Composite of Layered Double Hydroxide with Casein and Carboxymethylcellulose as a White Pigment for Food Application
Abstract
1. Introduction
2. Materials and Methodologies
2.1. Synthesis of Mg-Al LDH
2.2. Synthesis of Mg-Al-Casein LDH (CLDH)
2.3. Synthesis of Carboxymethyl Cellulose (CMC) Modified CLDH (CCLDH)
2.4. Materials Characterization
2.5. Transmittance and Opacity Measurements
2.6. Characterization of Stability of Particle Suspension to pH and Temperature
2.6.1. Effect of pH
2.6.2. Effect of Temperature
2.7. Casein Antigenicity Study
2.8. Cytotoxicity Study
2.9. Application of Alternatives Assessment Framework
3. Results
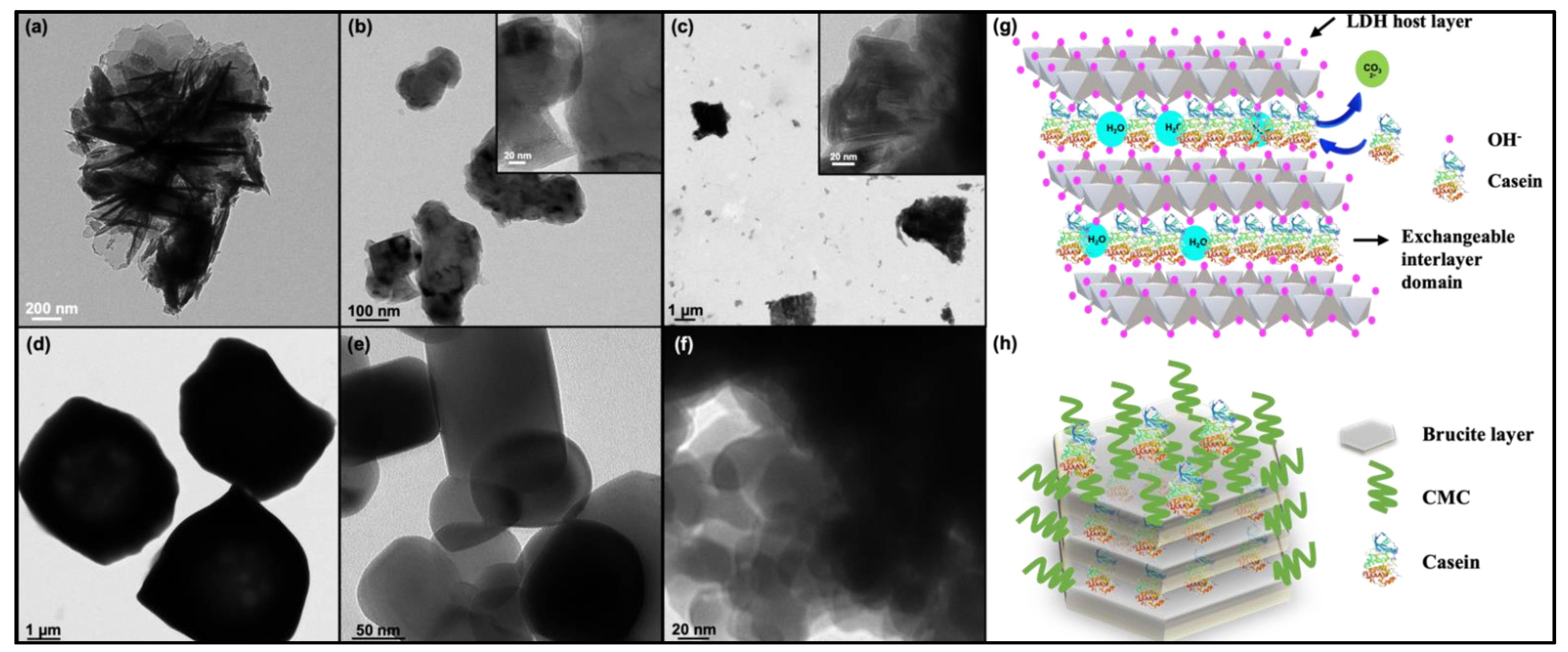
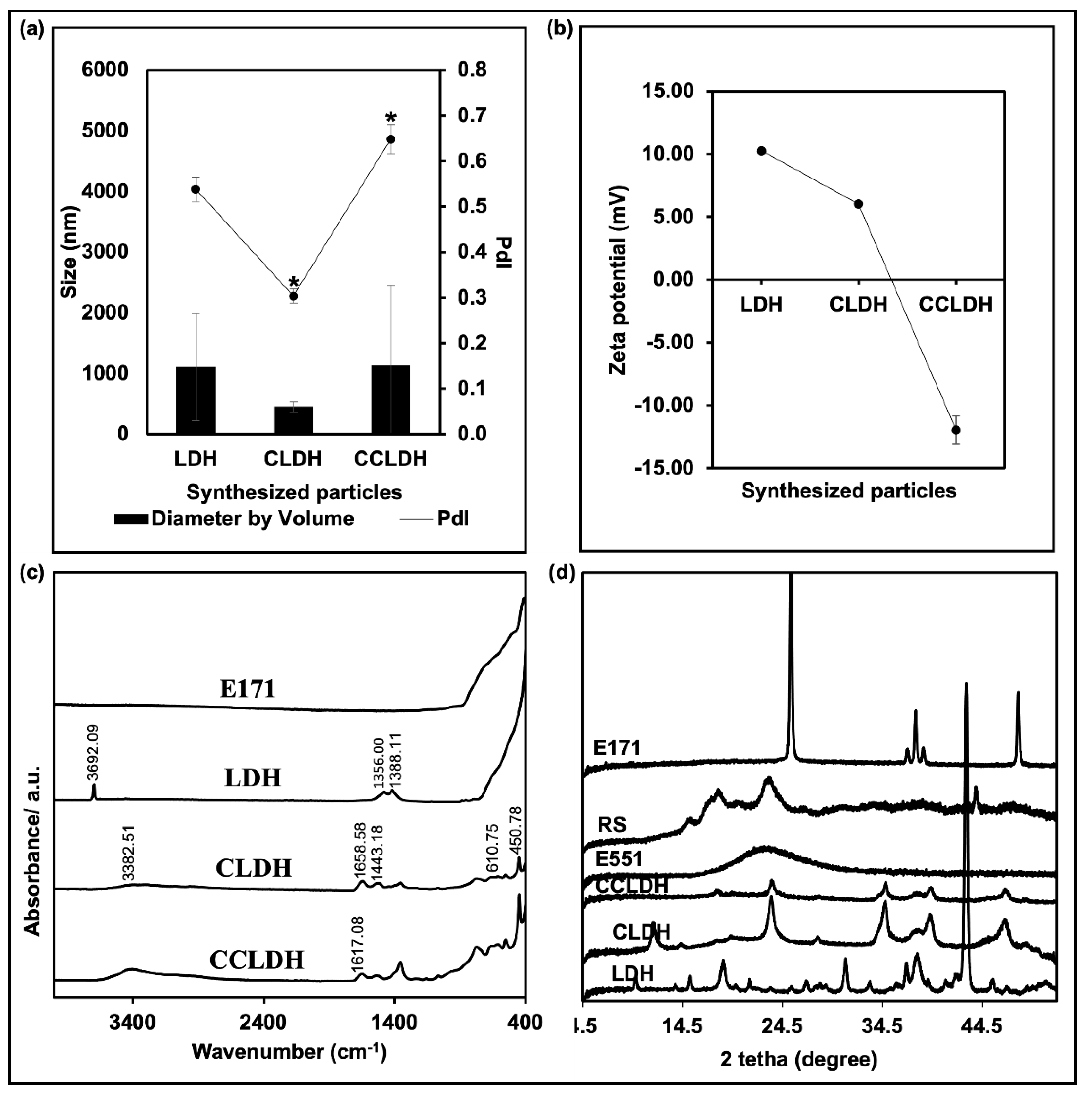
3.1. Synthesis and Characterization of Particles
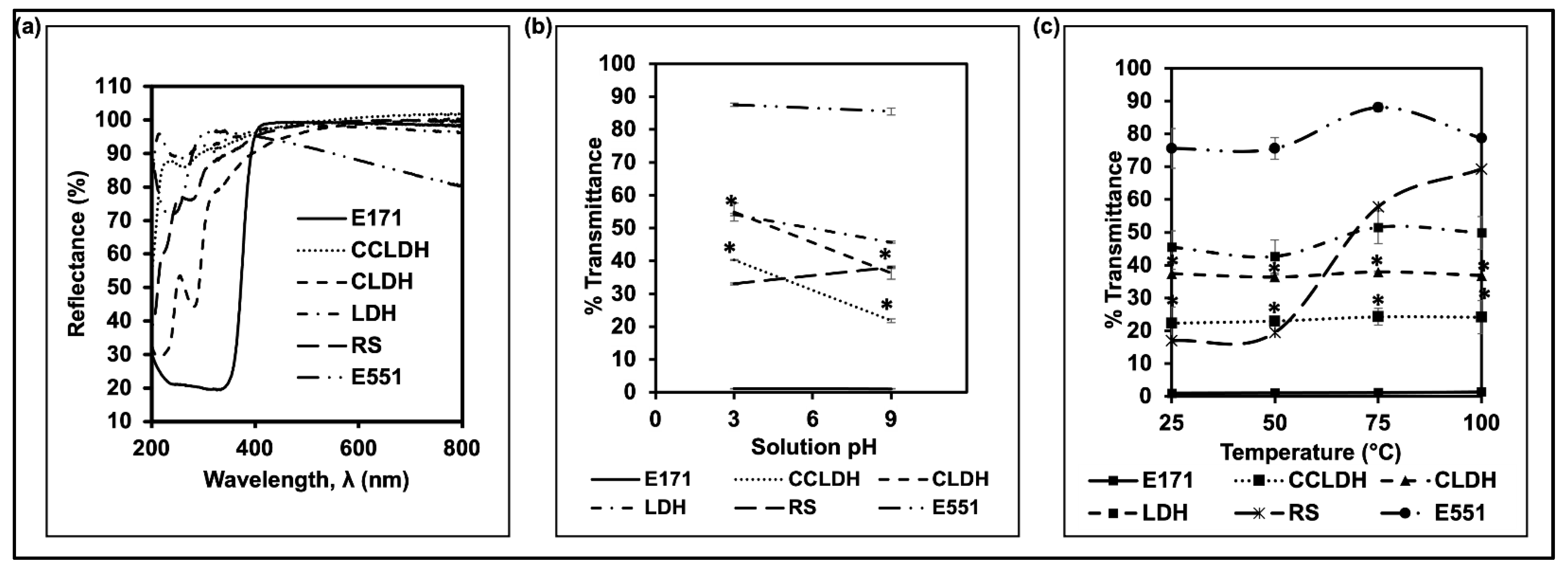
3.2. Reflectance, Stability of Particle Suspensions, and Masking Power
3.3. Antigenicity and Cytotoxicity Assessment of LDH Composites
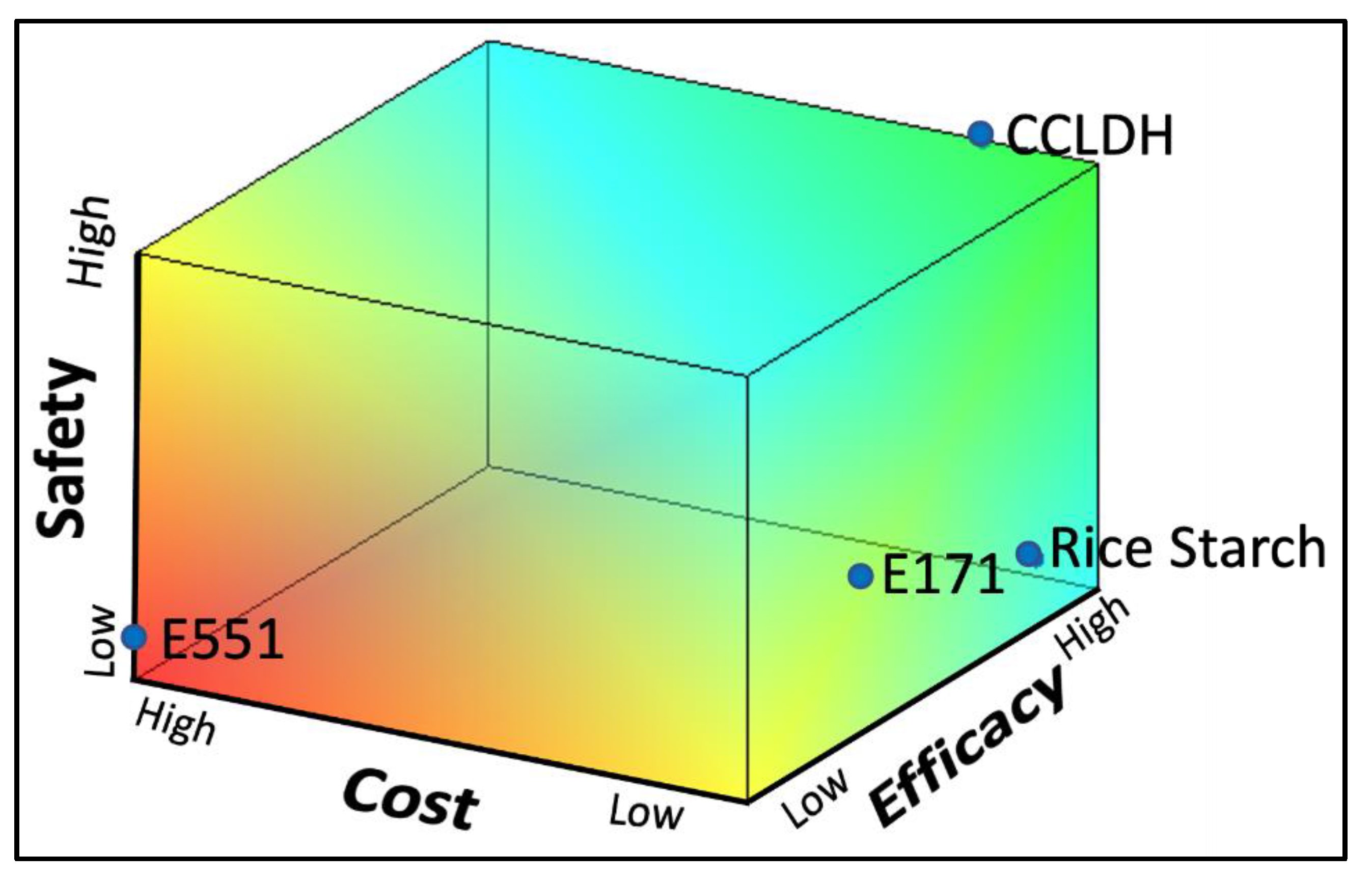
3.4. Comparison of Particles for Efficacy, Cost, and Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, S.; Kaptan, G.; Lee, J.; Frewer, L. Awareness on adverse effects of nanotechnology increases negative perception among public: Survey study from Singapore. J. Nanopart. Res. 2014, 16, 2751. [Google Scholar] [CrossRef]
- Sha, B.; Gao, W.; Wang, S.; Li, W.; Liang, X.; Xu, F.; Lu, T.J. Nano-titanium dioxide induced cardiac injury in rat under oxidative stress. Food Chem. Toxicol. 2013, 58, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Bianchi, I.; Senaldi, C.; Bucher, G.; Verleysen, E.; Waegeneers, N.; Brassinne, F.; Mast, J.; Loeschner, K.; Vidmar, J.; et al. Particle size analysis of pristine food-grade titanium dioxide and E 171 in confectionery products: Interlaboratory testing of a single-particle inductively coupled plasma mass spectrometry screening method and confirmation with transmission electron microscopy. Food Control 2021, 120, 107550. [Google Scholar] [PubMed]
