s milk mixtures | 5% of cow’s milk in ovine/bovine milk mixtures | [18] | |
| Cow | Bovine α-lactalbumin | Cow’s milk in buffalo’s milk | 1% of cow’s milk (limit of quantification of 3.1%) | [19] | |
| Cow | α-lactalbumins and β-lactoglobulins | Goat’s and ewe’s cheeses | 1% (cow’s milk) | [21] | |
| Capillary IEF | Cow | Products of plasmin hydrolysis of bovine and water buffalo β-casein | Water buffalo’s milk | 1% (cow’s milk) | [17] |
| ELISA | Goat | Caprine IgG | Sheep’s milk | 0.5% (of goat’s milk in sheep’s milk) | [28] |
| Indirect Competitive ELISA | Cow | Bovine IgG | Goat’s, sheep’s and buffalo’s milk | 1.0 µg/mL of bovine IgG (0.1%) | [26] |
| Cow | mAb 1-9B | Yak’s milk | 1% (10 µg/mL) of cow’s milk in yak’s milk | [36] | |
| Competitive ELISA | Cow | Bovine β-casein | Donkey’s milk | 0.5% of cow’s milk in donkey milk | [25] |
| Indirect ELISA | Cow | Bovine β-casein | Raw and heated goat’s milk | 2% of cow’s milk in goat’s milk | [29] |
| Cow | Bovine β-casein | Goat’s and sheep’s milk cheeses | - a | [37] | |
| Sandwich ELISA | Cow | Anti-bovine IgG antibody | Dairy products | 0.001% cow´s milk in buffalo or sheep milk; 0.01% cow’s milk in goat’s milk; 0.001% in goat cheeses and 0.01% in buffalo and sheep cheeses | [23] |
| ELISA kits | Cow and goat | Bovine or caprine protein β-lactoglobulin | Ewe’s milk and cheese | ∼0.2% of cow and goat’s milk in ewe’s milk Not adequate for quantitative measures in cheese | [30] |
| ELISA kits | Cow | Bovine IgG | Sheep’s milk and cheese, and commercial “Bryndza” | 0.5% raw and 50% pasteurized cow milk in sheep’s milk; 0.5% raw and low pasteurized and 5% high pasteurized cow milk in sheep’s cheese | [31] |
| Sandwich ELISA kit | Cow | Bovine β-lactoglobulin | Sheep’s dairy products | 0.2 ppm (mg/kg) | [34] |
| LFIA | Cow | Specific bovine immunoglobulins (IgG) | Buffalo, sheep and goat raw milks | 0.5% of cows’ milk | [35] |
| Optical immunoassay | Cow | Bovine k-casein | Raw and pasteurized cow’s and goat’s milks | 0.04% (cow’s milk in goat’s milk) | [38] |
| QCM immunosensor | Cow | Bovine k-casein | Cow’s and goat’s milks | 1 ppm (cow’s milk in goat’s milk) | [39] |
2.3. Chromatographic and Mass Spectrometry Techniques
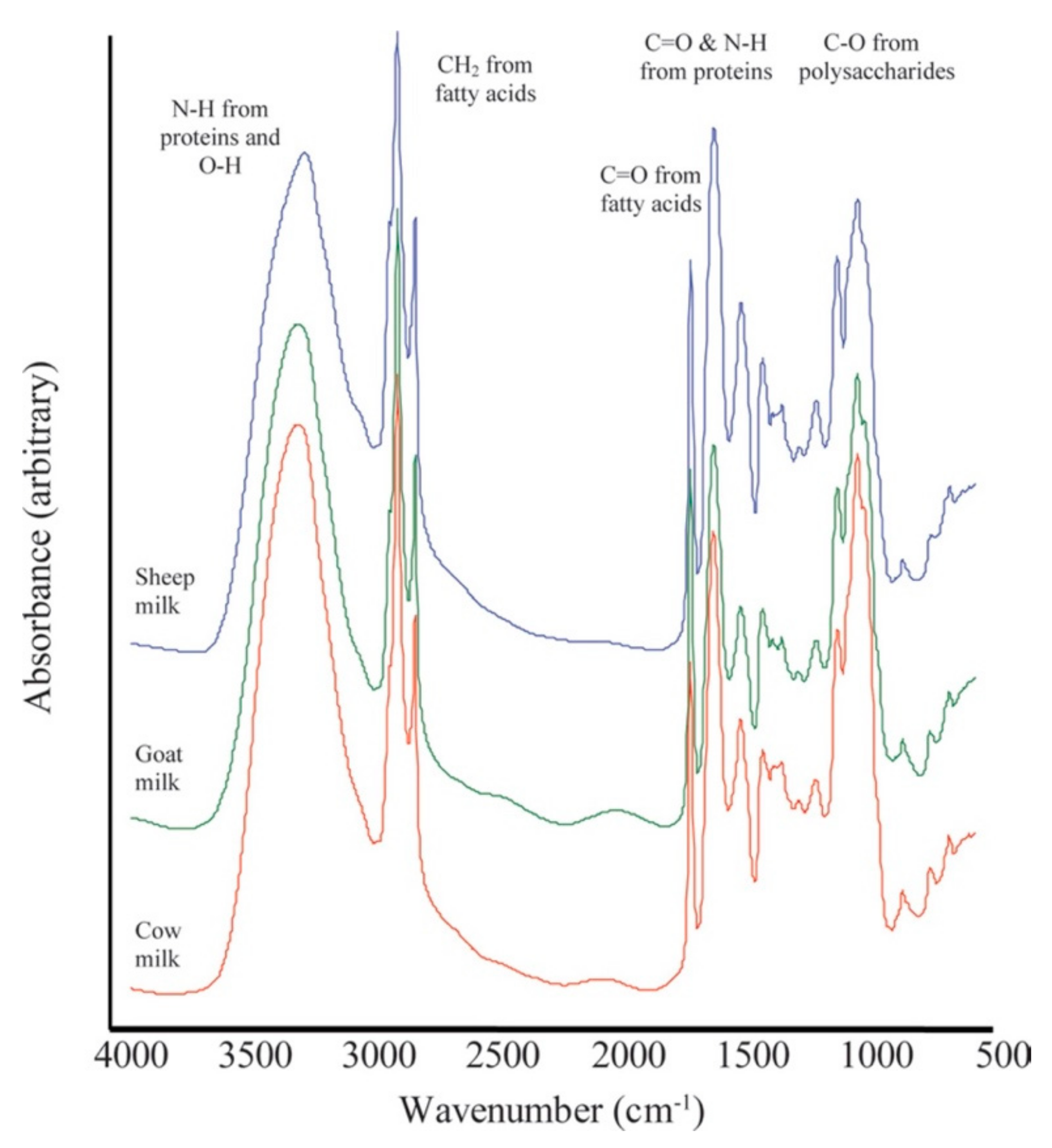
3. Spectroscopic Methods
| Method | Target Species | Target Molecule | Type of Product | Sensitivity | Reference |
|---|---|---|---|---|---|
| HPLC-DAD | Sheep, goat and cow | Albumines (β-lactoglobulin, α-lactoalbumin and serum albumine), globulins (immunoglobulin: IgG, IgA and IgM), proteoso-peptones and lactoferrin | Milk and cheeses | 3.92% (sheep’s milk in cheese) 2.81% (goat’s milk in cheese) 1.47% (cow’s milk in cheese) | [42] |
| Buffalo and cow | β-lactoglobulin | Creams | 1% (cow’s milk in buffalo’s cream) | [43] | |
| MALDI-TOF MS | Cow, buffalo, sheep, she-donkey and goat | Intact proteins | She-donkey’s and goat’s milk | 0.5% (cow’s milk in She-donkey’s and goat’s milk) | [45] |
| Goat, sheep and cow | Caseins and proteose peptone | Milk | 2% (cow’s milk in goat’s and sheep’s milk) | [47] | |
| Water buffalo and cow | Four signature unphosphorylated peptides derived from β-CN A, i.e., (f49-68) Asn68, (f1-28) Ser10, (f1-29) Ser10 and (f33-48) Thr41 and two from αs1-CN (f35-42), i.e., (f23-34) Met31 and (f43-58) Val44 | Mozzarella cheeses | 0.78% (cow’s milk in PDO water buffalo’s cheeses) | [51] | |
