Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture
Abstract
:1. Introduction
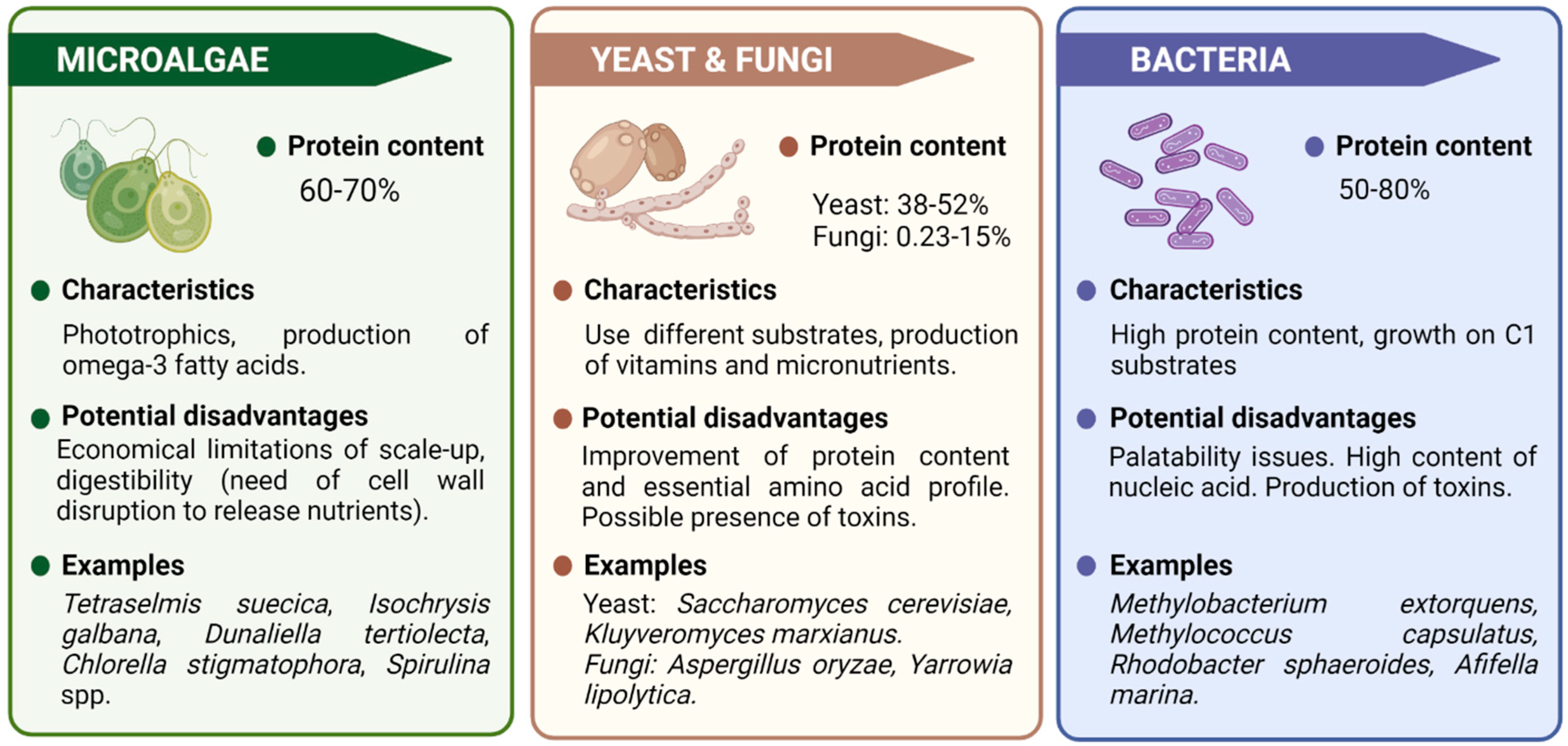
2. Single-Cell Proteins Production Systems
2.1. Microalgae
2.2. Yeast and Fungi
2.3. Bacteria
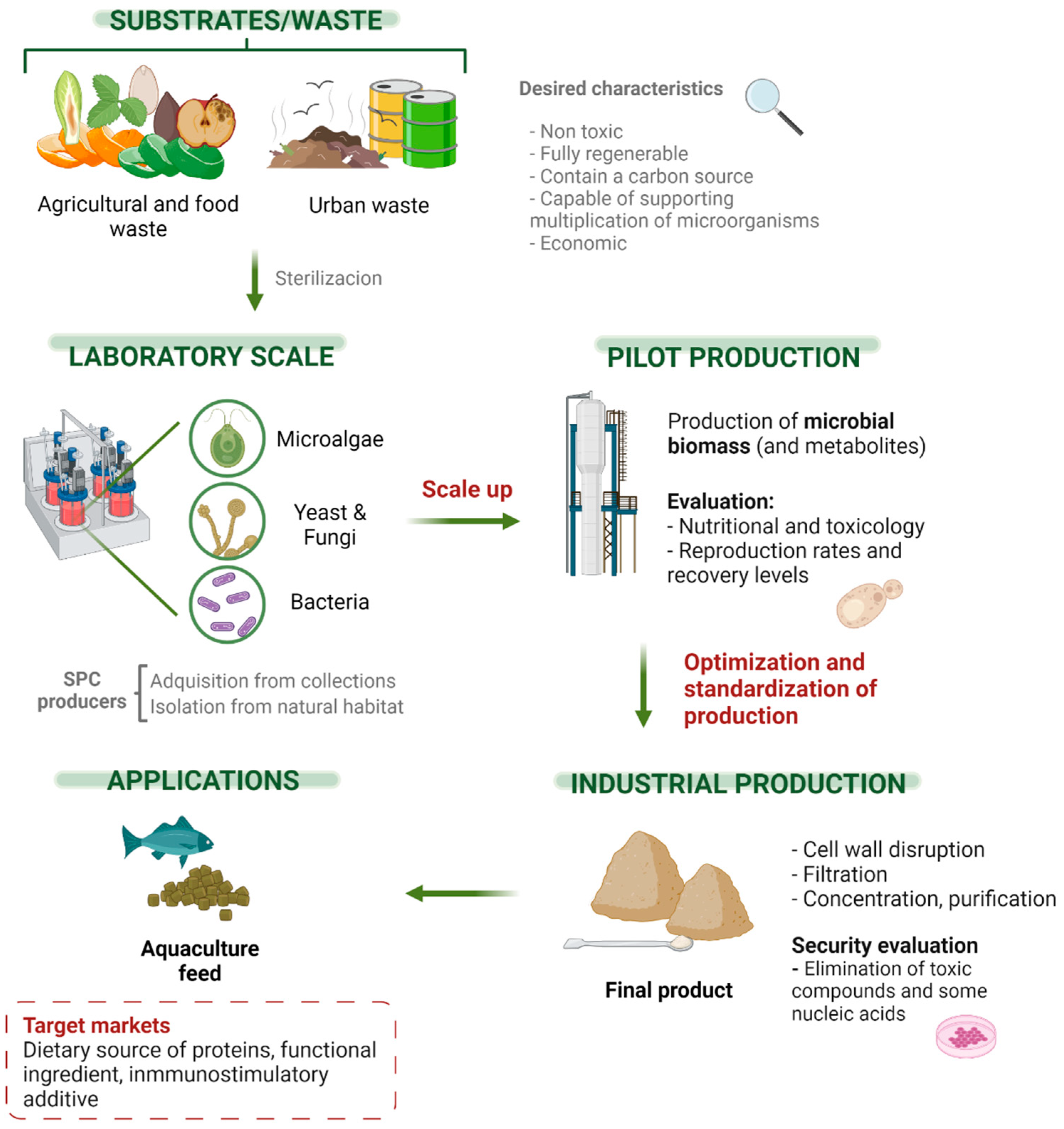
3. Opportunities to Meet Circular Economy: Revalorization of Industrial Sub-Products as Substrate for SCP Production Systems
3.1. Food and Agricultural Sub-Products
3.2. Urban or Industrial Wastes
4. Process Application
4.1. Design of a SCP Production Plant
4.2. Food-Safety of SCP
4.3. Benefits and Drawbacks of Using SCP as Aquaculture Feeds
4.4. Legislation of SCP
4.5. Environmental Impact
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dillard, H.R. Global food and nutrition security: From challenges to solutions. Food Secur. 2019, 11, 249–252. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gándara, J. Solutions for the Sustainability of the Food Production and Consumption System. Crit. Rev. Food Sci. Nutr. 2022, 62, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; Scherhaufer, S.; et al. FUSIONS—Estimates of European Food Waste Levels. IVL-Report C 186; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; ISBN 9789188319012. [Google Scholar]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2010. [Google Scholar]
- Ritchie, H.; Roser, M. Meat and Seafood Production and Consumption; Our World in Data: Oxford, UK, 2017. [Google Scholar]
- Pereira, A.G.; Jimenez-Lopez, C.; Fraga, M.; Lourenço-Lopes, C.; García-Oliveira, P.; Lorenzo, J.M.; Perez-Lamela, C.; Prieto, M.A.; Simal-Gandara, J. Extraction, Properties, and Applications of Bioactive Compounds Obtained from Microalgae. Curr. Pharm. Des. 2020, 26, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Ovando, D.; Hilborn, R.; Gaines, S.D.; Deschenes, O.; Lester, S.E. Status and solutions for the world’s unassessed fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef]
- Britten, G.L.; Duarte, C.M.; Worm, B. Recovery of assessed global fish stocks remains uncertain. Proc. Natl. Acad. Sci. USA 2021, 118, e2108532118. [Google Scholar] [CrossRef]
- Dabi, M.; Dzorvakpor, S.E.A. The Impact of Aquaculture on the Environment : A Ghanaian Perspective. Int. J. Sci. Technoledge 2015, 3, 106. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Russell, R.M. Essential nutrients: Food or supplements? Where should the emphasis be? J. Am. Med. Assoc. 2005, 294, 351–358. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.E.; Little, D.C. Intensifying aquaculture production through new approaches to manipulating natural food. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 2006. [Google Scholar] [CrossRef]
- Zhou, L. Investigations of Ammonia Nitrogen in Aquaculture: The Methodology, Concentrations, Removal, and Pond Fertilization. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2015. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, F.Y.; Rosentrate, K.A.; Muthukumar, K. Alternative Protein Sources for Aquaculture Feeds. J. Aquac. Feed Sci. Nutr. 2012, 4, 1–26. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.A.; Pulina, P. The introduction of insect meal into fish diet: The first economic analysis on European sea bass farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Feeds for the Aquaculture Sector; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-77940-9. [Google Scholar]
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. Single-cell protein. Yeasts and Bacteria. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5277–5284. [Google Scholar]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast. Fermentation 2022, 8, 355. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Marine microalgae as a potential source of minerals in fish diets. Aquaculture 1986, 51, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Fabregas, J.; Herrero, C. Marine microalgae as a potential source of single cell protein (SCP). Appl. Microbiol. Biotechnol. 1985, 23, 110–113. [Google Scholar] [CrossRef]