- Xu, K.; Basu, N.; George, S. Dietary nanoparticles compromise epithelial integrity and enhance translocation and antigenicity of milk proteins: An in vitro investigation. NanoImpact 2021, 24, 100369. [Google Scholar] [CrossRef]
- Phue, W.H.; Xu, K.; George, S. Inorganic food additive nanomaterials alter the allergenicity of milk proteins. Food Chem. Toxicol. 2022, 162, 112874. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered Double Hydroxide Nanostructured Photocatalysts for Renewable Energy Production. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Machado, J.P.E.; de Freitas, R.A.; Wypych, F. Layered clay minerals, synthetic layered double hydroxides and hydroxide salts applied as pickering emulsifiers. Appl. Clay Sci. 2019, 169, 10–20. [Google Scholar] [CrossRef]
- Guillot, S.; Bergaya, F.; de Azevedo, C.; Warmont, F.; Tranchant, J.-F. Internally structured pickering emulsions stabilized by clay mineral particles. J. Colloid Interface Sci. 2009, 333, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Reinholdt, M.X.; Kirkpatrick, R.J. Experimental Investigations of Amino Acid-Layered Double Hydroxide Complexes: Glutamate−Hydrotalcite. Chem. Mater. 2006, 18, 2567–2576. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Balcomb, B.; Singh, M.; Singh, S. Synthesis and characterization of layered double hydroxides and their potential as nonviral gene delivery vehicles. ChemistryOpen 2015, 4, 137–145. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V.; Yeroshkina, A.; Zaichuk, A. Synthesis and characterisation of dye-intercalated nickel-aluminium layered double hydroxide as a cosmetic pigment. East. J. Enterp. Technol. 