| Cow and buffalo | Region 149–162 of bovine β-lactoglobulin | Water buffalo’s ricotta PDO cheese | 5% (cow’s milk in buffalo’s cheese) | [52] | |
| Goat | αs1-CN f8-22 and αs1-CN f4-22 | Milk mixtures | 0.5% (goat’s milk in milk mixtures) | [54] | |
| Sheep, goat, buffalo and cow | γ2-caseins and γ3-caseins in the four species; α-lactalbumins in bovine, buffalo and goat milk; β- CN fragments (98–207) in goat and ovine milk; β-lactoglobulin in goat milk, proteoso peptones p.p.8.I., in bovine milk and β-casein fragments (1–68) and (69–209) in buffalo milk | Fresh raw cow’s, buffalo’s, sheep’s and goat’s milk | 5% (cow’s milk in goat’s milk) | [74] | |
| Goat, sheep and cow | Intact phospholipids | Milk | - a | [75] | |
| LC-MS | Sheep and cow | Fragments 1–14 and 1–23 from αS1 casein | Fresh sheep’s milk cheeses | 1% (cow’s milk in sheep’s cheese) | [59] |
| LC-MS/MS | Cow, buffalo, sheep and goat | β-lactoglobulin variants A and or α-lactalbumin | Buffalo’s, sheep’s and goat’s Italian ricotta cheese | 0.5% (cow’s whey in ricotta cheeses from the other species) | [55] |
| LC-ESI-MS | Goat | α1-CN f4-22 variant A and B | Milk mixtures | - a | [54] |
| LC-ESI-MS/MS | - | Caseinomacropeptide (CMP) and pseudo-CMP | Milk | 1 µg/mL (CMP and pseudo-CMP in milk) | [76] |
| Cow, buffalo, sheep and goat | Species-Specific Peptides: Goat (YLGYLEQLLK), sheep (TPEVDNEALEK), buffalo (AFKPTELGEVITK) and cow (AMKPWIQPK) | Milk and cheeses | - a | [77] | |
| HPLC-ESI-MS, MALDI-TOF MS and MS/MS | Goat | Variant D of caprine β-casein | Italian goat’s milk | - a | [56] |
| UPLC-ESI-MS/MS | Cow and buffalo | β-casein f33-48 transitions | PDO buffalo’s mozzarella | 0.001% (cow’s milk in buffalo’s cheese) | [57] |
| UHPLC-MS/MS | Goat, sheep and cow | Caseins (β-casein, αs1-casein, αs2-casein, and κ-casein) and major whey proteins (β-lactoglobulin and α lactalbumin) | Cow’s milk whey, whole milk powder and goat’s milk infant formula | 0.01–0.05 g/100 g (cow’s whey and whole milk powder in goat’s or sheep’s milk products including infant formula) | [62] |
| UHPLC-MS/MS | Cow | Peptide LRPVAAEIYGTK, VDSALYLGSR (corresponding to amino acid residues 93–104 and 333–342 of bovine lactoferrin, respectively) | Dairy products, include infant formula and whey proteins | 0.3 mg/100 mg (cow’s lactoferrin in infant formulas) | [78] |
4. DNA-Based Methods
4.1. PCR-RFLP
4.2. Species-Specific PCR
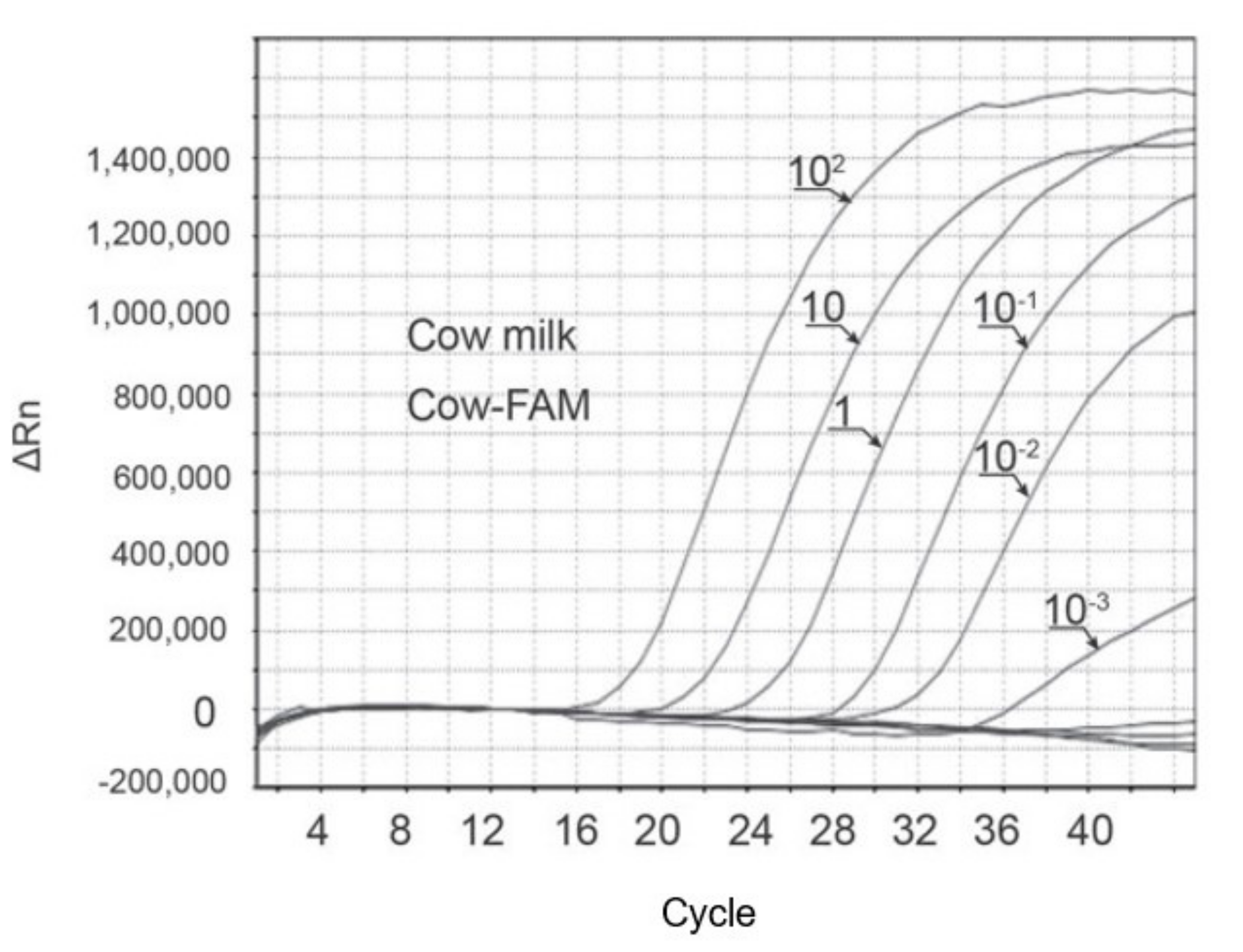
4.3. Real-Time PCR
4.4. HRM Analysis
4.5. ddPCR
4.6. LAMP
4.7. NGS
4.8. Fingerprint Techniques
5. (Bio)Sensors
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Downey, G. Advances in Food Authenticity Testing; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Amaral, J.S. Target and Non-Target Approaches for Food Authenticity and Traceability. Foods 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Environment, Public Health and Food Safety. Draft Report the Food Crisis, Fraud in the Food Chain and the Control Thereof (2013/2091(INI)). Available online: https://www.europarl.europa.eu/doceo/document/ENVI-PR-519759_EN.pdf?redirect (accessed on 11 June 2021).
