Anisakicidal Effects of R (+) Limonene: An Alternative to Freezing Treatment in the Industrial Anchovy Marinating Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Plan and Media Preparation
2.2. Anisakis and Anchovy Fillet Collection
Anisakis Molecular Identification
2.3. Preparation of Fillets Experimentally Parasitized with Anisakis Larvae
2.4. Evaluation of Anisakis Viability
2.5. Treatment 1: Addition of LMN during Marinating at 4 °C
2.6. Treatment 2: Addition of LMN during Storage in Sunflower Seed Oil at 4 °C
2.7. Treatment 3: Double Treatment with LMN
2.8. Sensory Evaluation of Marinated Anchovy Fillets
2.9. Data Processing
3. Results
3.1. Anisakis Molecular Identification
3.2. Treatment 1: Addition of LMN during Marinating at 4 °C
3.3. Treatment 2: Addition of LMN during Storage in Sunflower Seed Oil at 4 °C
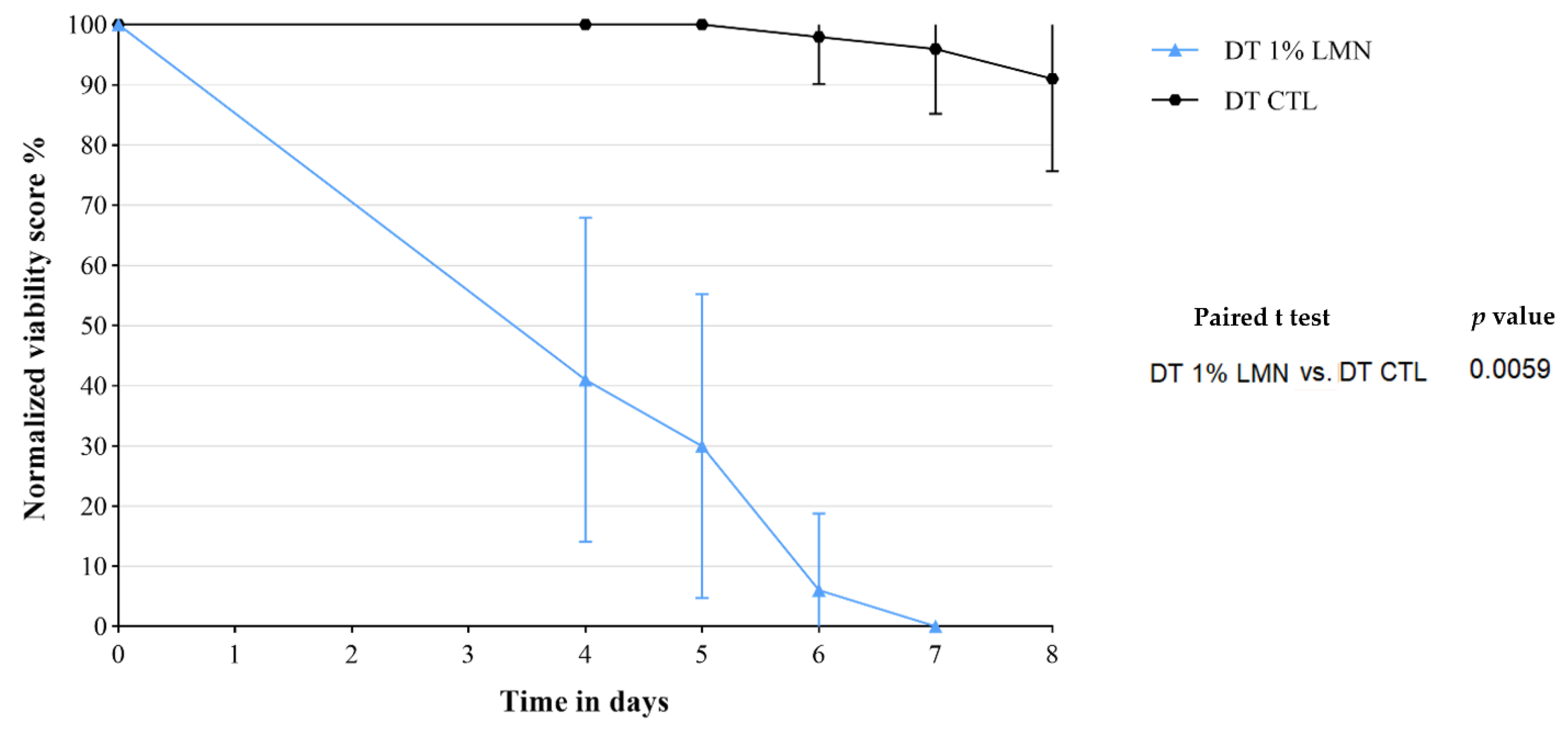
3.4. Treatment 3: Double Treatment with LMN
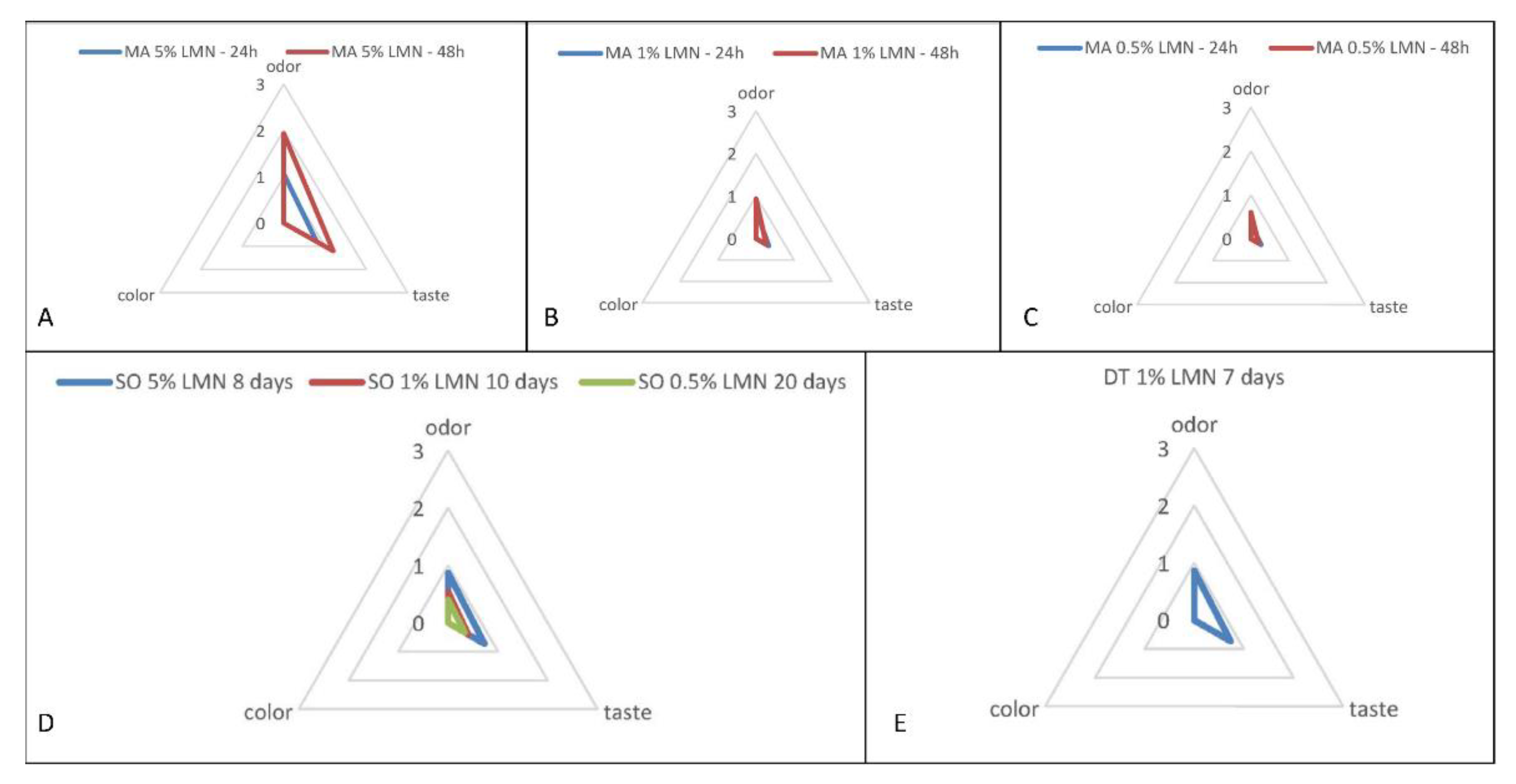
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef]
- Chai, J.Y.; Murrell, K.D.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Abollo, E.; Gestal, C.; Pascual, S. Anisakis infestation in marine fish and cephalopods from Galician waters: An updated perspective. Parasitol. Res. 2001, 87, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, A.; Ziino, G.; Pennisi, L. First report of Anisakis larva in one specimen of Sepia officinalis of Mediterranean sea. Ind. Aliment. 2002, 41, 1086–1088. [Google Scholar] [CrossRef]
- Morozinska-Gogol, J. Anisakis spp. as etiological agent of zoonotic disease and allergy in European region–an overview. Ann. Parasitol. 2019, 65, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Wootten, R.; Smith, J.W. Observational and experimental studies on the acquisition of Anisakis sp. larvae (Nematoda: Ascaridida) by trout in fresh water. Int. J. Parasitol. 1975, 5, 373–378. [Google Scholar] [CrossRef]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis nematodes in fish and shellfish-from infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef]