2017, 89, 27–33. [Google Scholar]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New Synthetic Routes to Hydrotalcite-Like Compounds−Characterisation and Properties of the Obtained Materials. Eur. J. Inorg. Chem. 1998, 1998, 1439–1446. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Hydrothermal Synthesis of Layered Double Hydroxides (LDHs) from Mixed MgO and Al2O3: LDH Formation Mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Wijitwongwan, R.; Intasa-ard, S.; Ogawa, M. Preparation of Layered Double Hydroxides toward Precisely Designed Hierarchical Organization. ChemEngineering 2019, 3, 68. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Gwak, G.-H.; Kim, H.-M.; Kim, T.-i.; Lee, G.J.; Oh, J.-M. Synthesis of hydrotalcite type layered double hydroxide with various Mg/Al ratio and surface charge under controlled reaction condition. Appl. Clay Sci. 2016, 134, 44–49. [Google Scholar] [CrossRef]
- Olfs, H.W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg–Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci. 2009, 43, 459–464. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S. Layered double hydroxide stability. 1. Relative stabilities of layered double hydroxides and their simple counterparts. Chem. Mater. 1999, 11, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, Y.; Jin, C.; Li, Y.; Shi, D.; Schmidt-Mende, L.; Gan, L.; Yang, J. Highly ordered monolayer/bilayer TiO2 hollow sphere films with widely tunable visible-light reflection and absorption bands. Nanoscale 2013, 5, 5009–5016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharafudeen, R. A spectroscopic method for quick evaluation of tint strength and tint tone of titania (rutile) pigment and factors affecting them. Color Res. Appl. 2019, 44, 44–49. [Google Scholar] [CrossRef]