- De la Fuente, M.A.; Juarez, M. Authenticity assessment of dairy products. Crit. Rev. Food Sci. 2005, 45, 563–585. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.M.I.; Pissard, A.; Pierna, J.A.F.; Baeten, V. Milk and milk products. In Foodintegrity Handbook; Morin, J.-F., Lees, M., Eds.; Eurofins Analytics France: Nantes, France, 2018; pp. 3–26. [Google Scholar]
- Kamal, M.; Karoui, R. Analytical methods coupled with chemometric tools for determining the authenticity and detecting the adulteration of dairy products: A review. Trends Food Sci. Technol. 2015, 46, 27–48. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Baptista, M.; Cunha, J.T.; Domingues, L. DNA-based approaches for dairy products authentication: A review and perspectives. Trends Food Sci. Technol. 2021, 109, 386–397. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. F 2018, 17, 137–164. [Google Scholar] [CrossRef]
- Ortea, I.; O’Connor, G.; Maquet, A. Review on proteomics for food authentication. J. Proteom. 2016, 147, 212–225. [Google Scholar] [CrossRef]
- Pesic, M.; Barac, M.; Vrvic, M.; Ristic, N.; Macej, O.; Stanojevic, S. Qualitative and quantitative analysis of bovine milk adulteration in caprine and ovine milks using native-PAGE. Food Chem. 2011, 125, 1443–1449. [Google Scholar] [CrossRef]
- Spoljaric, J.; Mikulec, N.; Plavljanic, D.; Radeljevic, B.; Havranek, J.; Antunac, N. Proving the adulteration of ewe and goat cheeses with cow milk using the reference method of isoelectric focusing of gamma-casein. Mljekarstvo 2013, 63, 115–121. [Google Scholar]
- Pizzano, R.; Nicolai, M.A.; Manzo, C.; Addeo, F. Authentication of dairy products by immunochemical methods: A review. Dairy Sci. Technol. 2011, 91, 77–95. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/150 of 30 January 2018 amending Implementing Regulation (EU) 2016/1240 as regards methods for the analysis and quality evaluation of milk and milk products eligible for public inter-vention and aid for private storage. Off. J. Eur. Union 2018, L26, 14–47. [Google Scholar]
- Addeo, F.; Pizzano, R.; Nicolai, M.A.; Caira, S.; Chianese, L. Fast Isoelectric Focusing and Antipeptide Antibodies for Detecting Bovine Casein in Adulterated Water Buffalo Milk and Derived Mozzarella Cheese. J. Agric. Food Chem. 2009, 57, 10063–10066. [Google Scholar] [CrossRef] [PubMed]
- Caira, S.; Nicolai, M.A.; Lilla, S.; Calabrese, M.G.; Pinto, G.; Scaloni, A.; Chianese, L.; Addeo, F. Eventual limits of the current EU official method for evaluating milk adulteration of water buffalo dairy products and potential proteomic solutions. Food Chem. 2017, 230, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Somma, A.; Ferranti, P.; Addeo, F.; Mauriello, R.; Chianese, L. Peptidomic approach based on combined capillary isoelectric focusing and mass spectrometry for the characterization of the plasmin primary products from bovine and water buffalo beta-casein. J. Chromatogr. A 2008, 1192, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, F.; Morittu, V.M.; Cicino, C.; Palmieri, C.; Britti, D. Rapid capillary electrophoresis approach for the quantification of ewe milk adulteration with cow milk. J. Chromatogr. A 2017, 1519, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, F.; Costanzo, N.; Lopreiato, V.; Ceniti, C.; Morittu, V.M.; Spina, A.; Britt, D. Detection of buffalo milk adulteration with cow milk by capillary electrophoresis analysis. J. Dairy Sci. 2019, 102, 5962–5970. [Google Scholar] [CrossRef]
- Molina, E.; Ramos, M.; Amigo, L. Characterisation of the casein fraction of Iberico cheese by electrophoretic techniques. J. Sci. Food Agric. 2002, 82, 1240–1245. [Google Scholar] [CrossRef]
- Herrero-Martinez, J.M.; Simo-Alfonso, E.F.; Ramis-Ramos, G.; Gelfi, C.; Righetti, P.G. Determination of cow’s milk and ripening time in nonbovine cheese by capillary electrophoresis of the ethanol-water protein fraction. Electrophoresis 2000, 21, 633–640. [Google Scholar] [CrossRef]
- Reid, L.M.; O’Donnell, C.P.; Downey, G. Recent technological advances for the determination of food authenticity. Trends Food Sci. Technol. 2006, 17, 344–353. [Google Scholar] [CrossRef]
- Hurley, I.P.; Coleman, R.C.; Ireland, H.E.; Williams, J.H.H. Use of sandwich IgG ELISA for the detection and quantification of adulteration of milk and soft cheese. Int. Dairy J. 2006, 16, 805–812. [Google Scholar] [CrossRef]
- Asensio, L.; Gonzalez, I.; Garcia, T.; Martin, R. Determination of food authenticity by enzyme-linked immunosorbent assay (ELISA). Food Control 2008, 19, 1–8. [Google Scholar] [CrossRef]
- Pizzano, R.; Salimei, E. Isoelectric Focusing and ELISA for Detecting Adulteration of Donkey Milk with Cow Milk. J. Agric. Food Chem. 2014, 62, 5853–5858. [Google Scholar] [CrossRef] [PubMed]
- Hurley, I.P.; Coleman, R.C.; Ireland, H.E.; Williams, J.H.H. Measurement of bovine IgG by indirect competitive ELISA as a means of detecting milk adulteration. J. Dairy Sci. 2004, 87, 543–549. [Google Scholar] [CrossRef]
- Hurley, I.P.; Ireland, H.E.; Coleman, R.C.; Williams, J.H.H. Application of immunological methods for the detection of species adulteration in dairy products. Int. J. Food Sci. Technol. 2004, 39, 873–878. [Google Scholar] [CrossRef]
- Zelenakova, L.; Golian, J.; Zaiac, P. Application of ELISA tests for the detection of goat milk in sheep milk. Milchwissenschaft 2008, 63, 137–141. [Google Scholar]
- Song, H.X.; Xue, H.Y.; Han, Y. Detection of cow’s milk in Shaanxi goat’s milk with an ELISA assay. Food Control 2011, 22, 883–887. [Google Scholar] [CrossRef]
- Costa, N.; Ravasco, F.; Miranda, R.; Duthoit, M.; Roseiro, L.B. Evaluation of a commercial ELISA method for the quantitative detection of goat and cow milk in ewe milk and cheese. Small Rumin. Res 2008, 79, 73–79. [Google Scholar] [CrossRef]
- Zeleňáková, L.; Židek, R.; Čanigová, M.; Ziarovska, J.; Zajác, P.; Marsalkova, L.; Fikselová, M.; Golian, J. Research And Practice: Quantification Of Raw And Heat-Treated Cow Milk in Sheep Milk, Cheese And Bryndza By ELISA Method. Potravinarstvo 2016, 10, 14–22. [Google Scholar] [CrossRef]
- Stanciuc, N.; Rapeanu, G. Identification of adulterated sheep and goat cheeses marketed in Romania by immunocromatographic assay. Food Agric. Immunol. 2010, 21, 157–164. [Google Scholar] [CrossRef]
- Zeleňáková, L.; Žıdek, R.; Čanigová, M.; Ziarovska, J.; Zajác, P.; Marsalkova, L.; Fikselová, M.; Golian, J. Reliability of cow casein quantitation in sheep milk and cheese by ELISA method. J. Food Phys. 2010, 23, 22–26. [Google Scholar]
- Zelenakova, L.; Zidek, R.; Canigova, M. Optimization of ELISA method for detection of bovine beta-lactoglobulin in sheep milk and sheep milk products. Milchwissenschaft 2011, 66, 278–281. [Google Scholar]
- Galan-Malo, P.; Mendiara, I.; Razquin, P.; Mata, L. Validation of a rapid lateral flow method for the detection of cows’ milk in water buffalo, sheep or goat milk. Food Addit. Contam. Part A 2018, 35, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.R.; Zhang, H.; Guo, H.Y.; Jiang, L.; Tian, M.; Ren, F.Z. Detection of cow milk adulteration in yak milk by ELISA. J. Dairy Sci. 2014, 97, 6000–6006. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calleja, I.M.; Gonzalez, I.; Fajardo, V.; Hernandez, P.E.; Garcia, T.; Martin, R. Application of an indirect ELISA and a PCR technique for detection of cows’ milk in sheep’s and goats’ milk cheeses. Int. Dairy J. 2007, 17, 87–93. [Google Scholar] [CrossRef]
- Angelopoulou, I.; Botsialas, A.; Salapatas, A.; Petrou, P.S.; Haasnoot, W.; Makarona, E.; Jobst, G.; Goustouridis, D.; Siafaka-Kapadai, A.; Raptis, I.; et al. Assessment of goat milk adulteration with a label-free monolithically integrated optoelectronic biosensor. Anal. Bioanal. Chem. 2015, 407, 3995–4004. [Google Scholar] [CrossRef]