- Cipriani, P.; Acerra, V.; Bellisario, B.; Sbaraglia, G.L.; Cheleschi, R.; Nascetti, G.; Mattiucci, S. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: Implications to seafood safety. Food Control 2016, 59, 148–157. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on risk assessment of parasites in fishery products. EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- Furuya, K.; Nakajima, H.; Sasaki, Y.; Urita, Y. Anisakiasis: The risks of seafood consumption. Niger. J. Clin. Pract. 2018, 21, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: Dangerous—dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speciale, A.; Trombetta, D.; Saija, A.; Panebianco, A.; Giarratana, F.; Ziino, G.; Minciullo, P.L.; Cimino, F.; Gangemi, S. Exposure to Anisakis extracts can induce inflammation on in vitro cultured human colonic cells. Parasitol. Res. 2017, 116, 2471–2477. [Google Scholar] [CrossRef]
- Jerončić, A.; Nonković, D.; Vrbatović, A.; Hrabar, J.; Bušelić, I.; Martínez-Sernández, V.; Lojo Rocamonde, S.A.; Ubeira, F.M.; Jaman, S.; Jeličić, E.C.; et al. Anisakis Sensitization in the Croatian fish processing workers: Behavioral instead of occupational risk factors? PLoS Negl. Trop. Dis. 2020, 14, e0008038. [Google Scholar] [CrossRef] [Green Version]
- Uña-Gorospe, M.; Herrera-Mozo, I.; Canals, M.L.; Martí-Amengual, G.; Sanz-Gallen, P. Occupational disease due to Anisakis simplex in fish handlers. Int. Marit. Health 2018, 69, 264–269. [Google Scholar] [CrossRef]
- Bao, M.; Pierce, G.J.; Strachan, N.J.; Pascual, S.; González-Muñoz, M.; Levsen, A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends Food Sci. Technol. 2019, 86, 298–310. [Google Scholar] [CrossRef]
- Guardone, L.; Armani, A.; Nucera, D.; Costanzo, F.; Mattiucci, S.; Bruschi, F. Human anisakiasis in Italy: A retrospective epidemiological study over two decades. Parasite 2018, 25, 41. [Google Scholar] [CrossRef] [Green Version]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Debenedetti, Á.L.; Madrid, E.; Trelis, M.; Codes, F.J.; Gil-Gómez, F.; Sáez-Durán, S.; Fuentes, M.V. Prevalence and risk of anisakid larvae in fresh fish frequently consumed in Spain: An Overview. Fishes 2019, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, P.; Sbaraglia, G.L.; Palomba, M.; Giulietti, L.; Bellisario, B.; Bušelić, I.; Mladineo, I.; Cheleschi, R.; Nascetti, G.; Mattiucci, S. Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy Engraulis encrasicolus from the Mediterranean Sea: Fishing ground as a predictor of parasite distribution. Fish. Res. 2018, 202, 59–68. [Google Scholar] [CrossRef]