- Coutinho, P.; Rema, P.; Otero, A.; Pereira, O.; Fábregas, J. Use of biomass of the marine microalga Isochrysis galbana in the nutrition of goldfish (Carassius auratus) larvae as source of protein and vitamins. Aquac. Res. 2006, 37, 793–798. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Nikolov, G. The effect of algae meal (Spirulina) on the growth performance and carcass parameters of rainbow trout (Oncorhynchus mykiss). J. Biosci. Biotechnol. 2012, 151–156. [Google Scholar]
- Kim, S.S.; Rahimnejad, S.; Kim, K.W.; Lee, K.J. Partial replacement of fish meal with Spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus). Turk. J. Fish. Aquat. Sci. 2013, 13, 197–204. [Google Scholar]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet color stability in rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 414, 224–228. [Google Scholar] [CrossRef]
- Kiron, V.; Sørensen, M.; Huntley, M.; Vasanth, G.K.; Gong, Y.; Dahle, D.; Palihawadana, A.M. Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for atlantic salmon. Front. Mar. Sci. 2016, 3, 67. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Larrán, A.M.; de Mercado, E.; Sanz-Calvo, M.A.; Hernández, D.; Riaño, B.; García-González, M.C. Scenedesmus almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 422–430. [Google Scholar] [CrossRef]
- Basri, N.A.; Shaleh, S.R.M.; Matanjun, P.; Noor, N.M.; Shapawi, R. The potential of microalgae meal as an ingredient in the diets of early juvenile Pacific white shrimp, Litopenaeus vannamei. J. Appl. Phycol. 2015, 27, 857–863. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Deng, D.F.; Dominy, W. A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 2012, 354, 50–55. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.Q.; Shao, R. Effect of dietary chlorella on the growth performance and physiological parameters of gibel carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sci. 2014, 14, 53–57. [Google Scholar]
- Namaei Kohal, M.; Esmaeili Fereidouni, A.; Firouzbakhsh, F.; Hayati, I. Effects of dietary incorporation of Arthrospira (Spirulina) platensis meal on growth, survival, body composition, and reproductive performance of red cherry shrimp Neocaridina davidi (Crustacea, Atyidae) over successive spawnings. J. Appl. Phycol. 2018, 30, 431–443. [Google Scholar] [CrossRef]
- Gong, Y.; Guterres, H.A.D.S.; Huntley, M.; Sørensen, M.; Kiron, V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquac. Nutr. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 2017, 479, 490–500. [Google Scholar] [CrossRef]
- Apandi, N.M.; Radin Mohamed, R.M.S.; Latiffi, N.A.A.; Rozlan, N.F.M.; Al-Gheethi, A.A.S. Protein and Lipid Content of Microalgae Scenedesmus sp. Biomass Grown in Wet Market Wastewater. In Proceedings of the MATEC Web of Conferences, Wuhan, China, 20–21 December 2017; Volume 103, p. 06011. [Google Scholar]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Pierre, J.F.; Wang, S.; Huang, H.; Zhang, J.; Liang, S.; Zeng, Q.; Zhang, C.; Huang, M.; et al. Marine microalgae bioengineered Schizochytrium sp. meal hydrolysates inhibits acute inflammation. Sci. Rep. 2018, 8, 9848. [Google Scholar] [CrossRef]
- Skalli, A.; Firmino, J.P.; Andree, K.B.; Salomón, R.; Estévez, A.; Puig, P.; Sabater-Martínez, M.; Hechavarria, T.; Gisbert, E. The inclusion of the microalga Scenedesmus sp. in diets for rainbow trout, Onchorhynchus mykiss, juveniles. Animals 2020, 10, 1656. [Google Scholar] [CrossRef]
- Jannathulla, R.; Dayal, J.S.; Ambasankar, K.; Eugine, A.C.; Muralidhar, M. Fungus, Aspergillus niger, fermented groundnut oil cake as a fishmeal alternative in the diet of Penaeus vannamei. Aquac. Res. 2018, 49, 2891–2902. [Google Scholar] [CrossRef]
- Dayal, J.S.; Jannathulla, R.; Ambasankar, K.; Muralidhar, M. Aspergillus niger fermented plant protein mix as a potential substitute for fishmeal in the diet of Penaeus vannamei (Boone, 1931). Aquac. Nutr. 2020, 26, 853–865. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Şişman, T.; Gür, Ö.; Doğan, N.; Özdal, M.; Algur, Ö.F.; Ergon, T. Single-cell protein as an alternative food for zebrafish, Danio rerio: A toxicological assessment. Toxicol. Ind. Health 2013, 29, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Langeland, M.; Vidakovic, A.; Vielma, J.; Lindberg, J.E.; Kiessling, A.; Lundh, T. Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis). Aquac. Nutr. 2016, 22, 485–495. [Google Scholar] [CrossRef]