- Sakti, S.P.; Chabibah, N.; Ayu, S.P.; Padaga, M.C.; Aulanni’am, A. Development of QCM Biosensor with Specific Cow Milk Protein Antibody for Candidate Milk Adulteration Detection. J. Sens. 2016, 2016, 1807647. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Teixeira, N.; Ferreira, I.M.P.L.V.O. Separation and quantification of the major casein fractions by reverse-phase high-performance liquid chromatography and urea-polyacrylamide gel electrophoresis—Detection of milk adulterations. J. Chromatogr. A 2002, 967, 209–218. [Google Scholar] [CrossRef]
- Enne, G.; Elez, D.; Fondrini, F.; Bonizzi, I.; Feligini, M.; Aleandri, R. High-performance liquid chromatography of governing liquid to detect illegal bovine milk’s addition in water buffalo Mozzarella: Comparison with results from raw milk and cheese matrix. J. Chromatogr. A 2005, 1094, 169–174. [Google Scholar] [CrossRef]
- Rodriguez, N.; Ortiz, M.C.; Sarabia, L.; Gredilla, E. Analysis of protein chromatographic profiles joint to partial least squares to detect adulterations in milk mixtures and cheeses. Talanta 2010, 81, 255–264. [Google Scholar] [CrossRef]
- Manzo, N.; Pizzolongo, F.; Montefusco, I.; Romano, A.; Masi, P.; Romano, R. Using whey proteins to detect the addition of bovine milk fat in buffalo cream destined for the butter-making process. Food Control 2017, 81, 164–167. [Google Scholar] [CrossRef][Green Version]
- Buckley, M.; Melton, N.D.; Montgomery, J. Proteomics analysis of ancient food vessel stitching reveals > 4000-year-old milk protein. Rapid Commun. Mass Spectrom. 2013, 27, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.; Masotti, A.; Salvatori, G.; Scapaticci, M.; Muraca, M.; Putignani, L. A Sensitive and Effective Proteomic Approach to Identify She-Donkey’s and Goat’s Milk Adulterations by MALDI-TOF MS Fingerprinting. Int. J. Mol. Sci. 2014, 15, 13697–13719. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, R.; Passalacqua, S.; Salemi, S.; Garozzo, D. Identification of adulteration in water buffalo mozzarella and in ewe cheese by using whey proteins as biomarkers and matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 2002, 37, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, N.; Xu, Y.; Goodacre, R. MALDI-MS and multivariate analysis for the detection and quantification of different milk species. Anal. Bioanal. Chem. 2011, 399, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Kuckova, S.; Zitkova, K.; Novotny, O.; Smirnova, T. Verification of cheeses authenticity by mass spectrometry. J. Sep. Sci. 2019, 42, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Korte, N.; Dyk, M.; Wenninger, O.; Schreiter, P.; Hiller, E. Rapid animal species identification of feta and mozzarella cheese using MALDI-TOF mass-spectrometry. Food Control 2020, 117, 107349. [Google Scholar] [CrossRef]
- Calvano, C.D.; De Ceglie, C.; Monopoli, A.; Zambonin, C.G. Detection of sheep and goat milk adulterations by direct MALDI-TOF MS analysis of milk tryptic digests. J. Mass Spectrom. 2012, 47, 1141–1149. [Google Scholar] [CrossRef]
- Caira, S.; Pinto, G.; Nicolai, M.A.; Chianese, L.; Addeo, F. Simultaneously tracing the geographical origin and presence of bovine milk in Italian water buffalo Mozzarella cheese using MALDI-TOF data of casein signature peptides. Anal. Bioanal. Chem. 2016, 408, 5609–5621. [Google Scholar] [CrossRef]
- Russo, R.; Rega, C.; Chambery, A. Rapid detection of water buffalo ricotta adulteration or contamination by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 497–503. [Google Scholar] [CrossRef]
- Nardiello, D.; Natale, A.; Palermo, C.; Quinto, M.; Centonze, D. Milk authenticity by ion-trap proteomics following multi-enzyme digestion. Food Chem. 2018, 244, 317–323. [Google Scholar] [CrossRef]
- Cuollo, M.; Caira, S.; Fierro, O.; Pinto, G.; Picariello, G.; Addeo, F. Toward milk speciation through the monitoring of casein proteotypic peptides. Rapid Commun. Mass Spectrom. 2010, 24, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Camerini, S.; Montepeloso, E.; Casella, M.; Crescenzi, M.; Marianella, R.M.; Fuselli, F. Mass spectrometry detection of fraudulent use of cow whey in water buffalo, sheep, or goat Italian ricotta cheese. Food Chem. 2016, 197, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Galliano, F.; Saletti, R.; Cunsolo, V.; Foti, S.; Marletta, D.; Bordonaro, S.; D’Urso, G. Identification and characterization of a new beta-casein variant in goat milk by high-performance liquid chromatography with electrospray ionization mass spectrometry and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Severino, V.; Mendez, A.; Lliberia, J.; Parente, A.; Chambery, A. Detection of buffalo mozzarella adulteration by an ultra-high performance liquid chromatography tandem mass spectrometry methodology. J. Mass Spectrom. 2012, 47, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Gunning, Y.; Fong, L.K.W.; Watson, A.D.; Philo, M.; Kemsley, E.K. Quantitative authenticity testing of buffalo mozzarella via alpha(s1)-Casein using multiple reaction monitoring mass spectrometry. Food Control 2019, 101, 189–197. [Google Scholar] [CrossRef]
- Sforza, S.; Aquino, G.; Cavatorta, V.; Galaverna, G.; Mucchetti, G.; Dossena, A.; Marchelli, R. Proteolytic oligopeptides as molecular markers for the presence of cows’ milk in fresh cheeses derived from sheep milk. Int. Dairy J. 2008, 18, 1072–1076. [Google Scholar] [CrossRef]
- Czerwenka, C.; Muller, L.; Lindner, W. Detection of the adulteration of water buffalo milk and mozzarella with cow’s milk by liquid chromatography-mass spectrometry analysis of beta-lactoglobulin variants. Food Chem. 2010, 122, 901–908. [Google Scholar] [CrossRef]
- Fuselli, F.; Tidona, F. Foreign milk in sheep’s, goat’s and water buffalo milk cheeses. In Handbook of Cheese in Health: Production, Nutrition and Medical Science; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 397–411. [Google Scholar]
- Ke, X.; Zhang, J.S.; Lai, S.Y.; Chen, Q.; Zhang, Y.; Jiang, Y.R.; Mo, W.M.; Ren, Y.P. Quantitative analysis of cow whole milk and whey powder adulteration percentage in goat and sheep milk products by isotopic dilution-ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 213–224. [Google Scholar] [CrossRef]
- Jia, W.; Dong, X.Y.; Shi, L.; Chu, X.G. Discrimination of Milk from Different Animal Species by a Foodomics Approach Based on High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 6638–6645. [Google Scholar] [CrossRef]
- Hrbek, V.; Vaclavik, L.; Elich, O.; Hajslova, J. Authentication of milk and milk-based foods by direct analysis in real time ionization-high resolution mass spectrometry (DART-HRMS) technique: A critical assessment. Food Control 2014, 36, 138–145. [Google Scholar] [CrossRef]
- Blasi, F.; Lombardi, G.; Damiani, P.; Simonetti, M.S.; Giua, L.; Cossignani, L. Triacylglycerol stereospecific analysis and linear discriminant analysis for milk speciation. J. Dairy Res. 2013, 80, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chmilenko, F.A.; Minaeva, N.P.; Sidorova, L.P. Complex chromatographic determination of the adulteration of dairy products: A new approach. J. Anal. Chem. 2011, 66, 572–581. [Google Scholar] [CrossRef]
- Cossignani, L.; Pollini, L.; Blasi, F. Invited review: Authentication of milk by direct and indirect analysis of triacylglycerol molecular species. J. Dairy Sci. 2019, 102, 5871–5882. [Google Scholar] [CrossRef] [PubMed]
- Bratu, A.; Mihalache, M.; Hanganu, A.; Chira, N.A.; Todasca, M.C.; Rosca, S. Gas Chromatography Coupled with Chemometric Method for Authentication of Romanian Cheese. Rev. Chim.-Buchar. 2012, 63, 1099–1102. [Google Scholar]