- Bechu, A.; Ghoshal, S.; Moores, A.; Basu, N. Are Substitutes to Cd-Based Quantum Dots in Displays More Sustainable, Effective, and Cost Competitive? An Alternatives Assessment Approach. ACS Sustain. Chem. Eng. 2022, 10, 2294–2307. [Google Scholar] [CrossRef]
- Yu, B.; Bian, H.; Plank, J. Self-assembly and characterization of Ca–Al–LDH nanohybrids containing casein proteins as guest anions. J. Phys. Chem. Solids 2010, 71, 468–472. [Google Scholar] [CrossRef]
- Phue, W.H.; Liu, M.; Xu, K.; Srinivasan, D.; Ismail, A.; George, S. A Comparative Analysis of Different Grades of Silica Particles and Temperature Variants of Food-Grade Silica Nanoparticles for Their Physicochemical Properties and Effect on Trypsin. J. Agric. Food Chem. 2019, 67, 12264–12272. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Uzzaman, S. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2017, 1, 42. [Google Scholar] [CrossRef]
- Xu, Z.P.; Kurniawan, N.D.; Bartlett, P.F.; Lu, G.Q. Enhancement of Relaxivity Rates of Gd-DTPA Complexes by Intercalation into Layered Double Hydroxide Nanoparticles. Chem. Eur. J. 2007, 13, 2824–2830. [Google Scholar] [CrossRef]
- Hernandez-Moreno, M.J.; Ulibarri, M.A.; Rendon, J.L.; Serna, C.J. IR characteristics of hydrotalcite-like compounds. Phys. Chem. Miner. 1985, 12, 34–38. [Google Scholar]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.J.; Choi, A.-J.; Kim, T.-H.; Kim, T.-i.; Oh, J.-M. Layered Double Hydroxide Nanomaterials Encapsulating Angelica gigas Nakai Extract for Potential Anticancer Nanomedicine. Front. Pharmacol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V. Controlling the Synthesis Conditions for Tuning the Properties of Hydrotalcite-like Materials at the Nano Scale. ChemEngineering 2018, 2, 31. [Google Scholar] [CrossRef]
- Behzadi Nia, S.; Pooresmaeil, M.; Namazi, H. Carboxymethylcellulose/layered double hydroxides bio-nanocomposite hydrogel: A controlled amoxicillin nanocarrier for colonic bacterial infections treatment. Int. J. Biol. Macromol. 2020, 155, 1401–1409. [Google Scholar] [CrossRef]