- Yamada, E.A.; Sgarbieri, V.C. Yeast (Saccharomyces cerevisiae) protein concentrate: Preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem. 2005, 53, 3931–3936. [Google Scholar] [CrossRef]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V.; Kiessling, A.; Lundh, T. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquac. Nutr. 2020, 26, 275–286. [Google Scholar] [CrossRef]
- Guo, J.; Qiu, X.; Salze, G.; Davis, D.A. Use of high-protein brewer’s yeast products in practical diets for the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2019, 25, 680–690. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture 2013, 402–403, 1–7. [Google Scholar] [CrossRef]
- Sahlmann, C.; Djordjevic, B.; Lagos, L.; Mydland, L.T.; Morales-Lange, B.; Øvrum Hansen, J.; Ånestad, R.; Mercado, L.; Bjelanovic, M.; Press, C.M.L.; et al. Yeast as a protein source during smoltification of Atlantic salmon (Salmo salar L.), enhances performance and modulates health. Aquaculture 2019, 513, 734396. [Google Scholar] [CrossRef]
- Øvrum Hansen, J.; Hofossæter, M.; Sahlmann, C.; Ånestad, R.; Reveco-Urzua, F.E.; Press, C.M.L.; Mydland, L.T.; Øverland, M. Effect of Candida utilis on growth and intestinal health of Atlantic salmon (Salmo salar) parr. Aquaculture 2019, 511, 734239. [Google Scholar] [CrossRef]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L. Evaluation of Kluyveromyces marxianus as a source of yeast autolysates. J. Ind. Microbiol. Biotechnol. 2003, 30, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.J.; Zhang, Z.H.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bitam, A.; Aissaoui, O. Spirulina platensis, oxidative stress, and diabetes. In Diabetes: Oxidative Stress and Dietary Antioxidants; Academic Press: Londom, UK, 2020; pp. 325–331. ISBN 9780128157763. [Google Scholar]
- Yang, P.; Li, X.; Song, B.; He, M.; Wu, C.; Leng, X. The potential of Clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (Micropterus salmoides): Growth, feed utilization and intestinal histology. Aquac. Fish. 2021, 8, 67–75. [Google Scholar] [CrossRef]
- Chen, Y.; Sagada, G.; Xu, B.; Chao, W.; Zou, F.; Ng, W.K.; Sun, Y.; Wang, L.; Zhong, Z.; Shao, Q. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2020, 51, 1000–1011. [Google Scholar] [CrossRef]
- Aas, T.S.; Grisdale-Helland, B.; Terjesen, B.F.; Helland, S.J. Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 2006, 259, 365–376. [Google Scholar] [CrossRef]
- Ekasari, J.; Suprayudi, M.A.; Elas, P.; Senja, R.K. The digestibility of biofloc meal from African catfish culture medium as a feed raw material for Pacific white shrimp. J. Akuakultur Indones. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Annamalai, S.N.; Das, P.; Thaher, M.I.A.; Abdul Quadir, M.; Khan, S.; Mahata, C.; Al Jabri, H. Nutrients and energy digestibility of microalgal biomass for fish feed applications. Sustainability 2021, 13, 13211. [Google Scholar] [CrossRef]
- Moreno, C.R.; Fernández, P.C.R.; Rodríguez, E.O.C.; Carrillo, J.M.; Rochín, S.M. Changes in Nutritional Properties and Bioactive Compounds in Cereals During Extrusion Cooking. In Extrusion of Metals, Polymers and Food Products; InTech: Nappanee, IN, USA, 2018; pp. 104–124. [Google Scholar]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Wallis, I.R.; Claridge, A.W.; Trappe, J.M. Nitrogen content, amino acid composition and digestibility of fungi from a nutritional perspective in animal mycophagy. Fungal Biol. 2012, 116, 590–602. [Google Scholar] [CrossRef]