- Vieitez, I.; Irigaray, B.; Callejas, N.; Gonzalez, V.; Gimenez, S.; Arechavaleta, A.; Grompone, M.; Gambaro, A. Composition of fatty acids and triglycerides in goat cheeses and study of the triglyceride composition of goat milk and cow milk blends. J. Food Compos. Anal. 2016, 48, 95–101. [Google Scholar] [CrossRef]
- Karoui, R.; De Baerdemaeker, J. A review of the analytical methods coupled with chemometric tools for the determination of the quality and identity of dairy products. Food Chem. 2007, 102, 621–640. [Google Scholar] [CrossRef]
- Luykx, D.M.A.M.; Van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Res. Int. 2014, 60, 131–139. [Google Scholar] [CrossRef]
- Sassi, M.; Arena, S.; Scaloni, A. MALDI-TOF-MS Platform for Integrated Proteomic and Peptidomic Profiling of Milk Samples Allows Rapid Detection of Food Adulterations. J. Agric. Food Chem. 2015, 63, 7093. [Google Scholar] [CrossRef]
- Calvano, C.D.; De Ceglie, C.; Aresta, A.; Facchini, L.A.; Zambonin, C.G. MALDI-TOF mass spectrometric determination of intact phospholipids as markers of illegal bovine milk adulteration of high-quality milk. Anal. Bioanal. Chem. 2013, 405, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Motta, T.M.C.; Hoff, R.B.; Barreto, F.; Andrade, R.B.S.; Lorenzini, D.M.; Meneghini, L.Z.; Pizzolato, T.M. Detection and confirmation of milk adulteration with cheese whey using proteomic-like sample preparation and liquid chromatography-electrospray-tandem mass spectrometry analysis. Talanta 2014, 120, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, N.; Benetti, G.; Haouett, N.M.; Sergi, M.; Grotta, L.; Marchetti, S.; Castellani, F.; Martino, G. A rapid high-performance liquid chromatography-tandem mass spectrometry assay for unambiguous detection of different milk species employed in cheese manufacturing. J. Dairy Sci. 2015, 98, 8405–8413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Lai, S.Y.; Cai, Z.X.; Chen, Q.; Huang, B.F.; Ren, Y.P. Determination of bovine lactoferrin in dairy products by ultra-high performance liquid chromatography-tandem mass spectrometry based on tryptic signature peptides employing an isotope-labeled winged peptide as internal standard. Anal. Chim. Acta 2014, 829, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trend Anal. Chem. 2016, 85, 123–132. [Google Scholar]
- Hruzikova, J.; Milde, D.; Krajancova, P.; Ranc, V. Discrimination of Cheese Products for Authenticity Control by Infrared Spectroscopy. J. Agric. Food Chem. 2012, 60, 1845–1849. [Google Scholar] [CrossRef][Green Version]
- Cirak, O.; Icyer, N.C.; Durak, M.Z. Rapid detection of adulteration of milks from different species using Fourier Transform Infrared Spectroscopy (FTIR). J. Dairy Res. 2018, 85, 222–225. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Kokkinofta, R.; Theocharis, C.R. Chemometric analysis combined with FTIR spectroscopy of milk and Halloumi cheese samples according to species’ origin. Food Sci. Nutr. 2020, 8, 3262–3273. [Google Scholar] [CrossRef]
- Teixeira, J.L.D.; Carames, E.T.D.; Baptista, D.P.; Gigante, M.L.; Pallone, J.A.L. Rapid adulteration detection of yogurt and cheese made from goat milk by vibrational spectroscopy and chemometric tools. J. Food Compos. Anal. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Brandao, M.P.; Neto, M.G.; dos Anjos, V.D.; Bell, M.J.V. Detection of adulteration of goat milk powder with bovine milk powder by front-face and time resolved fluorescence. Food Control 2017, 81, 168–172. [Google Scholar] [CrossRef]
- Velioglu, S.D.; Ercioglu, E.; Boyaci, I.H. Rapid discrimination between buffalo and cow milk and detection of adulteration of buffalo milk with cow milk using synchronous fluorescence spectroscopy in combination with multivariate methods. J. Dairy Res. 2017, 84, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Genis, D.O.; Sezer, B.; Bilge, G.; Durna, S.; Boyaci, I.H. Development of synchronous fluorescence method for identification of cow, goat, ewe and buffalo milk species. Food Control 2020, 108, 106808. [Google Scholar] [CrossRef]
- Grazina, L.; Amaral, J.S.; Mafra, I. Botanical origin authentication of dietary supplements by DNA-based approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1080–1109. [Google Scholar] [CrossRef] [PubMed]
- Mafra, I.; Ferreira, I.M.P.L.V.O.; Oliveira, M.B.P.P. Food authentication by PCR-based methods. Eur. Food Res. Technol. 2008, 227, 649–665. [Google Scholar] [CrossRef]
- Amaral, J.S.; Meira, L.; Oliveira, M.B.P.P.; Mafra, I. Advances in authenticity testing for meat speciation. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing Ltd.: Sawston, UK, 2016; pp. 369–414. [Google Scholar]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA barcode markers applied to seafood authentication: An updated review. Crit. Rev. Food Sci. 2021, 61, 3904–3935. [Google Scholar] [CrossRef]
- Kalogianni, D.P. DNA-based analytical methods for milk authentication. Eur. Food Res. Technol. 2018, 244, 775–793. [Google Scholar] [CrossRef]
- Plath, A.; Krause, I.; Einspanier, R. Species identification in daily products by three different DNA-based techniques. Z. Lebensm. Und-Forsch. A 1997, 205, 437–441. [Google Scholar] [CrossRef]
- Lanzilao, I.; Burgalassi, F.; Fancelli, S.; Settimelli, M.; Fani, R. Polymerase chain reaction-restriction fragment length polymorphism analysis of mitochondrial cytb gene from species of dairy interest. J. Aoac. Int. 2005, 88, 128–135. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.M.; Ahmed, M.M.M. Rapid and sensitive identification of buffalo’s, cattle’s and sheep’s milk using species-specific PCR and PCR-RFLP techniques. Food Control 2007, 18, 1246–1249. [Google Scholar] [CrossRef]
- Otaviano, A.R.; Lima, A.L.F.; Laureano, M.M.M.; Sena, J.A.D.; de Albuquerque, L.G.; Tonhati, H. beta-casein gene polymorphism permits identification of bovine milk mixed with bubaline milk in mozzarella cheese. Genet. Mol. Biol. 2008, 31, 902–905. [Google Scholar] [CrossRef]
- Abdelfatah, E.N.; El-Araby, I.E.; Mohamed, A.A. Identification of species adulteration in raw milk and butter using polymerase chain reaction—Restriction fragment length polymorphism. System 2015, 15, 332–338. [Google Scholar]
- Ewida RM, E.-M.D. Species adulteration in raw milk samples using polymerase chain reaction-restriction fragment length polymorphism. Vet. World 2018, 11, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Vafin, R.R.; Galstyan, A.G.; Tyulkin, S.V.; Gilmanov, K.K.; Yurova, E.A.; Semipyatniy, V.K.; Bigaeva, A.V. Species identification of ruminant milk by genotyping of the κ-casein gene. J. Dairy Sci. 2022, 105, 1004–1113. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calleja, I.; Gonzalez, I.; Fajardo, V.; Rodriguez, M.A.; Hernandez, P.E.; Garcia, T.; Martin, R. Rapid detection of cows’ milk in sheeps’ and goats’ milk by a species-specific polymerase chain reaction technique. J. Dairy Sci. 2004, 87, 2839–2845. [Google Scholar] [CrossRef]
- Lopez-Calleja, I.; Gonzalez, I.; Fajardo, V.; Martin, I.; Hernandez, P.E.; Garcia, T.; Martin, R. Application of polymerase chain reaction to detect adulteration of sheep’s milk with goats’ milk. J. Dairy Sci. 2005, 88, 3115–3120. [Google Scholar] [CrossRef]
- Lopez-Calleja, I.; Alonso, I.G.; Fajardo, V.; Rodriguez, M.A.; Hernandez, P.E.; Garcia, T.; Martin, R. PCR detection of cows’ milk in water buffalo milk and mozzarella cheese. Int. Dairy J. 2005, 15, 1122–1129. [Google Scholar] [CrossRef]
- Maskova, E.; Paulickova, I. PCR-based detection of cow’s milk in goat and sheep cheeses marketed in the Czech Republic. Czech J. Food Sci. 2006, 24, 127–132. [Google Scholar] [CrossRef]
- Diaz, I.L.C.; Alonso, I.G.; Fajardo, V.; Martin, I.; Hernandez, P.; Lacarra, T.G.; de Santos, R.M. Application of a polymerase chain reaction to detect adulteration of ovine cheeses with caprine milk. Eur. Food Res. Technol. 2007, 225, 345–349. [Google Scholar] [CrossRef]