- Mladineo, I.; Popović, M.; Drmić-Hofman, I.; Poljak, V. A case report of Anisakis pegreffii (Nematoda, Anisakidae) identified from archival paraffin sections of a Croatian patient. BMC Infect. Dis. 2015, 16, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardone, L.; Nucera, D.; Lodola, L.B.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int. J. Food Microbiol. 2018, 268, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šimat, V.; Trumbić, Ž. Viability of Anisakis spp. larvae after direct exposure to different processing media and non-thermal processing in anchovy fillets. Fishes 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- European Comission. Regulation (EC) 1276/2011. Commission Regulation (EU) No 1276/2011 of 8 December 2011 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the treatment to kill viable parasites in fishery products for human consumption. Off. J. Eur. Union 2011, 60, 289–291. [Google Scholar]
- FDA. Parasites. In Fish and Fishery Products Hazards and Controls Guidance Food and Drug Administration, 4th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2021; pp. 91–98. [Google Scholar]
- Serracca, L.; Battistini, R.; Rossini, I.; Carducci, A.; Verani, M.; Prearo, M.; Tomei, L.; De Montis, G.; Ercolini, C. Food safety considerations in relation to Anisakis pegreffii in anchovies (Engraulis encrasicolus) and sardines (Sardina pilchardus) fished off the Ligurian Coast (Cinque Terre National Park, NW Mediterranean). Int. J. Food Microbiol. 2014, 190, 79–83. [Google Scholar] [CrossRef]
- Duarte, A.M.; Silva, F.; Pinto, F.R.; Barroso, S.; Gil, M.M. Quality Assessment of Chilled and Frozen Fish—Mini Review. Foods 2020, 9, 1739. [Google Scholar] [CrossRef]
- Sampels, S. The effects of storage and preservation technologies on the quality of fish products: A review. J. Food Process. Preserv. 2015, 39, 1206–1215. [Google Scholar] [CrossRef]
- Anastasio, A.; Smaldone, G.; Cacace, D.; Marrone, R.; Voi, A.L.; Santoro, M.; Cringoli, G.; Pozio, E. Inactivation of Anisakis pegreffii larvae in anchovies (Engraulis encrasicolus) by salting and quality assessment of finished product. Food Control 2016, 64, 115–119. [Google Scholar] [CrossRef]