- Zhang, J.; Elser, J.J. Carbon: Nitrogen: Phosphorus stoichiometry in fungi: A meta-analysis. Front. Microbiol. 2017, 8, 1281. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of nutritional composition of pure filamentous fungal biomass as a novel ingredient for fish feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Jin, M.; Xiong, J.; Zhou, Q.C.; Yuan, Y.; Wang, X.X.; Sun, P. Dietary yeast hydrolysate and brewer’s yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 82, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Huyben, D.; Nyman, A.; Vidaković, A.; Passoth, V.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Effects of dietary inclusion of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus on gut microbiota of rainbow trout. Aquaculture 2017, 473, 528–537. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Mirvaghefi, A.; Merrifield, D.L. The effects of dietary inactive brewer’s yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 2011, 318, 90–94. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Fernández-Díaz, B.; Nieto-López, M.; Cruz-Suárez, L.E. Nutritional contribution of torula yeast and fish meal to the growth of shrimp Litopenaeus vannamei as indicated by natural nitrogen stable isotopes. Aquaculture 2016, 453, 116–121. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Rahman, A.M.; Ismael, N.E.M. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Ugalde, U.O.; Castrillo, J.I. Single cell proteins from fungi and yeasts. Appl. Mycol. Biotechnol. 2002, 2, 123–149. [Google Scholar]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa Moustafa, E.; Shahin, M.G. Effects of feeding regimen of dietary Aspergillus oryzae on the growth performance, intestinal morphometry and blood profile of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2019, 25, 1063–1072. [Google Scholar] [CrossRef]
- Patsios, S.I.; Dedousi, A.; Sossidou, E.N.; Zdragas, A. Sustainable animal feed protein through the cultivation of Yarrowia lipolytica on agro-industrial wastes and by-products. Sustainability 2020, 12, 1398. [Google Scholar] [CrossRef]
- Berge, G.M.; Hatlen, B.; Odom, J.M.; Ruyter, B. Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquac. Nutr. 2013, 19, 110–121. [Google Scholar] [CrossRef]
- Neuls, L.; de Souza, V.J.; Romão, S.; Bitencourt, T.B.; Ramos, C.J.R.; Parra, J.E.G.; Cazarolli, L.H. Immunomodulatory effects of Yarrowia lipolytica as a food additive in the diet of Nile tilapia. Fish Shellfish Immunol. 2021, 119, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Licona-Jain, A.; Campa-Córdova, Á.; Luna-González, A.; Racotta, I.S.; Tello, M.; Angulo, C. Dietary supplementation of marine yeast Yarrowia lipolytica modulates immune response in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 105, 469–476. [Google Scholar] [CrossRef]
- Tlusty, M.; Rhyne, A.; Szczebak, J.T.; Bourque, B.; Bowen, J.L.; Burr, G.; Marx, C.J.; Feinberg, L. A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds. PeerJ 2017, 2017, e3170. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W.; Patro, B.; Pujol-Baxley, C.; Marx, C.J.; Feinberg, L. Partial replacement of soybean meal with Methylobacterium extorquens single-cell protein in feeds for rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2018, 49, 2218–2224. [Google Scholar] [CrossRef]
- Øverland, M.; Tauson, A.H.; Shearer, K.; Skrede, A. Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch. Anim. Nutr. 2010, 64, 171–189. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Øverland, M.; Mydland, L.T.; Skrede, A.; Landsverk, T. Bacteria grown on natural gas prevent soybean meal-induced enteritis in atlantic salmon. J. Nutr. 2011, 141, 124–130. [Google Scholar] [CrossRef]
- Chumpol, S.; Kantachote, D.; Nitoda, T.; Kanzaki, H. Administration of purple nonsulfur bacteria as single cell protein by mixing with shrimp feed to enhance growth, immune response and survival in white shrimp (Litopenaeus vannamei) cultivation. Aquaculture 2018, 489, 85–95. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Yun, H.; Won, S.; Kim, S.K.; Farris, N.W.; Bai, S.C. Evaluation of a single-cell protein as a dietary fish meal substitute for whiteleg shrimp Litopenaeus vannamei. Fish. Sci. 2019, 85, 147–155. [Google Scholar] [CrossRef]