- Reale, S.; Campanella, A.; Merigioli, A.; Pilla, F. A novel method for species identification in milk and milk-based products. J. Dairy Res. 2008, 75, 107–112. [Google Scholar] [CrossRef]
- Bottero, M.T.; Civera, T.; Nucera, D.; Rosati, S.; Sacchi, P.; Turi, R.M. A multiplex polymerase chain reaction for the identification of cows’, goats’ and sheep’s milk in dairy products. Int. Dairy J. 2003, 13, 277–282. [Google Scholar] [CrossRef]
- Mafra, I.; Ferreira, I.M.P.L.V.O.; Faria, M.A.; Oliveira, B.P.P. A novel approach to the quantification of bovine milk in ovine cheeses using a duplex polymerase chain reaction method. J. Agric. Food Chem. 2004, 52, 4943–4947. [Google Scholar] [CrossRef] [PubMed]
- Mafra, I.; Roxo, A.; Ferreira, I.M.P.L.V.O.; Oliveira, M.B.P.P. A duplex polymerase chain reaction for the quantitative detection of cowsl milk in goats’ milk cheese. Int. Dairy J. 2007, 17, 1132–1138. [Google Scholar] [CrossRef]
- Bai, W.L.; Yin, R.H.; Zhao, S.J.; Dou, Q.L.; Yang, J.C.; Jiang, W.Q.; Zhao, Z.H.; Luo, G.B. Rapid detection of bovine milk in yak milk using a polymerase chain reaction technique. J. Dairy Sci. 2009, 92, 1354–1360. [Google Scholar] [CrossRef]
- De, S.; Brahma, B.; Polley, S.; Mukherjee, A.; Banerjee, D.; Gohaina, M.; Singh, K.P.; Singh, R.; Datta, T.K.; Goswami, S.L. Simplex and duplex PCR assays for species specific identification of cattle and buffalo milk and cheese. Food Control 2011, 22, 690–696. [Google Scholar] [CrossRef]
- Goncalves, J.; Pereira, F.; Amorim, A.; van Asch, B. New Method for the Simultaneous Identification of Cow, Sheep, Goat, and Water Buffalo in Dairy Products by Analysis of Short Species-Specific Mitochondrial DNA Targets. J. Agric. Food Chem. 2012, 60, 10480–10485. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.P.A.; Givisiez, P.E.N.; Queiroga, R.C.R.E.; Azevedo, P.S.; Gebreyes, W.A.; Oliveira, C.J.B. Milk adulteration: Detection of bovine milk in bulk goat milk produced by smallholders in northeastern Brazil by a duplex PCR assay. J. Dairy Sci. 2012, 95, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- Golinelli, L.P.; Carvalho, A.C.; Casaes, R.S.; Lopes, C.S.C.; Deliza, R.; Paschoalin, V.M.F.; Silva, J.T. Sensory analysis and species-specific PCR detect bovine milk adulteration of frescal (fresh) goat cheese. J. Dairy Sci. 2014, 97, 6693–6699. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, L.; Di Gerlando, R.; Tolone, M.; Mastrangelo, S.; Sardina, M.T. 12S rRNA mitochondrial gene as marker to trace Sicilian mono-species dairy products. Livest. Sci. 2016, 193, 39–44. [Google Scholar] [CrossRef][Green Version]
- Di Pinto, A.; Terio, V.; Marchetti, P.; Bottaro, M.; Mottola, A.; Bozzo, G.; Bonerba, E.; Ceci, E.; Tantillo, G. DNA-based approach for species identification of goat-milk products. Food Chem. 2017, 229, 93–97. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.F.; Ku, T.; Liu, M.H.; Huang, Y. Qualitative and quantitative identification of adulteration of milk powder using DNA extracted with a novel method. J. Dairy Sci. 2017, 100, 1657–1663. [Google Scholar] [CrossRef]
- Deng, L.; Li, A.L.; Gao, Y.; Shen, T.; Yue, H.T.; Miao, J.; Li, R.R.; Yang, J. Detection of the Bovine Milk Adulterated in Camel, Horse, and Goat Milk Using Duplex PCR. Food Anal. Method 2020, 13, 560–567. [Google Scholar] [CrossRef]
- Guerreiro, J.S.; Fernandes, P.; Bardsley, R.G. Identification of the species of origin of milk in cheeses by multivariate statistical analysis of polymerase chain reaction electrophoretic patterns. Int. Dairy J. 2012, 25, 42–45. [Google Scholar] [CrossRef]
- Lopparelli, R.M.; Cardazzo, B.; Balzan, S.; Giaccone, V.; Novelli, E. Real-time TaqMan polymerase chain reaction detection and quantification of cow DNA in pure water buffalo mozzarella cheese: Method validation and its application on commercial samples. J. Agric. Food Chem. 2007, 55, 3429–3434. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, A.; Walecka, E.; Bania, J.; Zelazko, M.; Szoltysik, M.; Chrzanowska, J. Quality of UHT goat’s milk in Poland evaluated by real-time PCR. Small Rumin. Res. 2010, 94, 32–37. [Google Scholar] [CrossRef]
- Marchetti, P.; Mottola, A.; Tantillo, G.; Castrica, M.; Di Pinto, A. Short communication: Detection of undeclared presence of bovine milk in buffalo yogurt. J. Dairy Sci. 2021, 104, 4056–4061. [Google Scholar] [CrossRef]
- Agrimonti, C.; Pirondini, A.; Marmiroli, M.; Marmiroli, N. A quadruplex PCR (qxPCR) assay for adulteration in dairy products. Food Chem. 2015, 187, 58–64. [Google Scholar] [CrossRef]
- Lopez-Calleja, I.; Gonzalez, I.; Fajardo, V.; Martin, I.; Hernandez, P.E.; Garcia, T.; Martin, R. Quantitative detection of goats’ milk in sheep’s milk by real-time PCR. Food Control 2007, 18, 1466–1473. [Google Scholar] [CrossRef]
- Lopez-Calleja, I.; Gonzalez, I.; Fajardo, V.; Martin, I.; Hernandez, P.E.; Garcia, T.; Martin, R. Real-time TaqMan PCR for quantitative detection of cows’ milk in ewes’ milk mixtures. Int. Dairy J. 2007, 17, 729–736. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fowler, M.R.; Scott, N.W.; Lawson, G.; Slater, A. A TaqMan real-time PCR system for the identification and quantification of bovine DNA in meats, milks and cheeses. Food Control 2007, 18, 1149–1158. [Google Scholar] [CrossRef]
- Dalmasso, A.; Civera, T.; La Neve, F.; Bottero, M.T. Simultaneous detection of cow and buffalo milk in mozzarella cheese by Real-Time PCR assay. Food Chem. 2011, 124, 362–366. [Google Scholar] [CrossRef]
- Dalmasso, A.; Sacchi, P.; Bottero, M.T. Development of a real-time PCR assay for the detection of cow and donkey milk. Eur. Food Res. Technol. 2012, 235, 47–52. [Google Scholar] [CrossRef]
- Drummond, M.G.; Brasil, B.S.A.F.; Dalsecco, L.S.; Brasil, R.S.A.F.; Teixeira, L.V.; Oliveira, D.A.A. A versatile real-time PCR method to quantify bovine contamination in buffalo products. Food Control 2013, 29, 131–137. [Google Scholar] [CrossRef]
- Di Domenico, M.; Di Giuseppe, M.; Rodriguez, J.D.W.; Camma, C. Validation of a fast real-time PCR method to detect fraud and mislabeling in milk and dairy products. J. Dairy Sci. 2017, 100, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, T.T.; Yu, W.J.; Qiao, L.; Liu, R.; Li, S.S.; Zhao, Y.; Yang, S.M.; Chen, A.L. Determination of content of camel milk in adulterated milk samples by normalized real-time polymerase chain reaction system based on single-copy nuclear genes. J. Sci. Food Agric. 2020, 100, 3465–3470. [Google Scholar] [CrossRef]
- Cottenet, G.; Blancpain, C.; Golay, P.A. Simultaneous detection of cow and buffalo species in milk from China, India, and Pakistan using multiplex real-time PCR. J. Dairy Sci. 2011, 94, 3787–3793. [Google Scholar] [CrossRef]
- Rentsch, J.; Weibel, S.; Ruf, J.; Eugster, A.; Beck, K.; Koppel, R. Interlaboratory validation of two multiplex quantitative real-time PCR methods to determine species DNA of cow, sheep and goat as a measure of milk proportions in cheese. Eur. Food Res. Technol. 2013, 236, 217–227. [Google Scholar] [CrossRef]
- Guo, L.; Qian, J.P.; Guo, Y.S.; Hai, X.; Liu, G.Q.; Luo, J.X.; Ya, M. Simultaneous identification of bovine and equine DNA in milks and dairy products inferred from triplex TaqMan real-time PCR technique. J. Dairy Sci. 2018, 101, 6776–6786. [Google Scholar] [CrossRef]
- Guo, L.; Ya, M.; Hai, X.; Guo, Y.S.; Li, C.D.; Xu, W.L.; Liao, C.S.; Feng, W.; Cai, Q. A simultaneous triplex TaqMan real-time PCR approach for authentication of caprine and bovine meat, milk and cheese. Int. Dairy J. 2019, 95, 58–64. [Google Scholar] [CrossRef]
- Guo, L.; Yu, Y.; Xu, W.L.; Li, C.D.; Liu, G.Q.; Qi, L.M.G.; Luo, J.X.; Guo, Y.S. Simultaneous detection of ovine and caprine DNA in meat and dairy products using triplex TaqMan real-time PCR. Food Sci. Nutr. 2020, 8, 6467–6476. [Google Scholar] [CrossRef]