- Sánchez-Monsalvez, I.; de Armas-Serra, C.; Martinez, J.; Dorado, M.; Sanchez, A.; Rodriguez-Caabeiro, F. A new procedure for marinating fresh anchovies and ensuring the rapid destruction of Anisakis larvae. J. Food Prot. 2005, 68, 1066–1072. [Google Scholar] [CrossRef]
- Trabelsi, N.; Marotta, S.M.; Giarratana, F.; Taamali, A.; Zarrouk, M.; Ziino, G.; Giuffrida, A. Use of Tunisian flavored olive oil as anisakicidal agent in industrial anchovy marinating process. J. Sci. Food Agric. 2018, 98, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Giuffrida, A.; Panebianco, A. Activity of Thymus vulgaris essential oil against Anisakis larvae. Exp. Parasitol. 2014, 142, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Panebianco, F.; Patania, A.; Benianti, C.; Ziino, G.; Giuffrida, A. Activity of R (+) limonene against Anisakis larvae. Ital. J. Food Saf. 2015, 4, 5499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giarratana, F.; Muscolino, D.; Ziino, G.; Giuffrida, A.; Marotta, S.M.; Lo Presti, V.; Chiofalo, V.; Panebianco, A. Activity of Tagetes minuta Linnaeus (Asteraceae) essential oil against L3 Anisakis larvae type 1. Asian Pac. J. Trop. 2017, 10, 461–465. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Ziino, G.; Lo Presti, V.; Rao, R.; Chiofalo, V.; Giuffrida, A.; Panebianco, A. Activity of Catmint (Nepeta cataria) essential oil against Anisakis larvae. Trop. Biomed. 2017, 34, 22–31. [Google Scholar]
- Trabelsi, N.; Nalbone, L.; Di Rosa, A.R.; Ed-Dra, A.; Nait-Mohamed, S.; Mhamdi, R.; Giuffrida, A.; Giarratana, F. Marinated anchovies (Engraulis encrasicolus) prepared with flavored olive oils (Chétoui cv.): Anisakicidal effect, microbiological, and sensory evaluation. Sustainability 2021, 13, 5310. [Google Scholar] [CrossRef]
- Trabelsi, N.; Nalbone, L.; Marotta, S.M.; Taamali, A.; Abaza, L.; Giarratana, F. Effectiveness of five flavored Tunisian olive oils on Anisakis larvae type 1: Application of cinnamon and rosemary oil in industrial anchovy marinating process. J. Sci. Food Agric. 2019, 99, 4808–4815. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Presti, V.L.; Zekkori, B.; Nalbone, L.; Elsharkawy, E.R.; Bentayeb, A.; Giarratana, F. Effectiveness of essential oil from the Artemisia herba-alba aerial parts against multidrug-resistant bacteria isolated from food and hospitalized patients. Biodiversitas J. 2021, 22, 2995–3005. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; El Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive evaluation on the use of Thymus vulgaris essential oil as natural additive against different serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Muscolino, D.; Giarratana, F.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Effects of allyl isothiocyanate on the shelf-life of gilthead sea bream (Sparus aurata) fillets. Czech J. Food Sci. 2016, 34, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Pezeshk, S.; Ojagh, S.M.; Alishahi, A. Effect of plant antioxidant and antimicrobial compounds on the shelf-life of seafood-a review. Czech J. Food Sci. 2015, 33, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, A.; Panebianco, A. Igiene e tecnologie dei prodotti della pesca freschi e trasformati. In Igiene e Tecnologia dei Prodotti di Origine Animale, 1st ed.; Colavita, G., Ed.; Le Point Vétérinarie Italie: Milan, Italy, 2012; pp. 274–276. [Google Scholar]
- Cammilleri, G.; Pulvirenti, A.; Costa, A.; Graci, S.; Collura, R.; Buscemi, M.D.; Badaco, V.V.; Vazzana, M.; Brunone, M.; Vella, A.; et al. Seasonal trend of Anisakidae infestation in South Mediterranean bluefish. Nat. Prod. Res. 2020, 34, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Ferrantelli, V.; Cicero, A.; Costa, A.; Alongi, A.; Palumbo, P.; Graci, S.; Giangrosso, G. Anisakidae in fishing products sold in Sicily. Ital. J. Food Saf. 2014, 3, 1719. [Google Scholar] [CrossRef] [Green Version]
- D’amelio, S.; Mathiopoulos, K.D.; Santos, C.P.; Pugachev, O.N.; Webb, S.C.; Picanço, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000, 30, 223–226. [Google Scholar] [CrossRef]
- Bao, M.; Cipriani, P.; Giulietti, L.; Roiha, I.S.; Paoletti, M.; Palomba, M.; Levsen, A. Air-dried stockfish of Northeast Arctic cod do not carry viable anisakid nematodes. Food Control 2020, 116, 107322. [Google Scholar] [CrossRef]
- Giarratana, F.; Giuffrida, A.; Gallo, F.; Ziino, G.; Panebianco, A. Study of the resistance variability of Anisakis larvae to some technological stressors. In Veterinary Science, 1st ed.; Pugliese, A., Gaiti, A., Boiti, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 155–159. [Google Scholar]