- Glencross, B.; Irvin, S.; Arnold, S.; Blyth, D.; Bourne, N.; Preston, N. Effective use of microbial biomass products to facilitate the complete replacement of fishery resources in diets for the black tiger shrimp, Penaeus monodon. Aquaculture 2014, 431, 12–19. [Google Scholar] [CrossRef]
- Hülsen, T.; Barnes, A.C.; Batstone, D.J.; Capson-Tojo, G. Creating value from purple phototrophic bacteria via single-cell protein production. Curr. Opin. Biotechnol. 2022, 76, 102726. [Google Scholar] [CrossRef] [PubMed]
- Balagurunathan, B.; Ling, H.; Choi, W.J.; Chang, M.W. Potential use of microbial engineering in single-cell protein production. Curr. Opin. Biotechnol. 2022, 76, 102740. [Google Scholar] [CrossRef] [PubMed]
- Dangelico, R.M.; Pujari, D. Mainstreaming green product innovation: Why and how companies integrate environmental sustainability. J. Bus. Ethics 2010, 95, 471–486. [Google Scholar] [CrossRef]
- Urbinati, A.; Chiaroni, D.; Chiesa, V. Towards a new taxonomy of circular economy business models. J. Clean. Prod. 2017, 168, 487–498. [Google Scholar] [CrossRef]
- Raimbault, M. General and microbiological aspects of solid substrate fermentation. Electron. J. Biotechnol. 1998, 1, 26–27. [Google Scholar] [CrossRef]
- Carter, C.G.; Codabaccus, M.B. Assessing the value of single-cell ingredients in aquafeeds. Curr. Opin. Biotechnol. 2022, 76, 102734. [Google Scholar] [CrossRef]
- Smil, V. Feeding the World: A Challenge for the Twenty-First Century. Issues Sci. Technol. 2004, 20, 93–96. [Google Scholar]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. European Comission: Brussels, Belgium, 2008; Volume 312. Available online: https://eur-lex.europa.eu (accessed on 30 August 2022).
- COM(2005)670/F1; EC Commission Communication: Thematic Strategy on the Sustainable Use of Natural Resuources. European Comission: Brussels, Belgium, 2005.
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single cell protein production from waste biomass: Review of various agricultural by-products. Agron. Res. 2018, 16, 1493–1508. [Google Scholar]
- Ukaegbu-Obi, K.M. Ukaegbu-Obi Single Cell Protein: A Resort to Global Protein Challenge and Waste Management. J. Microbiol. Microb. Technol. 2016, 1, 251–262. [Google Scholar]
- Finco, A.M.D.O.; Mamani, L.D.G.; Carvalho, J.C.D.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Bacha, U.; Nasir, M.; Khalique, A.; Anjum, A.A.; Jabbar, M.A. Comparative assessment of various agro-industrial wastes for Saccharomyces cerevisiae biomass production and its quality evaluation as single cell protein. J. Anim. Plant Sci. 2011, 21, 844–849. [Google Scholar]
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of fruit wastes producing single cell protein. Int. J. Sci. Environ. Technol. 2012, 1, 430–438. [Google Scholar]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of single cell protein (SCP) from food and agricultural waste by using Saccharomyces cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef]
- Mahan, K.M.; Le, R.K.; Wells, T.; Anderson, S.; Yuan, J.S.; Stoklosa, R.J.; Bhalla, A.; Hodge, D.B.; Ragauskas, A.J. Production of single cell protein from agro-waste using Rhodococcus opacus. J. Ind. Microbiol. Biotechnol. 2018, 45, 795–801. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tsapekos, P.; Zhang, Y.; Valverde-Pérez, B.; Angelidaki, I. Urban biowaste valorization by coupling anaerobic digestion and single cell protein production. Bioresour. Technol. 2019, 290, 121743. [Google Scholar] [CrossRef]
- Zha, X.; Tsapekos, P.; Zhu, X.; Khoshnevisan, B.; Lu, X.; Angelidaki, I. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 2021, 320, 124351. [Google Scholar] [CrossRef]
- Saejung, C.; Thammaratana, T. Biomass recovery during municipal wastewater treatment using photosynthetic bacteria and prospect of production of single cell protein for feedstuff. Environ. Technol. 2016, 37, 3055–3061. [Google Scholar] [CrossRef]
- European Union Food Waste. Available online: https://ec.europa.eu/food/safety/food-waste_en (accessed on 9 May 2022).