- Hai, X.; Liu, G.Q.; Luo, J.X.; Guo, Y.S.; Qian, J.P.; Ya, M.; Guo, L. Triplex real-time PCR assay for the authentication of camel-derived dairy and meat products. J. Dairy Sci. 2020, 103, 9841–9850. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Sakaridis, I.; Argiriou, A.; Madesis, P.; Tsaftaris, A. A novel closed-tube method based on high resolution melting (HRM) analysis for authenticity testing and quantitative detection in Greek PDO Feta cheese. Food Chem. 2013, 141, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Sakaridis, I.; Ganopoulos, I.; Argiriou, A.; Tsaftaris, A. High resolution melting analysis for quantitative detection of bovine milk in pure water buffalo mozzarella and other buffalo dairy products. Int. Dairy J. 2013, 28, 32–35. [Google Scholar] [CrossRef]
- Cutarelli, A.; Fulgione, A.; Fraulo, P.; Serpe, F.P.; Gallo, P.; Biondi, L.; Corrado, F.; Citro, A.; Capuano, F. Droplet Digital PCR (ddPCR) Analysis for the Detection and Quantification of Cow DNA in Buffalo Mozzarella Cheese. Animals 2021, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Sengar, G.S.; Singh, U.; Kumar, S.; Alyethodi, R.R.; Alex, R.; Raja, T.V.; Das, A.K.; Prakash, B. Application of a Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Cow Components Adulterated in Buffalo Milk/Meat. Mol. Biotechnol. 2016, 58, 850–860. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.Y. Direct duplex real-time loop mediated isothermal amplification assay for the simultaneous detection of cow and goat species origin of milk and yogurt products for field use. Food Chem. 2018, 246, 26–31. [Google Scholar] [CrossRef]
- Ribani, A.; Schiavo, G.; Utzeri, V.J.; Bertolini, F.; Geraci, C.; Bovo, S.; Fontanesi, L. Application of next generation semiconductor based sequencing for species identification in dairy products. Food Chem. 2018, 246, 90–98. [Google Scholar] [CrossRef]
- Beltramo, C.; Riina, M.V.; Colussi, S.; Campia, V.; Maniaci, M.G.; Biolatti, C.; Trisorio, S.; Modesto, P.; Peletto, S.; Acutis, P.L. Validation of a DNA biochip for species identification in food forensic science. Food Control 2017, 78, 366–373. [Google Scholar] [CrossRef]
- Kounelli, M.L.; Kalogianni, D.P. A sensitive DNA-based fluorometric method for milk authenticity of dairy products based on spectrally distinct microspheres. Eur. Food Res. Technol. 2017, 243, 1773–1781. [Google Scholar] [CrossRef]
- Bougadi, E.T.; Kalogianni, D.P. Paper-based DNA biosensor for food authenticity testing. Food Chem. 2020, 322, 126758. [Google Scholar] [CrossRef]
- Navarro, E.; Serrano-Heras, G.; Castano, M.J.; Solera, J. Real-time PCR detection chemistry. Clin. Chim. Acta 2015, 439, 231–250. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. Lupine allergens: Clinical relevance, molecular characterization, cross-reactivity, and detection strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar] [CrossRef] [PubMed]
- Druml, B.; Cichna-Markl, M. High resolution melting (HRM) analysis of DNA—Its role and potential in food analysis. Food Chem. 2014, 158, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Grazina, L.C.J.; Amaral, J.S.; Mafra, I. High-Resolution Melting Analysis as a Tool for Plant Species Authentication. In Crop Breeding: Genetic Improvement Methods; Tripodi, P., Ed.; Springer: New York, NY, USA, 2021; pp. 55–73. [Google Scholar]
- Pereira, L.; Gomes, S.; Barrias, S.; Fernandes, J.R.; Martins-Lopes, P. Applying high-resolution melting (HRM) technology to olive oil and wine authenticity. Food Res. Int. 2018, 103, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Kosir, A.B.; Demsar, T.; Stebih, D.; Zel, J.; Milavec, M. Digital PCR as an effective tool for GMO quantification in complex matrices. Food Chem. 2019, 294, 73–78. [Google Scholar] [CrossRef]
- Shehata, H.R.; Li, J.P.; Chen, S.; Redda, H.; Cheng, S.M.; Tabujara, N.; Li, H.H.; Warriner, K.; Hanner, R. Droplet digital polymerase chain reaction (ddPCR) assays integrated with an internal control for quantification of bovine, porcine, chicken and turkey species in food and feed. PLoS ONE 2017, 12, e0182872. [Google Scholar] [CrossRef]
- Zhang, H.W.; Li, J.; Zhao, S.B.; Yan, X.H.; Si, N.W.; Gao, H.F.; Li, Y.J.; Zhai, S.S.; Xiao, F.; Wu, G.; et al. An Editing-Site-Specific PCR Method for Detection and Quantification of CAO1-Edited Rice. Foods 2021, 10, 1209. [Google Scholar] [CrossRef]
- Huang, T.Z.; Li, L.Z.; Liu, X.; Chen, Q.; Fang, X.E.; Kong, J.L.; Draz, M.S.; Cao, H.M. Loop-mediated isothermal amplification technique: Principle, development and wide application in food safety. Anal. Methods 2020, 12, 5551–5561. [Google Scholar] [CrossRef]
- Plácido, A.; Amaral, J.S.; Costa, J.; Fernandes, T.J.R.; Oliveira, M.B.P.P.; Delerue-Matos, C.; Mafra, I. Novel strategies for genetically modified organism detection. In Genetically Modified Organisms in Foods; Watson, R.R., Preedy, V.R., Eds.; Academic ress: London, UK, 2016; pp. 119–131. [Google Scholar]
- Haynes, E.; Jimenez, E.; Pardo, M.A.; Helyar, S.J. The future of NGS (Next Generation Sequencing) analysis in testing food authenticity. Food Control 2019, 101, 134–143. [Google Scholar] [CrossRef]
- Mayo, B.; Rachid, C.T.C.C.; Alegria, A.; Leite, A.M.O.; Peixoto, R.S.; Delgado, S. Impact of Next Generation Sequencing Techniques in Food Microbiology. Curr. Genom. 2014, 15, 293–309. [Google Scholar] [CrossRef]
- Cunha, J.T.; Ribeiro, T.I.B.; Rocha, J.B.; Nunes, J.; Teixeira, J.A.; Domingues, L. RAPD and SCAR markers as potential tools for detection of milk origin in dairy products: Adulterant sheep breeds in Serra da Estrela cheese production. Food Chem. 2016, 211, 631–636. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Sardina, M.T.; Tortorici, L.; Mastrangelo, S.; Di Gerlando, R.; Tolone, M.; Portolano, B. Application of microsatellite markers as potential tools for traceability of Girgentana goat breed dairy products. Food Res. Int. 2015, 74, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Meshram, B.D.; Agrawal, A.K.; Adil, S.; Ranvir, S.; Sande, K.K. Biosensor and its Application in Food and Dairy Industry: A Review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3305–3324. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Veloso, A.C.A.; Reis, F.S.; Vilas-Boas, M.; Machado, A.A.S.C. An electronic tongue taste evaluation: Identification of goat milk adulteration with bovine milk. Sens. Actuat. B-Chem. 2009, 136, 209–217. [Google Scholar] [CrossRef]
- Costa, J.; Fernandes, T.J.R.; Villa, C.; Oliveira, M.B.P.P.; Mafra, I. Advances in food allergen analysis. In Food Analysis: Innovative Analytical Tools for Safety Assessment; Spizzirri, U.G., Cirillo, G., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017; pp. 305–360. [Google Scholar]

| Technique | Target Species | Application | Target Gene | Sensitivity | Reference |
|---|---|---|---|---|---|
| PCR-RFLP | Cow, sheep and goat | Milk and cheese | β-casein | 0.5% (cow’s milk in goat’s and sheep’s milk) | [92] |
| Cow, sheep, goat and buffalo | Meat and milk | cytb | - a | [93] | |
| Buffalo, cow and sheep | Milk | SSR marker and cytb | - a | [94] | |
| Cow and buffalo | Mozzarella cheeses | α-, β-and κ-casein | 1% (cow’s milk in buffalo’s milk mozzarella cheese) | [95] | |
| Cow and buffalo | Milk and butter | cytb | 5% (cow’s milk in buffalo’s milk and butter) | [96] | |
| Cow and buffalo | Raw milk | cytb | - a | [97] | |
| Cow, goat, and sheep | Raw and powder milks, pasteurized cream, and hard and semi-hard cheeses. | κ-casein | - a | [98] | |
| Species-specific PCR | Sheep and goat | Raw, thermally and process milk, milk mixtures and cheeses | 12S rRNA | 0.1% (cow’s milk in sheep’s and goat’s milk) | [99] |