- Musa, A.; Imam, M.; Ismail, M. Physicochemical properties of germinated brown rice (Oryza sativa L.) starch. Afr. J. Biotechnol. 2011, 10, 6281–6291. [Google Scholar]
- Farnood, R. Optical properties of paper: Theory and practice. Pulp Pap. Fud. Res. Soc. Bury 2009, 273. [Google Scholar] [CrossRef]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Ortega, I.M.U.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2016, 32, 139–149. [Google Scholar] [CrossRef]
- Xu, Z.P.; Braterman, P.S. Synthesis, structure and morphology of organic layered double hydroxide (LDH) hybrids: Comparison between aliphatic anions and their oxygenated analogs. Appl. Clay Sci. 2010, 48, 235–242. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach to Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, K.; Guo, S.; Duan, X. Nanostructural drug-inorganic clay composites: Structure, thermal property and in vitro release of captopril-intercalated Mg–Al-layered double hydroxides. J. Solid State Chem. 2006, 179, 1792–1801. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Hu, J.; Jia, Y.-B.; Liu, R.-T.; Cai, T.; Xu, Z.P. Two-Dimensional Layered Double Hydroxide Nanoadjuvant: Recent Progress and Future Direction. Nanoscale 2021, 13, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Fakurazi, S.; Zainal, Z. Development of drug delivery systems based on layered hydroxides for nanomedicine. Int. J. Mol. Sci. 2014, 15, 7750–7786. [Google Scholar] [CrossRef]
- Arancibia, C.; Costell, E.; Bayarri, S. Fat replacers in low-fat carboxymethyl cellulose dairy beverages: Color, rheology, and consumer perception. J. Dairy Sci. 2011, 94, 2245–2258. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Liu, T.; Kong, J.; Long, Z.; Zhao, M. Sodium caseinate/carboxymethylcellulose interactions at oil–water interface: Relationship to emulsion stability. Food Chem. 2012, 132, 1822–1829. [Google Scholar] [CrossRef]