- Guan, A.; Van Damme, I.; Devlieghere, F.; Gabriël, S. Effect of temperature, CO2 and O2 on motility and mobility of Anisakidae larvae. Sci. Rep. 2021, 11, 4279. [Google Scholar] [CrossRef]
- Hirasa, K.; Takemasa, M. Spice Science and Technology, 1st ed.; Hirasa, K., Takemasa, M., Eds.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Kroeger, M.; Karl, H.; Simmler, B.; Singer, P. Viability Test Device for anisakid nematodes. Heliyon 2018, 4, e00552. [Google Scholar] [CrossRef] [Green Version]
- Smaldone, G.; Ambrosio, R.L.; Marrone, R.; Ceruso, M.; Anastasio, A. Anisakis spp. Larvae in deboned, in-oil fillets made of anchovies (Engraulis encrasicolus) and sardines (Sardina pilchardus) sold in EU retailers. Animals 2020, 10, 1807. [Google Scholar] [CrossRef]
- Ksouda, G.; Sellimi, S.; Merlier, F.; Falcimaigne-Cordin, A.; Thomasset, B.; Nasri, M.; Hajji, M. Composition, antibacterial and antioxidant activities of Pimpinella saxifraga essential oil and application to cheese preservation as coating additive. Food Chem. 2019, 288, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Avato, P.; Laquale, S.; Argentieri, M.P.; Lamiri, A.; Radicci, V.; D’Addabbo, T. Nematicidal activity of essential oils from aromatic plants of Morocco. J. Pest Sci. 2017, 90, 711–722. [Google Scholar] [CrossRef]
- European Comission. Regulation (EC) No 872/2012. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. Off. J. Eur. Union 2012, 50, 152–312. [Google Scholar]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [Green Version]
- Garre, A.; Espín, J.F.; Huertas, J.P.; Periago, P.M.; Palop, A. Limonene nanoemulsified with soya lecithin reduces the intensity of non-isothermal treatments for inactivation of Listeria monocytogenes. Sci. Rep. 2020, 10, 3656. [Google Scholar] [CrossRef]
- Zahi, M.R.; Liang, H.; Yuan, Q. Improving the antimicrobial activity of D-limonene using a novel organogel-based nanoemulsion. Food Control 2015, 50, 554–559. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Activity of R (+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int. J. Food Microbiol. 2016, 237, 109–113. [Google Scholar] [CrossRef]
- Del Carmen Romero, M.; Valero, A.; Martín-Sánchez, J.; Navarro-Moll, M.C. Activity of Matricaria chamomilla essential oil against anisakiasis. Phytomedicine 2012, 19, 520–523. [Google Scholar] [CrossRef]