- Blakeney, M. Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing: Northampton, MA, USA, 2019; ISBN 9781788975391. [Google Scholar]
- ONU. Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021; ISBN 9789280738513. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Gupta, S.; Varjani, S.; Pandey, A.; Gnansounou, E.; You, S.; Ngo, H.H.; Wong, J.W.C. Trends in mitigation of industrial waste: Global health hazards, environmental implications and waste derived economy for environmental sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Ahmadi, A.R.; Ghoorchian, H.; Hajihosaini, R.; Khanifar, J. Determination of the amount of protein and amino acids extracted from the microbial protein (SCP) of lignocellulosic wastes. Pak. J. Biol. Sci. 2010, 13, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hülsen, T.; Hsieh, K.; Lu, Y.; Tait, S.; Batstone, D.J. Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae—A comparison. Bioresour. Technol. 2018, 254, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 251–262. [Google Scholar]
- Chama, N.T. Production of single-cell protein from different substrates. Aust. J. Sci. Technol. 2019, 3, 2208–6404. [Google Scholar]
- Brijwani, K.; Vadlani, P.V. Solid State Fermentation of Soybean Hulls for Cellulolytic Enzymes Production. In Soybean—Applications and Technology; InTech: Nappanee, IN, USA, 2011. [Google Scholar]
- The World Bank Solid Waste Management. Available online: https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management (accessed on 19 August 2022).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2018; ISBN 5856420187. [Google Scholar]
- EPA National Overview: Facts and Figures on Materials, Wastes and Recycling. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 9 May 2022).
- Tawfik, N.I.; Khalil, A.A.-G.M.; Abou-Zeid, A.-Z.A. Utilization of Petroleum Fractions for the Production of Single-Cell Protein. Zent. für Bakteriol. Parasitenkd. Infekt. und Hyg. Zweite Nat. Abt. Mikrobiol. der Landwirtsch. der Technol. und des Umweltschutzes 1981, 136, 433–448. [Google Scholar] [CrossRef]
- Spalvins, K.; Zihare, L.; Blumberga, D. Single cell protein production from waste biomass: Comparison of various industrial by-products. Energy Procedia 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Bessa, L.A. Technological microbiology: Development and applications. Front. Microbiol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Akanni, G.B.; Ntuli, V. Cactus pear biomass, a potential lignocellulose raw material for Single Cell Protein production (SCP): A Review Quantitative microbial risk assessment of Salmonella spp. in lettuce irrigated from surface water in South Africa View project. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 171–197. [Google Scholar]
- Bjorn, K.; Bu’Lock, J. Biotecnología Básica; Editorial Acribia: Navarra, Spain, 1991. [Google Scholar]
- Chacón Villalobos, A. Agronomía Mesoamericana. Agron. Mesoam. 2004, 15, 93–106. [Google Scholar] [CrossRef]
- Van Der Ha, D.; Nachtergaele, L.; Kerckhof, F.M.; Rameiyanti, D.; Bossier, P.; Verstraete, W.; Boon, N. Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef] [PubMed]
- Anupama; Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiebe, M.G. QuornTM myco-protein—Overview of a successful fungal product. Mycologist 2004, 18, 17–20. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Saljooghi, S.; Mansouri-Najand, L.; Ebrahimnejad, H.; Doostan, F.; Askari, N. Microbiological, biochemical and organoleptic properties of fermented-probiotic drink produced from camel milk. Vet. Res. Forum Int. Q. J. 2017, 8, 313–317. [Google Scholar]
- Cox, B.M.; Jamrog, D.E.; Zurcher, K.R. Euglena Lysate Composition. U.S. Patent No. 9,901,606, 27 February 2018. [Google Scholar]
- Cyanotech Spirulina Process—Cyanotech. Available online: https://www.cyanotech.com/spirulina/spirulina-process/ (accessed on 21 September 2021).
- Jackson, L.U.S. Biotech Firm Targets Shrimp with SCP Ingredient—Responsible Seafood Advocate. Available online: https://www.globalseafood.org/advocate/u-s-biotech-firm-targets-shrimp-scp-ingredient/ (accessed on 27 September 2021).
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Cuadrado, C.; Arribas, C.; Pedrosa, M.M.; Berrios, J.D.J.; Pan, J.; Morales, P. Novel gluten-free formulations from lentil flours and nutritional yeast: Evaluation of extrusion effect on phytochemicals and non-nutritional factors. Food Chem. 2020, 315, 126175. [Google Scholar] [CrossRef]
- Hälvin, K.; Paalme, T.; Nisamedtinov, I. Comparison of different extraction methods for simultaneous determination of B complex vitamins in nutritional yeast using LC/MS-TOF and stable isotope dilution assay. Anal. Bioanal. Chem. 2013, 405, 1213–1222. [Google Scholar] [CrossRef]
- Drouault, A.; Glenn, E. ARBIOM SylPro® Enhanced Torula Yeast. Product Fact Sheet. ARBIOM Prod. Fact Sheets 2018, 1–2. Available online: https://arbiom.com/ (accessed on 30 August 2022).
- Hülsen, T.; Carvalho, G.; Egger, F.; Cruz, H.; Vertstraete, W.; Batstone, D.J.; Pikaar, I. Production of single-cell proteins from organic matter and residual nitrogen. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 355–389. ISBN 9780128162040. [Google Scholar]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Nucelis LLC. Nucelis Our Products. Available online: https://www.nucelis.com/products.php?product=oils#circles (accessed on 27 September 2021).
- Yan, J.; Han, B.; Gui, X.; Wang, G.; Xu, L.; Yan, Y.; Madzak, C.; Pan, D.; Wang, Y.; Zha, G.; et al. Engineering Yarrowia lipolytica to Simultaneously Produce Lipase and Single Cell Protein from Agro-industrial Wastes for Feed. Sci. Rep. 2018, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Unibio Group UniProtein®. Available online: https://www.unibio.dk/end-product/protein/ (accessed on 21 September 2021).
- Silverman, J.; Product, C.; Benemann, J.; Engineering, M. Innovating for alternatives to marine proteins at Aquaculture 2016. AQUA Cult. Asia Pac. 2016, 6, 51–52. [Google Scholar]
- Kumar, V. String Bio—An Unreasonable Company. Available online: https://unreasonablegroup.com/companies/string-bio (accessed on 27 September 2021).