| Goat | Dairy products | 12S rRNA | 0.1% (goat’s milk in sheep’s milk) | [100] | |
| Cow and buffalo | Mozzarella cheese | 12S rRNA | 0.1% (cow’s milk in mozzarella cheeses) | [101] | |
| Goat, sheep and cow | Goat’s and sheep’s cheeses | cytb | 1% (cow’s milk in goat’s cheeses) | [102] | |
| Goat and ovine | Ovine cheeses | 12S rRNA | 1% (goat’s milk in sheep’s cheeses) | [103] | |
| Cow, goat and sheep | Cheeses and other dairy products | 12S rRNA | 1% (cow’s milk in cheeses) | [37] | |
| Cow, sheep, goat and buffalo | Raw and pasteurized milks and cheese | k-casein | 0.1% (cow’s milk in buffalo’s milk) | [104] | |
| Multiplex PCR | Cow, goat and sheep | Mixture cheeses | 12S rRNA (cow, sheep and goat) and 16S rRNA (sheep) | 0.125 ng (DNA from the three species) 0.5% (cow’s milk in goat’s milk) | [105] |
| Cow and sheep | Ovine cheeses | 12S rRNA (cow, sheep) and 16S rRNA (sheep) | 0.1% (cow’s milk in ovine cheeses) | [106] | |
| Cow and goat | Goat cheeses | 12S rRNA | 0.1% (cow’s milk in goat’s cheese) | [107] | |
| Cow and yak | Raw, pasteurized, and sterilized milk mixtures | 12S rRNA | 0.1% (cow’s milk in yak’s milk) | [108] | |
| Cow and buffalo | Raw and heat treated milks and cheeses | D-Loop | 0.1% (both species in milk and cheese) 0.15 ng of buffalo’s and 0.04 ng cow’s DNA). | [109] | |
| Cow, goat, sheep and water buffalo | Dairy products (butter, cheese, cottage cheese, cream, milk (fresh, UHT, powdered) and yogurt | mtDNA | 1% (in two-species milk mixtures) | [110] | |
| Cow and goat | Goat’s milk | mtDNA | 0.5% (cow’s milk) | [111] | |
| Goat and cow | Goat’s cheese | 12S rRNA | 0.5% (cow’s milk in goat cheeses) | [112] | |
| Cow, sheep and goat | Mono-species Sicilian dairy products | 12S rRNA (cow, goat) 12S rRNA and 16S rRNA (sheep) | 0.1% (milk all species in cheeses) | [113] | |
| Cow, sheep and goat | Goat’s milk products (aged cheese, fresh cheese, yogurt, UHT milk and powder milk) | 12S rRNA (cow and goat) and cytb (sheep) | 0.05 ng (DNA of each species) | [114] | |
| Cow and goat | Milk powder | 12S rRNA | 0.1% (cow’s milk in goat’s milk) | [115] | |
| Cow, camel, horse and goat | Raw, freeze-dried, pasteurized and ultra-high temperature (UHT) milk | 16S rRNA (camel and cow) and D-Loop (horse and goat) | 0.1%, 0.2% and 0.5% (cow’s milk in raw milk and freeze-dried milk mixtures, pasteurized milk and UHT milk, respectively) | [116] | |
| Cow, sheep and goat | PDO Portuguese cheeses | cytb | - a | [117] | |
| Real-time PCR—SYBR Green dye | Cow and buffalo | Mozzarella cheeses | cytb | 0.1% (cow’s milk) | [118] |
| Cow and goat | UHT goat’s milk | 12S rRNA | 0.5% (cow’s milk) | [119] | |
| Cow, sheep and goat | Goat’s milk products (aged cheese, fresh cheese, yogurt, UHT milk and powder milk) | 12S rRNA (cow and goat) and cytb (sheep) | 0.005 ng (DNA of each species) | [114] | |
| Cow and buffalo | buffalo yogurt | cytb | 0.015 ng of DNA for both species | [120] | |
| Multiplex real-time PCR—SYBR Green dye | Cow, sheep, goat and buffalo | Milk mixtures and cheeses | 12S rRNA (cow and goat) and cytb (sheep and buffalo) | 0.1% (all species) | [121] |
| Real-time PCR—TaqMan probes | Goat and sheep | Raw and heat-treated milk mixtures | 12S rRNA | 0.5% (goat’s DNA) 0.6% (goat’s milk in raw and pasteurized mixtures) | [122] |
| Cow and sheep | Raw and heat-treated milk mixtures | 12S rRNA | 0.5% (cow’s milk in raw and pasteurized sheep’s milk) | [123] | |
| Cow | Fresh and processed meats, milks and cheeses | cytb | 35 pg cow’s DNA | [124] | |
| Bovine and buffalo | Cheese samples | cytb | 2% (cow’s milk in buffalo’s milk) | [125] | |
| Cow and donkey | Raw, pasteurized and autoclaved milks | COI | 2% (cow’s milk in donkey’s milk) | [126] | |
| Bovine and buffalo | Dairy products and meat | cytb (cow) and 16S rRNA (buffalo) | 1% (cow’s milk in buffalo cheese) | [127] | |
| Cow, goat, sheep and buffalo | Dairy products | 12S rRNA | ≤25 ng (DNA of all species) | [128] | |
| Cow and goat | Milk powder | 12S rRNA | 0.1% (cow’s milk in goat’s milk) | [115] | |
| Camel | Milk mixtures | Heart development protein with EGF-like domain 1 (HEG1) (camel) Myostatin (mammalian species) | 1% (camel’s milk in cow’s milk) | [129] | |
| Multiplex real-time PCR—TaqMan probes | Cow and buffalo | milk | cytb | 1% (cow DNA in buffalo DNA and vice versa) | [130] |
| Cow, goat, sheep and buffalo | Milk and cheeses | Allmilk: tRNA-Lys (cow), cytb (goat, sheep and buffalo) Allcheese: β-actine (cow), prolactic receptor (sheep), grwoth hormone receptor (goat) | 0.32–32 ng of DNA of all species (Allmilk) | [131] | |
| Cow and mare | Dairy products | 12S rRNA | 0.001 ng (DNA of cow milk, yogurt, and mare milk) 0.005 ng (DNA of sour soup and Koumiss) | [132] | |
| Cow and goat | Dairy and meat products | 12S rRNA | 0.005 ng and 0.01 ng (DNA of goat’s milk and cheese, respectively) 0.01 ng and 0.05 ng (DNA of cow’s milk and cheese, respectively) | [133] | |
| Sheep and goat | Dairy and meat products | 12S rRNA | 0.001 ng and 0.01 ng (DNA of fresh and processed ovine meats, respectively) 0.00025 ng, 0.005 ng and 0.01 ng (DNA of caprine meat, milk and cheese, respectively) | [134] | |
| Camel and cow | Dairy and meat products | 12S rRNA | 1% (camel and cow milk in milk mixtures) 0.005–0.0025 ng (DNA of camel milk) 0.05–0.001 ng (DNA of camel yogurt) 0.001–0.0005 ng (DNA of camel milk beverage), 0.00025–0.0001 ng (DNA of camel meat), 0.0025–0.001 ng (DNA of cow milk), 0.5–0.001 ng (DNA of cow yogurt), 1–0.05 ng (DNA of cow cheese), 0.01 ng (DNA of cow acidic whey), 0.001 ng (DNA of cow milk powder), 0.0005–0.00025 ng (DNA of beef and beef jerky), 0.005 ng (DNA of beef sausage) | [135] | |
| HRM analysis | Cow, sheep and goat | Cheeses | D-loop | 0.1% (cow’s milk in mixed-milk) | [136] |
| Cow and buffalo | Buffalo dairy products | 12S rRNA and D-loop | 1% (cow’s milk in mozzarella cheese) | [137] | |
| ddPCR | Cow and buffalo | Mozzarella cheeses | cytb | 0.1% (cow’s milk in buffalo’s milk mozzarella cheese) | [138] |
| LAMP | Cow and buffalo | Milk and meat mixtures | D-loop | 5% (cow’s milk in buffalo’s milk) | [139] |
| Cow and goat | Milk and yogurt | cytb | 2% (cow’s and goat’s milk) | [140] | |
| NGS | Goat, sheep, cow and buffalo | Milk mixtures and cheeses | 12S and 16S rRNA | -a | [141] |
| DNA biochip (microarray) kit | Cow, pig, horse, donkey, sheep, goat, water buffalo, hare, rabbit, deer, chicken, turkey, ostrich, cat, and dog | Milk and meat mixtures, and dairy and meat products | 16S rRNA | 0.1% (Cow’s, goat’s and buffalo’s milk) | [142] |
| DNA hybridization on microspheres | Cow, sheep and goat | Milk mixtures and yogurts | cytb | 0.01% (cow’s milk in goat’s yogurt and 0.05% (cow’s milk in sheep’s yogurt) | [143] |
| Paper-based DNA biosensor | Cow, sheep and goat | Milk mixture yogurts | cytb (cow and sheep) and prolactic receptor (sheep), | 0.01% of cow’s yogurt | [144] |
| Technique | Pros | Cons |
|---|---|---|
| Electrophoretic techniques |
|
|
| Immunochemical techniques |
|
|
| Chromatography coupled to mass spectrometry |
|
|
| Spectroscopy |
|
|
| PCR-RFLP |
|
|
| Species-specific PCR |
|
|
| Real-time PCR |
|
|
| Biosensors |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods 2022, 11, 1124. https://doi.org/10.3390/foods11081124
Mafra I, Honrado M, Amaral JS. Animal Species Authentication in Dairy Products. Foods. 2022; 11(8):1124. https://doi.org/10.3390/foods11081124
Chicago/Turabian StyleMafra, Isabel, Mónica Honrado, and Joana S. Amaral. 2022. "Animal Species Authentication in Dairy Products" Foods 11, no. 8: 1124. https://doi.org/10.3390/foods11081124
APA StyleMafra, I., Honrado, M., & Amaral, J. S. (2022). Animal Species Authentication in Dairy Products. Foods, 11(8), 1124. https://doi.org/10.3390/foods11081124