- FDA. Food and Drug Administration. Title 21CFR182.60. 2012. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.60&SearchTerm=limonene (accessed on 1 March 2022).
- Lipsa, D.; Lev, P.; Barrero-Moreno, J.; Coelhan, M. Inflammatory effects induced by selected limonene oxidation products: 4-OPA, IPOH, 4-AMCH in human bronchial (16HBE14o) and alveolar (A549) epithelial cell lines. Toxicol. Lett. 2016, 262, 70–79. [Google Scholar] [CrossRef]
- Vidaček, S.; de las Heras, C.; Solas, M.T.; Mendizabal, A.; Rodriguez-Mahillo, A.I.; González-Munoz, M.; Tejada, M. Anisakis simplex allergens remain active after conventional or microwave heating and pepsin treatments of chilled and frozen L3 larvae. J. Sci. Food Agric. 2009, 89, 1997–2002. [Google Scholar] [CrossRef] [Green Version]



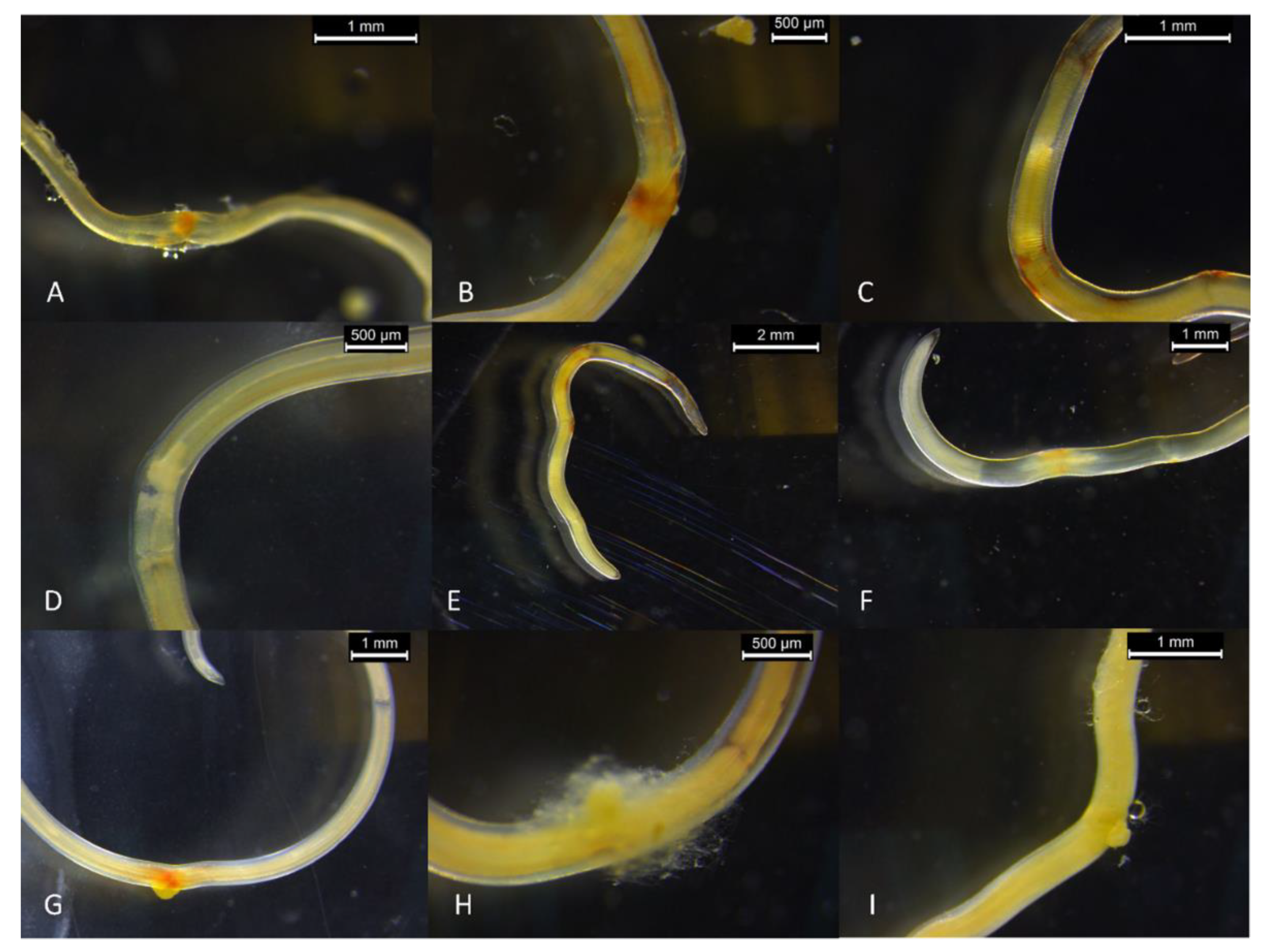
| Viability Score | Score Descriptor | Criteria |
|---|---|---|
| 3 | Viable | In situ movement of the whole larval body |
| 2 | Reduction of motility | In situ movement of at least one part of the larval body |
| 1 | Motile only after stimulation | In situ movement of at least one part of the larval body only after mechanical stimulation |
| 0 | Death | No in situ movement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalbone, L.; Panebianco, F.; Cammilleri, G.; Ferrantelli, V.; Giarratana, F. Anisakicidal Effects of R (+) Limonene: An Alternative to Freezing Treatment in the Industrial Anchovy Marinating Process. Foods 2022, 11, 1121. https://doi.org/10.3390/foods11081121
Nalbone L, Panebianco F, Cammilleri G, Ferrantelli V, Giarratana F. Anisakicidal Effects of R (+) Limonene: An Alternative to Freezing Treatment in the Industrial Anchovy Marinating Process. Foods. 2022; 11(8):1121. https://doi.org/10.3390/foods11081121
Chicago/Turabian StyleNalbone, Luca, Felice Panebianco, Gaetano Cammilleri, Vincenzo Ferrantelli, and Filippo Giarratana. 2022. "Anisakicidal Effects of R (+) Limonene: An Alternative to Freezing Treatment in the Industrial Anchovy Marinating Process" Foods 11, no. 8: 1121. https://doi.org/10.3390/foods11081121
APA StyleNalbone, L., Panebianco, F., Cammilleri, G., Ferrantelli, V., & Giarratana, F. (2022). Anisakicidal Effects of R (+) Limonene: An Alternative to Freezing Treatment in the Industrial Anchovy Marinating Process. Foods, 11(8), 1121. https://doi.org/10.3390/foods11081121