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein enrichment of yam peels by fermentation with Saccharomyces cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of single-cell ingredients in aquaculture feeds—A review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Hanlon, P.; Sewalt, V. GEMs: Genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021, 61, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Dantas, E.M.; Valle, B.C.S.; Brito, C.M.S.; Calazans, N.K.F.; Peixoto, S.R.M.; Soares, R.B. Partial replacement of fishmeal with biofloc meal in the diet of postlarvae of the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2016, 22, 335–342. [Google Scholar] [CrossRef]
- Singh, J.; Sarma, K.; Kumar, T.; Ahirwal, S.K.; Keer, S.R.N.R. Bio-floc Technology (BFT): An Intensive Eco Sustainable and Cost-Effective Tool for Aquaculture. Food Sci. Rep. 2020, 10, 11–14. [Google Scholar]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The prospects of biofloc technology (BFT) for sustainable aquaculture development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Smedley, K.O. Comparison of Regulatory Management of Authorized Ingredients, Approval Processes, and Risk-Assessment Procedures for Feed Ingredients Jurisdictions Covered: On behalf of International Feed Industry Federation; The International Feed Industry Federation: Sun City, South Africa, 2013. [Google Scholar]
- Matassa, S.; Papirio, S.; Pikaar, I.; Hülsen, T.; Leijenhorst, E.; Esposito, G.; Pirozzi, F.; Verstraete, W. Upcycling of biowaste carbon and nutrients in line with consumer confidence: The “full gas” route to single cell protein. Green Chem. 2020, 22, 4912–4929. [Google Scholar] [CrossRef]
- Puyol, D.; Batstone, D.J.; Hülsen, T.; Astals, S.; Peces, M.; Krömer, J.O. Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Front. Microbiol. 2017, 7, 2106. [Google Scholar] [CrossRef] [PubMed]
- Matassa, S.; Batstone, D.J.; Hülsen, T.; Schnoor, J.; Verstraete, W. Can direct conversion of used nitrogen to new feed and protein help feed the world? Environ. Sci. Technol. 2015, 49, 5247–5254. [Google Scholar] [CrossRef] [PubMed]
- LaTurner, Z.W.; Bennett, G.N.; San, K.Y.; Stadler, L.B. Single cell protein production from food waste using purple non-sulfur bacteria shows economically viable protein products have higher environmental impacts. J. Clean. Prod. 2020, 276, 123114. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Spiller, M.; Muys, M.; Papini, G.; Sakarika, M.; Buyle, M.; Vlaeminck, S.E. Environmental impact of microbial protein from potato wastewater as feed ingredient: Comparative consequential life cycle assessment of three production systems and soybean meal. Water Res. 2020, 171, 115406. [Google Scholar] [CrossRef] [PubMed]
| Feed | Protein Content (%) | Species | Dose | Digestibility (%) | Process | Ref. |
|---|---|---|---|---|---|---|
| Microalgae | ||||||
| Nannochloropsis spp. | 39.3 | S. salar | 30% diet | 72.0 | Extrusion | Ref. [40] |
| Nannochloropsis gaditana | 39.3 | Oreochromis niloticus, Clarus gariepinus | 30% diet | 72.4; 74.7, respectively | Dry | Ref. [41] |
| Desmodesmus sp. | 37.3 | S. salar | 30% diet | 67.0 | Extrusion | Refs. [34,40,42] |
| Schizochytrium sp. | 9.4–42.5 | O. niloticus | Fish oil | 82.0 | Extrusion | Refs. [43,44] |
| Chlorella vulgaris | 17.9 | O. niloticus, C. gariepinus | 30% diet | 80.7; 80.9, respectively | Dry | Ref. [41] |
| Scenedesmus sp. | 48 | Onchorhynchus mykiss | 5% diet | No data | Pellets | Ref. [45] |
| Scenedesmus dimorphus | 48 | O. niloticus, C. gariepinus | 30% diet | 67.0; 68.3, respectively | Dry | Ref. [41] |
| Fungi | ||||||
| Aspergillus niger | 17–50 | Penaeus vannamei | 50–60% diet | 80.7; 81.7, respectively | Dry | Refs. [46,47,48] |
| Fusarium venenatum | 50 | Melanogrammus aeglefinus | No data | No data | QUORN | Ref. [49] |
| Trichoderma harzianum | 34 | Danio rerio | 24.0 g/L | No data | Pellets | Ref. [50] |
| Yeast | ||||||
| S. cerevisiae | 44.4 | Salvelinus alpinus, Perca fluviatilis | 30% diet | 86; 83, respectively | Dry | Refs. [51,52] |
| S. cerevisiae | 44.4 | S. salar | 40% diet | 73.0 | Spray-drying | Ref. [34] |
| S. cerevisiae | 44.4 | O. mykiss | 40% diet | 91.0 | Dry | Ref. [53] |
| S. cerevisiae | 44.4 | L. vannamei | 30% diet | 74.4 | Dry | Ref. [54] |
| C. utilis | 40 | S. salar | 40% diet | 88.0 | Spray-drying | Ref. [55] |
| C. utilis | 40 | S. salar | 25% diet | No data | Drum drying | Ref. [56] |
| C. utilis | 40 | S. salar | 40% diet | 23.0 | Extrusion | Ref. [57] |
| Kluyveromyces marxianus | 9.5–12 | S. salar | 40% diet | 86.0 | Spray-drying | Refs. [55,58] |
| Rhodotorula mucilaginosa | No data | O. niloticus | 1% diet | No data | Hydrolyzed | Ref. [59] |
| Bacteria | ||||||
| Arthrospira maxima | 60–70 | O. niloticus, C. gariepinus | 30% diet | 81.4; 82.5, respectively | Extrusion | Refs. [41,60] |
| Clostridium autoethanogenum | 83 | Micropterus salmoides | 50% diet | 92 | Dry | Ref. [61] |