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Zein-layered hydroxide biohybrids: Strategies of synthesis and characterization. Materials 2020, 13, 825. [Google Scholar] [CrossRef]
- Rojas, R.; Palena, M.C.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Giacomelli, C.E. Modeling drug release from a layered double hydroxide–ibuprofen complex. Appl. Clay Sci. 2012, 62–63, 15–20. [Google Scholar] [CrossRef]
- SIAM. Organoclays Category. In SIDS Initial Assessment Profile; The Institute of Certified Cost & Management Accountants: DE, USA, 2007. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA J. 2017, 15, e04911. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, e04545. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngew, E.; Phue, W.H.; Liu, Z.; George, S. Composite of Layered Double Hydroxide with Casein and Carboxymethylcellulose as a White Pigment for Food Application. Foods 2022, 11, 1120. https://doi.org/10.3390/foods11081120
Ngew E, Phue WH, Liu Z, George S. Composite of Layered Double Hydroxide with Casein and Carboxymethylcellulose as a White Pigment for Food Application. Foods. 2022; 11(8):1120. https://doi.org/10.3390/foods11081120
Chicago/Turabian StyleNgew, Estee, Wut Hmone Phue, Ziruo Liu, and Saji George. 2022. "Composite of Layered Double Hydroxide with Casein and Carboxymethylcellulose as a White Pigment for Food Application" Foods 11, no. 8: 1120. https://doi.org/10.3390/foods11081120
APA StyleNgew, E., Phue, W. H., Liu, Z., & George, S. (2022). Composite of Layered Double Hydroxide with Casein and Carboxymethylcellulose as a White Pigment for Food Application. Foods, 11(8), 1120. https://doi.org/10.3390/foods11081120