| C. autoethanogenum | 85 | Acanthopagrus schlegelii | 58.2% diet | No data | Extrusion | Ref. [62] |
| Biofloc | 70% | S. salar | 36% diet | 88.0 | Extrusion | Ref. [63] |
| Biofloc | No data | L. vannamei | 30% diet | 76.3 | Extrusion | Ref. [64] |
| Waste | Strain | Production System | Protein Yield | Characteristics | Ref. |
|---|---|---|---|---|---|
| FOOD | |||||
| Orange pulp and brewer’s spent grain | S. cerevisiae | Solid state fermentation | 38.5% | Significant content of fat (12.9%). | Ref. [103] |
| Dried potato and carrot skins | S. cerevisiae | Flask fermentation | 49.3% | Quantitative quality parameters comparable with casein. | Ref. [104] |
| Cucumber and orange peels | S. cerevisiae | Submerged fermentation | 53.4% | Addition of glucose enhanced the protein content (60.31%). | Ref. [105] |
| Discarded foods (mixtures of fruits and vegetables) | S. cerevisiae | Simple aerobic fermentation | 39.0% | Protein percentage in starting material less or equal to 8%. | Ref. [106] |
| Whey and potato pulp | K. marxianus | Solid state fermentation | 33.7% | High yields of fat (25.5%). | Ref. [103] |
| Juice, pulp, and peel from oranges and lemons | R. opacus | Flask fermentation | 42.0–56.9% | Protein production can be increased optimizing production conditions. | Ref. [107] |
| Corn stover effluent | R. opacus | Flask fermentation | 47.0–52.7% | Protein production can be dramatically optimizing production conditions. | Ref. [107] |
| URBAN | |||||
| Organic fraction of municipal solid waste | Methanotroph mixed culture | Anaerobic digestion | 20.6% | Methane derived from anaerobic digestion can be considered as carbon source for SCP production. | Ref. [108] |
| Methane | Methylococcales and Methylophilales | Anaerobic digestion | 8.0–20.0% | Better yields at higher concentrations CO2 in gas. | Ref. [108] |
| End-products of sludge | Methanotrophic bacteria | Anaerobic digestion | 41.0% | Potential alternative to partially replace soya in aquaculture. | Ref. [109] |
| Municipal wastewater | Rhodopseudomonas sp. | Anoxygenic condition | 60.1% | All essential amino acids produced. | Ref. [110] |
| Trade Name | Organism | Company | Country | Protein Content | Production | Other | Ref. |
|---|---|---|---|---|---|---|---|
| Microalgae | |||||||
| Algaeon | Euglena gracillis | Algaeon Inc. | USA | No data | Fermentation process | β-glucan and whole cell products | Ref. [136] |
| Cyanotech’s spirulina | Arthrospira platensis | Cyanotech Corporation | USA | 60% | Deep ocean water | One of the most commercialized products | Ref. [137] |
| ProTyton | Clostridium spp. | Biotech | USA | 85% | Ethanol plant | Atlantic salmon, shrimp feed | Ref. [138] |
| Yeast and fungi | |||||||
| Lynside® Nutri | Saccharomyces cerevisiae | LeSaffre | USA | 55.7% | Extrusion | Dried inactive yeast | Ref. [139] |
| Engevita™ | S. cerevisiae | Lallemand Inc | Canada | No data | Extrusion | Dried inactive yeast | Ref. [140] |
| SylPro | Candida utilis | Arbiom | USA | >60% | Forestry by-products | Comparable to soy | Ref. [141] |
| Quorn™ | Fusarium venenatum | Marlow Foods Ltd. | UK | 70% | Airlift reactor | Over 17% of the global meat substitute market (2016) | Refs. [142,143] |
| Yarrowia flour | Yarrowia lipolytica | Nucelis Inc. | USA | 45–55% | Agro-industrial wastes | 151.2 g/L of single-cell protein at 10 L fermentation scale | Refs. [144,145] |
| Bacteria | |||||||
| UniProtein® | Methylococcus capsulatus | UniBio A/S | Denmark | 70% | Natural gas | Particle size of 150–200 μm | Ref. [146] |
| ProFloc™ | Bacteria | Nutrinsic | USA | 60% | Wastewater from a local brewery | Replaced up to 100% fish meal in feeds for L. vannamei shrimp | Ref. [143] |
| FeedKind® | Bacteria | Calysta Inc. | UK | 70% | Methane | Satisfactory results in Atlantic salmon | Refs. [143,147] |
| String Pro | Bacteria | String Bio | India | No data | Methane | Animal feed | Ref. [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Otero, P.; Soria-Lopez, A.; Cassani, L.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods 2022, 11, 2831. https://doi.org/10.3390/foods11182831
Pereira AG, Fraga-Corral M, Garcia-Oliveira P, Otero P, Soria-Lopez A, Cassani L, Cao H, Xiao J, Prieto MA, Simal-Gandara J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods. 2022; 11(18):2831. https://doi.org/10.3390/foods11182831
Chicago/Turabian StylePereira, Antia G., Maria Fraga-Corral, Paula Garcia-Oliveira, Paz Otero, Anton Soria-Lopez, Lucia Cassani, Hui Cao, Jianbo Xiao, Miguel A. Prieto, and Jesus Simal-Gandara. 2022. "Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture" Foods 11, no. 18: 2831. https://doi.org/10.3390/foods11182831
APA StylePereira, A. G., Fraga-Corral, M., Garcia-Oliveira, P., Otero, P., Soria-Lopez, A., Cassani, L., Cao, H., Xiao, J., Prieto, M. A., & Simal-Gandara, J. (2022). Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods, 11(18), 2831. https://doi.org/10.3390/foods11182831











